Abstract
Background
The purpose of this report is to summarize our clinical experience of patients with stage I/II extranodal natural killer (NK)/T-cell lymphoma, nasal type, treated using sequential chemotherapy followed by radiotherapy (SCRT) or concurrent chemoradiotherapy (CCRT).
Methods
Forty-three patients with stage I/II extranodal NK/T-cell lymphoma, nasal type, who received SCRT (16 patients) or CCRT (27 patients) were included in the present analysis.
Results
The median follow-up time was 39 months (range, 4-171 months) for all patients, 77 months (range, 4-171 months) for the SCRT group, and 31 months (range, 6-132 months) for the CCRT group. There were no statistically significant differences between the SCRT and CCRT groups with regard to the 3-year progression-free survival (PFS) (56% vs. 41%, P=0.823) and 3-year overall survival (OS) (75% vs. 59%, P=0.670). Univariate analysis revealed that patients with tumors confined to the nasal cavity and patients achieved complete remission had better PFS and OS rates, regardless of the treatment sequence. Multivariate analysis revealed that patients with tumors confined to the nasal cavity and patients aged ≤60 years had better OS rates.
Conclusion
The effect of SCRT and CCRT are similar in terms of survival outcomes of patients with stage I/II extranodal NK/T-cell lymphoma, nasal type. Our results show that tumors confined to the nasal cavity and an age ≤60 years were associated with a better prognosis.
Keywords: Extranodal NK/T-cell lymphoma, Nasal type, Chemoradiotherapy, Treatment outcome
INTRODUCTION
Extranodal natural killer (NK)/T-cell lymphoma, nasal type, is a rare subtype of non-Hodgkin lymphoma that is more common in Asia and Latin America than in Western countries [1]. In South Korea, the crude incidence rate of extranodal NK/T-cell lymphoma has increased 3-fold over the past 10 years, to 0.38 cases per 100,000 population in 2010 [2]. Approximately 80% of patients with extranodal NK/T-cell lymphoma present with localized disease (stage I/II). In general, extranodal NK/T-cell lymphoma, nasal type, has a poor prognosis, with a 3-year overall survival (OS) rate of 57% to 70% for stage I/II disease and 20% for stage III/IV disease [3-5].
Extranodal NK/T-cell lymphoma that occurs as localized disease (stage I/II) responds well to radiation therapy (RT) with or without chemotherapy [2-4]. Combination therapy that includes both RT and chemotherapy, which is administered either sequentially or concurrently, is the current treatment of choice [5, 6]. No randomized trials have compared treatment sequences for extranodal NK/T cell lymphoma because of the rarity of this disease. Therefore, the optimal combination and sequence of RT and chemotherapy have not been fully defined.
To address this issue, we conducted a retrospective study to compare the treatment outcomes of patients with extranodal NK/T-cell lymphoma, nasal type, who received either sequential chemotherapy followed by RT (SCRT) or concurrent chemoradiotherapy (CCRT). The purpose of this study was to summarize our clinical experience with SCRT and CCRT for treatment of patients with extranodal NK/T-cell lymphoma. We hope this information will aid clinicians in their efforts to determine the type of patients who would most likely benefit from these treatment regimens.
MATERIALS AND METHODS
Patients
This study was performed in accordance with the guidelines of the Institutional Review Board of the Korea University Medical Center. The study population consisted of 49 consecutive patients diagnosed with Ann Arbor stage I/II extranodal NK/T-cell lymphoma, nasal type, at the Korea University Anam Hospital, Korea, between 1998 and 2012. Four patients whose medical records were not accessible and two patients who refused treatment were excluded from this study. The remaining 43 patients with extranodal NK/T-cell lymphoma, nasal type who received SCRT or CCRT were included in the present analysis.
Evaluation
All patients underwent a standardized pre-treatment workup that included recording of medical history, a complete physical examination, determination of B symptoms and performance status, laboratory studies (complete blood cell count, differential platelet count, comprehensive metabolic panel, evaluation of serum lactate dehydrogenase [LDH] and β2 microglobulin levels, and Epstein-Barr virus [EBV] RNA in situ hybridization), imaging studies (computed tomography and magnetic resonance imaging), positron emission tomography, and bone marrow aspiration and biopsy.
Response was evaluated according to the revised International Working Group guidelines [6]. Complete response (CR) was defined as the disappearance of all evidence of disease, partial response (PR) was defined as a 50% decrease in measurable disease, progressive disease (PD) was defined as the appearance of any new lesion or a ≥50% increase in previously involved sites, and stable disease (SD) was defined as failure to attain CR/PR or PD. A complete physical examination, laboratory studies, and imaging studies were performed 1 month after completion of the planned treatment, and they were repeated every 3 to 6 months thereafter.
Treatment and toxicity
Radiotherapy was delivered using either the 3-field technique (1 anterior portal and 2 lateral portals) or the parallel-opposed 2-field technique with 4- or 6-MV photon beams. The median radiation doses were 54 Gy (range, 44-61.2 Gy) and 45 Gy (range, 44-54 Gy) for the SCRT and CCRT groups, respectively. Elective nodal irradiation was performed if the primary lesion was located in the oropharynx or hypopharynx or if lymph node metastasis was clinically suspected.
Prior to 2006, SCRT was used to treat Ann Arbor stage I/II extranodal NK/T-cell lymphoma, nasal type, at our institution, whereas clinicians began to use CCRT after 2006. Sixteen patients (37%) were treated with SCRT, and 27 patients (63%) were treated with CCRT. In the SCRT group, 14 patients were treated with a median of 4 cycles (range, 1-6 cycles) of cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisolone ([CHOP]-like regimen). Of these 14 patients, 11 received cyclophosphamide, epirubicin, vincristine, prednisolone, and bleomycin, and 3 received cyclophosphamide, epirubicin, vincristine, prednisolone, and etoposide. The remaining 2 patients in the SCRT group received a non-doxorubicin-containing chemotherapy regimen. CCRT comprised RT with weekly intravenous administration of cisplatin (30 mg/m2) as a radiosensitizer. Of the 27 patients in the CCRT group, 18 (67%) received a median of 3 cycles (range, 1-4 cycles) of additional chemotherapy. Of these 18 patients, 11 patients received a regimen of etoposide, ifosfamide, cisplatin, and dexamethasone (VIPD); 4 patients received a regimen of etoposide, ifosfamide, dexamethasone, and L-asparaginase; and 3 patients received a regimen of dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE). Treatment compliance for additional chemotherapy was 56%, which was defined as the proportion of patients who completed all 3 planned cycles of chemotherapy according to a study by Kim et al., in which 3 cycles of VIPD was chosen to avoid unnecessary toxicities [7]. Acute toxicity was graded according to the Radiation Therapy Oncology Group criteria [8].
Statistics
The primary endpoint of the study was progression-free survival (PFS), which was defined as the time interval between diagnosis and tumor progression or death from any cause. OS was defined as the time interval from the diagnosis to the date of death from any cause or the last follow-up. Survival rates were estimated using the Kaplan-Meier method, with differences compared using the log-rank test. The Cox proportional hazards regression model was used for multivariate analysis. The chi-square test, Fisher exact test, or Student t-test was used for intergroup comparisons. A P-value<0.05 was considered statistically significant. Statistical analysis was performed using the IBM SPSS statistics release 20 (IBM Inc., Somers, NY, USA).
RESULTS
Patient characteristics
The clinical characteristics of the 43 patients included in the study are summarized in Table 1. There were no significant differences in patient characteristics between the SCRT and CCRT groups. The male to female ratio was 1.5:1, and all patients had an Eastern Cooperative Oncology Group performance score of 0 or 1. The nasal cavity was the most common site of primary disease (32 patients [74%]). Of these patients, 18 had tumors confined to the nasal cavity (7 in the SCRT group and 11 in the CCRT group), 2 had tumors with nasopharyngeal extension (1 in each of the SCRT and CCRT groups), 4 patients had tumors extending to the oropharynx (1 in the SCRT group and 3 in the CCRT group), and 8 patients had tumors with paranasal sinus extension (2 in the SCRT group and 6 in the CCRT group). EBV RNA in situ hybridization was performed in 29 patients (67%) and yielded positive results in 26 patients (3 in the SCRT group and 23 in the CCRT group). No patients showed evidence of bone marrow involvement.
Table 1.
Patient characteristics.
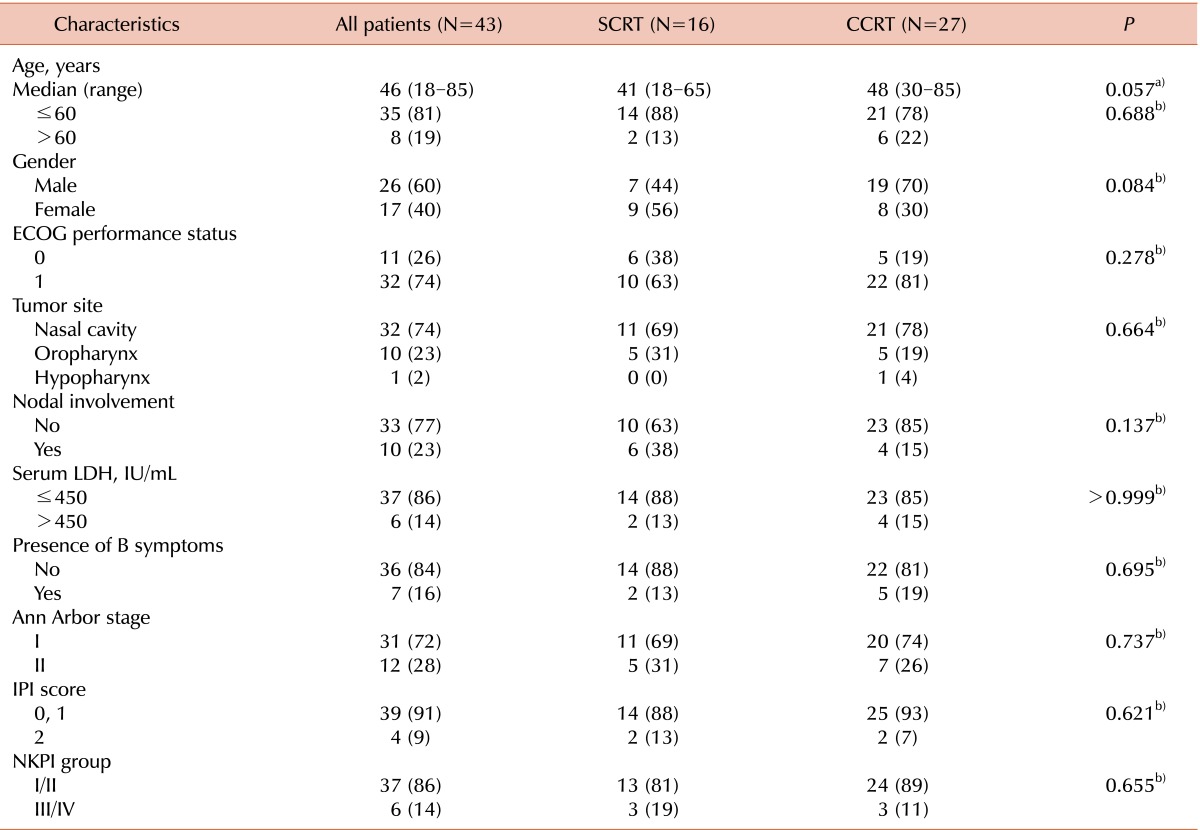
a)Student t-test, b)Pearson χ2 test, or Fisher exact test. Data are presented as number of patients (%) unless otherwise indicated.
Abbreviations: SCRT, sequential chemotherapy followed by radiotherapy; CCRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; IPI, International Prognostic Index; NKPI, natural killer/T cell Prognostic Index.
Response to treatment
In the SCRT group, CR, PR, SD, and PD were observed in 4 (25%), 8 (50%), 2 (13%), and 2 (31%) patients, respectively, after completion of chemotherapy. After completion of SCRT, CR, PR, and PD were observed in 10 (63%), 5 (31%), and 1 (6%) patient, respectively. Seven patients showed improved response after completion of RT: 4 patients with initial PR, 1 patient with SD, and 1 patient with PD showed CR and 1 patient with SD showed PR. Response in 9 patients did not change after completion of RT: 4 patients with CR, 4 patients with PR, and 1 patient with PD. In the CCRT group, CR, PR, and PD were observed in 19 (70%), 7 (26%), and 1 (4%) patient, respectively, after the completion of CCRT. There was no significant difference in the CR rate between the 2 groups (P=0.597). In the entire study population, CR, PR, and PD were observed in 29 (67%), 12 (28%), and 2 (5%) patients, respectively. The overall response rate (CR or PR) was 95%. Of the 18 patients who received additional chemotherapy in the CCRT group, 1 patient with a PR achieved CR and the initial response did not change in 13 patients (10 patients with CR, 2 patients with PR, and 1 patient with PD). However, response in 4 patients deteriorated despite additional chemotherapy: 1 patient with CR showed PR and 3 patients with PR showed PD. After additional chemotherapy, CR, PR, and PD were observed in 11 (61%), 3 (17%), and 4 (22%) patients, respectively.
Toxicity
Patients experienced the acute toxicities commonly associated with SCRT or CCRT. Grade 1 or 2 mucositis and leukopenia were the most common acute toxicities. In 8 patients, RT was suspended due to toxicity, but was restarted within 10 days. In the SCRT group, 4 patients (25%) had grade 4 leukopenia during chemotherapy, but they were all able to continue treatment without interruption. All the patients with this condition fully recovered with supportive care during hospitalization. Grade 3 toxicities were observed in 13 patients (30%). In the SCRT group, grade 3 leukopenia, anemia, thrombocytopenia, pancreatitis, and mucositis were observed in 3 (19%), 2 (6%), 1 (6%), 1 (6%), and 1 (6%) patient, respectively. In the CCRT group, grade 3 leukopenia and mucositis were observed in 1 (4%) and 5 (19%) patients, respectively. No patients experienced acute nephrotoxicity during CCRT. No patients experienced treatment-related mortality during SCRT, CCRT or additional chemotherapy in the CCRT group.
Patterns of failure
The median follow-up time was 39 months (range, 4-171 months) for all patients, 77 months (range, 4-171 months) for the SCRT group, and 31 months (range, 6-132 months) for the CCRT group. During the follow-up period, a total of 23 patients (53%; 8 of 16 [50%] in the SCRT group and 15 of 27 [56%] in the CCRT group) experienced disease recurrence. Eleven patients (26%) experienced local failure (5 of 16 [31%] in the SCRT group vs. 6 of 27 [22%] in the CCRT group, P=0.719). Two patients (5%) treated with CCRT experienced regional failure. Eleven patients (26%) experienced distant failure (3 of 16 [19%] in the SCRT group and 8 of 27 [30%] in the CCRT group, P=0.494), and 1 patient (2%) in the CCRT group had both local and regional failure. Among the 23 patients with disease recurrence, 19 received salvage treatment (successful in 8 patients), 3 received conservative management, and 1 refused treatment. For salvage treatment, 6 patients received local therapy, excision, or radiotherapy, which was successful in 4 patients (67%). Thirteen patients received chemotherapy, which was successful in 4 patients (31%). However, 1 patient with local recurrence and 1 with regional recurrence died of complications that arose during salvage chemotherapy with the SMILE regimen. No significant difference in the overall treatment failure rate was observed between the 2 groups (P=0.761). Although local failure was more frequent in the SCRT group (5 of 8 [63%] with recurrence) than in the CCRT group (6 of 15 [40%] with recurrence), there was no significant difference between the 2 groups (P=0.400). The treatment failure rates, survival outcomes, and follow-up results for each treatment group are summarized in Tables 2 and 3.
Table 2.
Treatment failure, survival outcomes, and follow-up results for sequential chemotherapy followed by radiotherapy (SCRT) group.
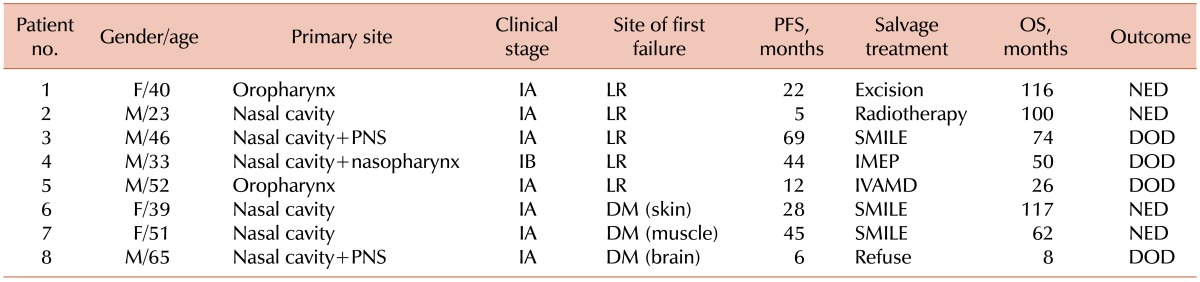
Abbreviations: PFS, progression-free survival; OS, overall survival; LR, local recurrence; NED, no evidence of disease; PNS, paranasal sinus; DOD, died of disease; DM, distant metastasis.
Table 3.
Treatment failure, survival outcomes, and follow-up results for the concurrent chemoradiotherapy (CCRT) group.
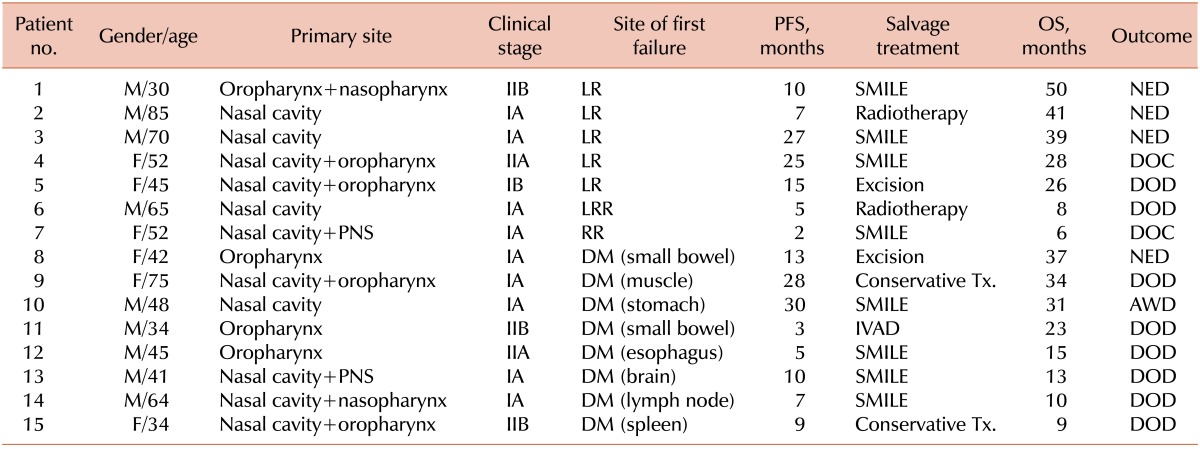
Abbreviations: PFS, progression-free survival; OS, overall survival; LR, local recurrence; NED, no evidence of disease; DOC, died of complication; DM, distant metastasis; DOD, died of disease; LRR, locoregional recurrence; RR, regional recurrence; PNS, paranasal sinus; Tx, treatment.
Survival
The 3-year PFS rate of all patients was 48%; there was no statistical difference between the 2 groups (56% and 41% for the SCRT and CCRT groups, respectively; P=0.823) (Fig. 1). The 3-year OS rate of all patients was 66%; there was no significant difference between the 2 treatment groups (75% and 59% for the SCRT and CCRT groups, respectively; P=0.670) (Fig. 2). At the time of analysis, 7 patients (44%) in the SCRT group and 10 patients (37%) in the CCRT group had died. Twelve patients (4 in the SCRT group and 8 in the CCRT group) died of disease recurrence or progression. Three patients in the SCRT group died of unknown causes or cardiovascular events without evidence of disease recurrence. There was no significant difference in the 3-year OS rate of stage I patients between the 2 treatment groups (82% and 67% for the SCRT and CCRT groups, respectively; P=0.687).
Fig. 1.
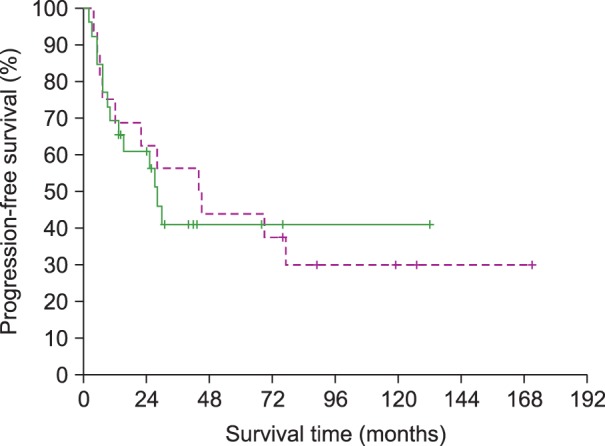
Comparison of progression-free survival between patients treated with sequential chemotherapy followed by radiotherapy (SCRT, dotted line) and those treated with concurrent chemoradiotherapy (CCRT, solid line) (P=0.823).
Fig. 2.
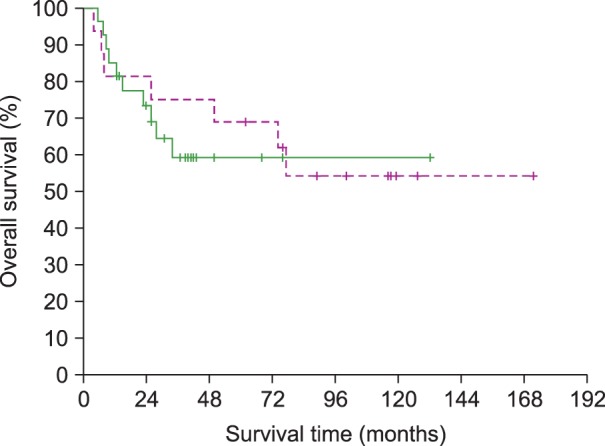
Comparison of overall survival between patients treated with sequential chemotherapy followed by radiotherapy (SCRT, dotted line) and those treated with concurrent chemoradiotherapy (CCRT, solid line) (P=0.670).
Factors affecting progression-free survival and overall survival
Table 4 summarizes the results of the univariate analysis of clinical variables associated with the PFS and OS rates in all patients. Age (≤60 years), tumor extension (confinement to the nasal cavity), and the absence of B symptoms were correlated with better PFS and OS. A low International Prognostic Index score and NK/T-cell Prognostic Index grade predicted a statistically significant superior OS. The sequence of treatment (SCRT or CCRT) was not associated with PFS or OS. The median survival time was 42 months for patients who achieved CR compared to 26 months for those showing either PR or PD (P=0.016). The 3-year PFS rate for patients achieving a CR was 63%, whereas the 3-year PFS rate for those showing PR or PD was 15% (P=0.005). The 3-year OS rate for patients achieving a CR was 82%, whereas the 3-year OS rate for those showing a PR or PD was 34% (P=0.016). The clinical variables significantly associated with survival outcomes were included in a multivariate analysis. The factors of age ≤60 years (relative risk, 0.20 [95% confidence interval, 0.06-0.72]; P=0.014) and tumor confinement to the nasal cavity (relative risk, 0.15 [95% confidence interval, 0.03-0.69]; P=0.015) were correlated with better OS.
Table 4.
Univariate analysis of clinical variables associated with progression-free survival and overall survival for all patients (N=43).
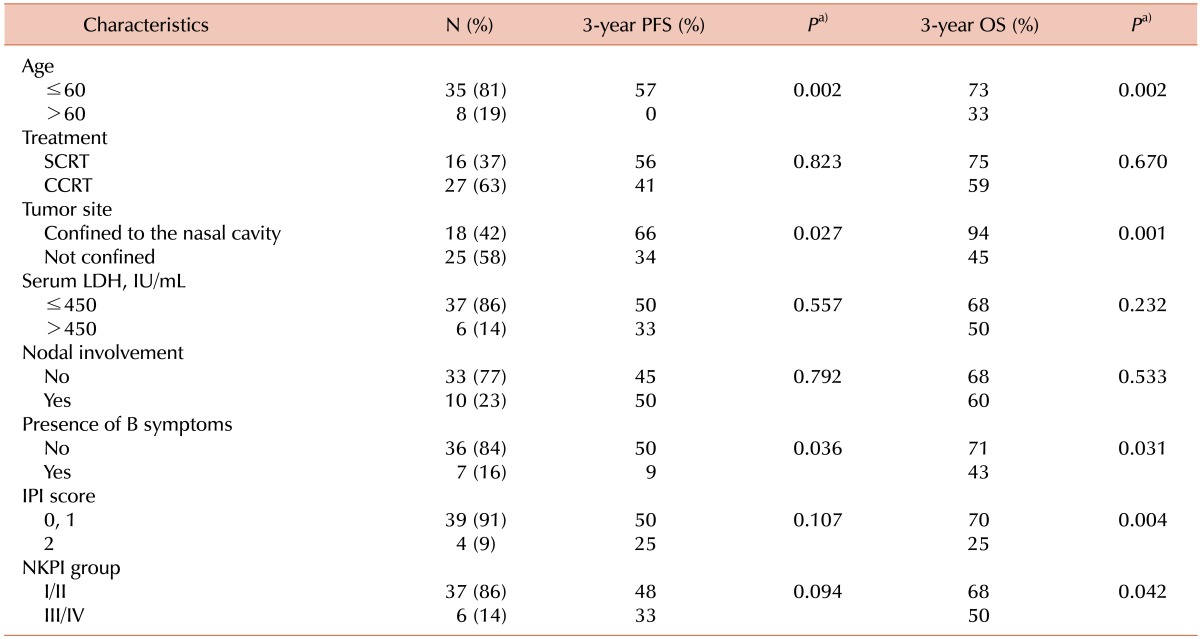
a)Log-rank test.
Abbreviations: PFS, progression-free survival; OS, overall survival; SCRT, sequential chemotherapy followed by radiotherapy; CCRT, concurrent chemoradiotherapy; LDH, lactate dehydrogenase; IPI, International Prognostic Index; NKPI, natural killer/T cell Prognostic Index.
DISCUSSION
The standard treatment for extranodal NK/T-cell lymphoma, nasal type, is RT combined with chemotherapy. Various factors, including age, B symptoms, performance status, an elevated LDH level, and regional lymph node involvement, are known to affect the survival outcome [3]. Patients with stage I disease and without the risk factors can be treated with RT alone, whereas those with stage I disease and poor risk factors or stage II disease can be treated with SCRT or CCRT [9].
Several studies have reported that patients treated with SCRT have a 2-year PFS rate of 60% to 62% and a 2-year OS rate of 70% to 76% [10-13]. Kim et al. reported that 17 patients with stage IE/IIE, non-bulky nasal NK/T-cell lymphoma had a 3-year OS rate of 59% when they were treated with a CHOP-based chemotherapy followed by a total dose of 45 Gy using involved field RT [10]. Wang et al. reported the outcome of 53 patients with stage IE/IIE NK/T-cell lymphoma arising in the nasal cavity treated with CHOP-based chemotherapy followed by involved field RT. A median of 45 Gy (range, 36-50 Gy) was delivered using a Cobalt-60 teletherapy unit or a linear accelerator. The 2-year PFS and OS rates were 62% and 76%, respectively. No grade 3, 4, or 5 toxicities occurred [11]. In our present study, the median radiation dose for the SCRT group was 54 Gy, which was higher than the dose prescribed in these previous studies. Nonetheless, there were no significant differences in survival outcomes or toxicity rates compared to previous studies.
Several researchers have reported a beneficial role for CCRT in the treatment of stage I/II nasal NK/T-cell lymphoma. Yamaguchi et al. reported the outcome of 33 patients with stage I/II nasal NK/T-cell lymphoma, who were treated with 3 cycles of dexamethasone, etoposide, ifosfamide, and carboplatin chemotherapy and concurrent radiotherapy (50 Gy). Grade 3 or greater acute toxicities commonly occurred; however, there were no grade 3 or greater late toxicities. Treatment compliance for CCRT was 94%, and the 2-year OS rate was 79% [14]. Kim et al. conducted a phase II trial that included 30 patients with stage I/II nasal NK/T-cell lymphoma, who were treated with RT (40 Gy) administered concurrently with weekly cisplatin, followed by 3 cycles of VIPD chemotherapy. Grade 3 or greater toxicities were minimal during CCRT but were frequent during VIPD. Grade 4 leukopenia was observed in 27% of the patients. The 3-year PFS and OS rates were 85% and 86%, respectively [7]. Our study had an inferior outcome when compared to these studies. However, these findings must be interpreted with caution. Several factors in the present analysis may have affected the treatment outcomes. First, 67% of the patients in the CCRT group received additional chemotherapy, where compliance was only 56%. Second, the CCRT group in this study included a higher percentage (21%) of relatively older patients (>60 years) compared to the previous study (13%). Third, the previous CCRT trial only included tumors that arose from the nasal cavity, while our study included not only nasal cavity tumors but also oropharynx and hypopharynx tumors. This heterogeneity in the primary tumor site may have contributed to the differences in survival outcomes. Finally, the small number of patients in the present analysis was also a limitation.
To date, there have been few reports comparing SCRT and CCRT. Lee et al. retrospectively analyzed the outcomes of 46 patients with stage I/II nasal NK/T-cell lymphoma, where 33 patients were treated with SCRT (50.4 Gy in 28 fractions) and 13 patients were treated with CCRT (40 Gy in 20 fractions). There was no statistically significant difference in survival outcome between the SCRT and CCRT groups. However, the CCRT group tended to show a better CR rate, local recurrence-free survival, and distant metastasis-free survival than the SCRT group. It was concluded that RT is important in local control, and treatment with RT should not be delayed [15]. Similarly, no survival difference between the SCRT and CCRT groups was observed in our study. Chemotherapy may contribute to treatment outcomes, as different chemotherapy regimens were used for the SCRT and CCRT groups. Anthracycline-based chemotherapy is known to have limited efficacy in NK/T-cell lymphoma due to the frequent expression of multidrug-resistance protein 1 [16]. Lee et al. evaluated the treatment outcome of the non-anthracycline-based regimen, ifosfamide, methotrexate, etoposide, and prednisolone, followed by RT. With this regimen, patients showed a superior CR rate of 93% [17].
Our study showed that a high incidence of local or distant failure remains the most serious problem in patients with stage I/II NK/T-cell lymphoma, nasal type, in contrast to the relatively low rate of regional failure. Although several studies report that a delay in administration of RT might contribute to a decrease in local control, our study did not show a statistically significant finding [4, 18]. Li et al. reported the treatment outcomes of initial RT (RT alone or RT followed by chemotherapy) and initial chemotherapy (chemotherapy followed by RT and chemotherapy alone) groups, and demonstrated that patients who received initial RT had a superior CR rate (83%) than patients who received initial chemotherapy (20%) (P=0.0001) [18]. Huang et al. also demonstrated that patients who received initial RT treatment had improved OS compared to patients who received SCRT, particularly in cases of stage I NK/T-cell lymphoma, nasal type (5-year OS of 90% vs. 49%, P=0.012) [4]. They concluded that a delay in RT should be avoided in cases of stage I/II extranodal NK/T-cell lymphoma, particularly in patients with stage I disease.
Some patients in the CCRT group received additional chemotherapy, but only half of the patients completed the planned chemotherapy in our study. Some strategies to improve compliance with additional chemotherapy have been suggested. Kim et al. reduced the dosage of ifosfamide and adjusted the dosage of ifosfamide or etoposide, according to the absolute neutrophil count. Administration of granulocyte colony-stimulating factor is another option that can improve compliance with VIPD chemotherapy [7]. Au et al. reported that autologous hematopoietic stem cell transplantation at the first observation of CR led to better OS [19].
We found that tumor confinement to the nasal cavity and a lack of extension to adjacent structures were associated with improved OS, regardless of the treatment modality. These differences in survival outcome may be attributed to a rich lymphatic supply in the upper aerodigestive tract as compared to the nasal cavity [20]. However, the prognostic significance of primary tumor location remains controversial [4, 12, 16, 21, 22]. A recent intensity-modulated RT study of stage I/II NK/T-cell lymphoma from the nasal cavity reported a 2-year locoregional control rate of 93% and an OS rate of 78% [23]. However, Li et al. reported no survival difference in cases of stage I NK/T-cell lymphoma between tumors in the nasal cavity and those in Waldeyer's ring [22].
In conclusion, both SCRT and CCRT are similar with regard to survival outcomes of patients with stage I/II extranodal NK/T-cell lymphoma, nasal type. Tumors confined to the nasal cavity and age ≤60 years were associated with a better prognosis. As most patients died due to local progression or distant metastases, further improvement in survival outcomes will depend on the intensification of RT and the development of effective and safe systemic chemotherapy regimens. Further studies are required to understand the predictive or prognostic markers for better selection of therapy.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 2.Annual report of cancer statistics in Korea in 2010. Sejong-si, Korea: Ministry of Health and Welfare; 2012. [Accessed August 22, 2013]. at http://download.mw.go.kr/front_new/modules/download.jsp?BOARD_ID=1003&CONT_SEQ=283331&FILE_SEQ=131977. [Google Scholar]
- 3.Kohrt H, Lee M, Advani R. Risk stratification in extranodal natural killer/T-cell lymphoma. Expert Rev Anticancer Ther. 2010;10:1395–1405. doi: 10.1586/era.10.130. [DOI] [PubMed] [Google Scholar]
- 4.Huang MJ, Jiang Y, Liu WP, et al. Early or up-front radiotherapy improved survival of localized extranodal NK/T-cell lymphoma, nasal-type in the upper aerodigestive tract. Int J Radiat Oncol Biol Phys. 2008;70:166–174. doi: 10.1016/j.ijrobp.2007.05.073. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Cho SG, Chung SM, et al. Retrospective analysis of treatment outcomes for extranodal NK/T-cell lymphoma (ENKL), nasal type, stage I-IIE: single institute experience of combined modality treatment for early localized nasal extranodal NK/T-cell lymphoma (ENKL) Ann Hematol. 2013;92:333–343. doi: 10.1007/s00277-012-1630-z. [DOI] [PubMed] [Google Scholar]
- 6.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 7.Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol. 2009;27:6027–6032. doi: 10.1200/JCO.2009.23.8592. [DOI] [PubMed] [Google Scholar]
- 8.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 9.Zelenetz AD, Wierda WG, Abramson JS, et al. Non-Hodgkin's lymphomas, version 1.2013. J Natl Compr Canc Netw. 2013;11:257–272. doi: 10.6004/jnccn.2013.0037. [DOI] [PubMed] [Google Scholar]
- 10.Kim WS, Song SY, Ahn YC, et al. CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T-cell lymphoma? Ann Oncol. 2001;12:349–352. doi: 10.1023/a:1011144911781. [DOI] [PubMed] [Google Scholar]
- 11.Wang B, Lu JJ, Ma X, et al. Combined chemotherapy and external beam radiation for stage IE and IIE natural killer T-cell lymphoma of nasal cavity. Leuk Lymphoma. 2007;48:396–402. doi: 10.1080/10428190601059795. [DOI] [PubMed] [Google Scholar]
- 12.Kim SJ, Kim BS, Choi CW, et al. Treatment outcome of front-line systemic chemotherapy for localized extranodal NK/T cell lymphoma in nasal and upper aerodigestive tract. Leuk Lymphoma. 2006;47:1265–1273. doi: 10.1080/10428190600565651. [DOI] [PubMed] [Google Scholar]
- 13.Kim K, Chie EK, Kim CW, Kim IH, Park CI. Treatment outcome of angiocentric T-cell and NK/T-cell lymphoma, nasal type: radiotherapy versus chemoradiotherapy. Jpn J Clin Oncol. 2005;35:1–5. doi: 10.1093/jjco/hyi006. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi M, Tobinai K, Oguchi M, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol. 2009;27:5594–5600. doi: 10.1200/JCO.2009.23.8295. [DOI] [PubMed] [Google Scholar]
- 15.Lee HJ, Lee SW, Suh C, et al. Treatment outcome of nasal natural killer/T-cell lymphoma. Radiat Oncol J. 2011;29:174–180. doi: 10.3857/roj.2011.29.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim TM, Heo DS. Extranodal NK / T-cell lymphoma, nasal type: new staging system and treatment strategies. Cancer Sci. 2009;100:2242–2248. doi: 10.1111/j.1349-7006.2009.01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KW, Yun T, Kim DW, et al. First-line ifosfamide, methotrexate, etoposide and prednisolone chemotherapy +/- radiotherapy is active in stage I/II extranodal NK/T-cell lymphoma. Leuk Lymphoma. 2006;47:1274–1282. doi: 10.1080/10428190600562823. [DOI] [PubMed] [Google Scholar]
- 18.Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24:181–189. doi: 10.1200/JCO.2005.03.2573. [DOI] [PubMed] [Google Scholar]
- 19.Au WY, Lie AK, Liang R, et al. Autologous stem cell transplantation for nasal NK/T-cell lymphoma: a progress report on its value. Ann Oncol. 2003;14:1673–1676. doi: 10.1093/annonc/mdg458. [DOI] [PubMed] [Google Scholar]
- 20.Parsons JT, Mendenhall WM, Mancuso AA, Cassisi NJ, Million RR. Malignant tumors of the nasal cavity and ethmoid and sphenoid sinuses. Int J Radiat Oncol Biol Phys. 1988;14:11–22. doi: 10.1016/0360-3016(88)90044-2. [DOI] [PubMed] [Google Scholar]
- 21.Li YX, Fang H, Liu QF, et al. Clinical features and treatment outcome of nasal-type NK/T-cell lymphoma of Waldeyer ring. Blood. 2008;112:3057–3064. doi: 10.1182/blood-2008-05-160176. [DOI] [PubMed] [Google Scholar]
- 22.Li YX, Liu QF, Fang H, et al. Variable clinical presentations of nasal and Waldeyer ring natural killer/T-cell lymphoma. Clin Cancer Res. 2009;15:2905–2912. doi: 10.1158/1078-0432.CCR-08-2914. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Li YX, Wang WH, et al. Mild toxicity and favorable prognosis of high-dose and extended involved-field intensity-modulated radiotherapy for patients with early-stage nasal NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2012;82:1115–1121. doi: 10.1016/j.ijrobp.2011.02.039. [DOI] [PubMed] [Google Scholar]


