Abstract
Objective To document trajectories of paternal involvement in diabetes management and examine bidirectional associations with diabetes outcomes across early adolescence. Methods 3-year prospective assessment of paternal involvement, diabetes self-management, and glycemic control among 136 youth (age 9–12 at baseline) and their mothers and fathers. Results Unconditional growth curves demonstrated decreasing amount (maternal report: F(1,128) = 14.79; paternal report: F(1,111) = 12.95, ps < 0.01) and level of contribution (maternal report: F(1,131) = 23.6, p < .01) of paternal involvement. Controlling for covariates, lower youth self-management predicted an increasing slope in fathers’ self-reported amount of involvement (b = −0.15 to −0.22, p < .05), and higher levels of fathers’ self-reported level of contribution predicted a decreasing slope in youths’ self-reported self-management (b = −0.01, p < .05). Conclusions Like mothers, fathers’ involvement declines modestly during early adolescence. Different aspects of paternal involvement influence or are influenced by youths’ self-management. Communication about ways to enhance fathers’ involvement before this transition may help prevent or reduce declining diabetes management and control common in adolescence.
Keywords: adherence, children and adolescents, fatherhood, type 1 diabetes
Parents’ ongoing, supportive involvement consistently benefits children and adolescents’ management and control of type 1 diabetes (Jaser, 2011; Palmer et al., 2011; Wysocki et al., 2009). Research has historically emphasized the important role of mothers, and empirical interest in fathers has surged over the past decade (Dashiff, 2003; Dashiff, Morrison, & Rowe, 2008; Gavin & Wysocki, 2006; Hansen, Weissbrod, Schwartz, & Taylor, 2012; Hilliard et al., 2011; Jaser, 2011; Palmer et al., 2009; Phares, Lopez, Fields, Kamboukos, & Duhig, 2005; Sullivan-Bolyai, Rosenberg, & Bayard, 2006; Sullivan-Bolyai, Bova, Lee, & Gruppuso, 2011; Wysocki & Gavin, 2006).
Fathers’ involvement has shown mixed associations with youths’ diabetes management and control. Hansen et al. (2012) reported associations between mothers’ ratings of fathers’ “helpfulness” and better youth-reported adherence, whereas paternal ratings of their own helpfulness were associated with poorer glycemic control. Our own research with early adolescents demonstrated that more frequent and more helpful paternal involvement ratings were linked with worse glycemic control (Hilliard et al., 2011). Berg and colleagues have demonstrated associations among less monitoring by fathers and poorer diabetes outcomes (Berg et al., 2008, 2011; King et al., 2012). One hypothesis for these diverse findings is that a father’s involvement in daily diabetes care may be influenced by his child’s diabetes outcomes (Hilliard et al., 2011; Jaser, 2011). Anecdotal evidence suggests that some fathers may increase their involvement when problems arise, such as when the teen is struggling with adherence or glycemic control. At such challenging times, parents may feel that a new approach to diabetes management is needed, and fathers may decide or be called on to become more involved. Much remains to be studied regarding how fathers’ involvement unfolds across adolescence, what influences the amount and quality of their involvement, and the impact on clinical outcomes.
Because previous research has been largely cross-sectional, it has been difficult to describe change in paternal involvement over time or disentangle the directionality of associations with youths’ diabetes management and control. Given mounting evidence supporting the importance of parents’ involvement during the transition to adolescence, it is necessary to evaluate and understand relations with diabetes management and control during this vulnerable period. In addition to physiological and developmental changes, shifts in youths’ autonomy and parents’ responsibility for diabetes self-management often begin to emerge (Anderson et al., 2009; Palmer et al., 2004), and declining adherence and glycemic control are common from this point forward (Helgeson et al., 2010; Kovacs, Goldston, Obrosky, & Iyengar, 1992). Although mothers’ roles in this process have been studied, understanding the patterns and predictors of fathers’ involvement has the potential to inform clinical research and practice by identifying new intervention targets to help prevent or reduce poor outcomes.
Thus, the primary goal of this study was to describe the trajectory of paternal involvement in diabetes care over 3 years during the transition to adolescence. To tease apart potential contributors to and consequences of paternal involvement, a secondary goal was to examine unidirectional and bidirectional associations between fathers’ involvement and key indices of diabetes management and control. Our data are uniquely poised to meet these aims, given the relatively large sample of families (n = 240, of which 136 include 2 participating caregivers) of youth at the brink of adolescence (Hilliard et al., 2011), and the prospective 3-year observation of these families as they manage diabetes at and across the point of transition from childhood to adolescence. These data include a comprehensive assessment of multiple aspects of diabetes management and control, including adherence to specific management tasks [e.g., blood glucose monitoring (BGM) frequency], overall diabetes self-management across multiple tasks, and glycemic control, as well as a range of contextual, developmental factors that may contribute to and co-vary with diabetes management and control.
Based on evidence of declining maternal involvement during adolescence (Palmer et al., 2004), it was hypothesized that fathers’ involvement (as reported by both mothers and fathers) would decrease over 3 years in early adolescence. In addition, it was hypothesized that there would be bidirectional associations among paternal involvement and key indices of diabetes management and control (i.e., BGM adherence, overall self-management, and glycemic control). Specifically, poorer diabetes management and control would predict increases in fathers’ involvement over the subsequent 3 years, and higher paternal involvement would predict improvements in diabetes management and control over 3 years.
Methods
Participants
Participants were 136 families of children or preadolescents (at baseline) with type 1 diabetes followed at pediatric diabetes clinics at one of three university-affiliated medical centers in the United States. Participating families were enrolled in a multisite, prospective, observational study investigating developmental and family factors associated with self-management patterns during the transition to adolescence (Drotar et al., 2013; Rausch et al., 2012). Of the 361 families approached about the study, 240 (66.5%) completed baseline data. Because the goal of the current research was to conduct dyadic and family-level analyses focusing on the role of paternal involvement in diabetes management and control, data from only those families with both a participating male and female caregiver (n = 146, 60.8%) were included. Following exclusions based on eligibility and data completeness, the sample consisted of 136 (56.7%) families at baseline (see Hilliard et al., 2011, for a detailed description of the baseline sample). For the current longitudinal analysis, we used data from baseline and annual study visits over 3 years. Five families (3.7%) dropped from the study because they were too busy, no longer interested in research, or moved to a different hospital. Including families enrolled in the study with missing data at one or more follow-up assessments, retention rates were 97, 93, and 95% at 12, 24, and 36 months, respectively.
At baseline, the mean youth age was 10.5 years (SD = 0.9) and 54% were female. The majority (88.4%) were of non-Hispanic, Caucasian ethnicity. The mean diabetes duration was 4.1 years (SD = 2.4). Two-thirds received insulin via continuous subcutaneous insulin infusion (CSII), and the remainder received multiple daily injections of insulin. The mean Tanner stage of pubertal development was 1.7 (SD = 0.9), placing this sample in the preadolescent range. Given the focus of this analysis, nearly all youth (95.6%) lived in a two-parent household, with the remainder of the sample having two participating caregivers who resided in separate homes. The majority of caregivers (primary: 68.4%; secondary: 75.7%) reported at least some college education, a college degree, or beyond.
Procedure
As part of the larger multisite study’s protocol (procedure first and fully described by McNally, Rohan, Pendley, Delamater, & Drotar, 2010), potentially eligible participants were identified from the diabetes clinic rosters of the three participating children’s hospitals. Eligibility requirements included: (a) ≥1 year duration of type 1 diabetes, (b) age 9–11 years at recruitment (9–12 at baseline), and (c) English fluency. Exclusionary criteria included plans to move from catchment area in upcoming 3 years and comorbid chronic physical or psychiatric condition, including secondary cause of diabetes diagnosis (e.g., cystic fibrosis), or diagnosis of intellectual disability. Parents and children aged 11 years or older provided written consent and assent and children aged <11 years provided verbal assent. The participating hospitals’ institutional review boards approved this study.
Measures
Paternal involvement in diabetes management was assessed with the Dads’ Active Disease Support scale (DADS; Wysocki & Gavin, 2004). Mothers and fathers rated the frequency (“DADS-Amount” scale) and perceived level of contribution (“DADS-Helpfulness” scale1) of fathers’ involvement in 24 diabetes care tasks. For those items that were endorsed as being needed over the past 6 months, respondents used a 5-point Likert scale to rate the frequency of paternal involvement in the task (“never” to “always”) and the degree to which the contribution made family coping with the disease harder or easier (“harder” to “much easier”). Possible scores for each scale range from 24 to 120, with higher scores indicating each reporter’s perception of greater frequency or helpfulness of paternal involvement. The DADS has excellent psychometric properties (Wysocki & Gavin, 2004). In the current sample, internal consistency coefficients were excellent at all time points (amount scale α range = maternal report: 0.86–0.96, paternal report: 0.84–0.95; helpfulness scale α range = maternal report: 0.91–0.96, paternal report: 0.90–0.94). There is no parallel measure of maternal involvement available.
Overall diabetes self-management was measured via the Diabetes Self-Management Profile (DSMP; Harris et al., 2000). Trained research staff administered this 25-item structured interview to all youth and parents. Open-ended questions assess the completion of a range of diabetes management tasks (e.g., exercise, insulin administration and adjustment, diet, hypoglycemia management) over the previous 3 months, and each item is scored as yes/no or on a 3- or 5-point Likert scale. The range of possible scores is 0–88, with higher scores denoting better self-management. The DSMP has demonstrated adequate psychometric properties and good predictive validity with glycemic control (Harris et al., 2000). In the present sample, internal consistencies were moderate at all time points (α range = maternal report: 0.62–0.71; paternal report: 0.62–0.72; youth report: 0.61–0.69). Research staff members were trained to a 100% criterion for inter-rater reliability with an observer.
BGM frequency was used as a specific behavioral indicator of diabetes treatment adherence. At each assessment, the average daily BGM frequency was calculated from the previous 2 weeks’ worth of data downloaded from blood glucose meters. Glycosylated hemoglobin (HbA1c) provided an estimate of glycemic control over the previous 2–3 months. Blood samples were obtained by a finger stick during each study visit and were shipped to a central laboratory where they were analyzed with high-performance liquid chromatography using the TOSOH-G7 analyzer (reference range: 4.0–6.0%).
At baseline, parents completed a background information form regarding family demographic characteristics (e.g., child age, race/ethnicity, maternal education level). Clinical information (e.g., date of diagnosis, insulin delivery method, Tanner stage of pubertal development) was obtained through medical chart review.
Data Analysis
For the first aim, unconditional growth curve models were used to examine changes in maternal and paternal reports of fathers’ involvement in the child’s diabetes care over the observation period of 36 months. Individual growth curve models (i.e., level 1) measured change over time for each individual in the sample as well as for the population of individuals in the entire sample. Growth was summarized for the population and for each individual using two terms: Fitted intercept (i.e., baseline value) and fitted slope (i.e., rate of change over time) (Singer & Willett, 2003). Unconditional growth curve modeling was performed using SAS Proc Mixed (SAS Institute, 1990). Restricted maximum likelihood estimations were used to avoid biased estimates of the variance components. Unstructured covariance matrices were used to allow variances and covariances to vary rather than to conform to a priori constraints (Singer & Willett, 2003).
For the second aim, bidirectional regression models were used to examine the longitudinal associations among paternal involvement and diabetes management and control. Relations between the intercept for each score (i.e., maternal and paternal ratings of fathers’ involvement; maternal, paternal, and youth ratings of diabetes self-management; and BGM frequency) and the slope of each of the other scores were individually examined to determine unidirectional and/or bidirectional associations. Demographic and clinical characteristics (i.e., primary caregiver education, household composition, youth age, gender, race, pubertal level/Tanner stage, diabetes duration, and insulin regimen) were included as covariates based on documented associations with glycemic control and self-management, and study site was included to control for regional differences. Unstandardized coefficients (b) are reported. Regression analyses were conducted in MPlus version 5.2 using Maximum Likelihood Estimation to account for missing data and estimation of parameters for trajectories.
Results
Unconditional Growth Models
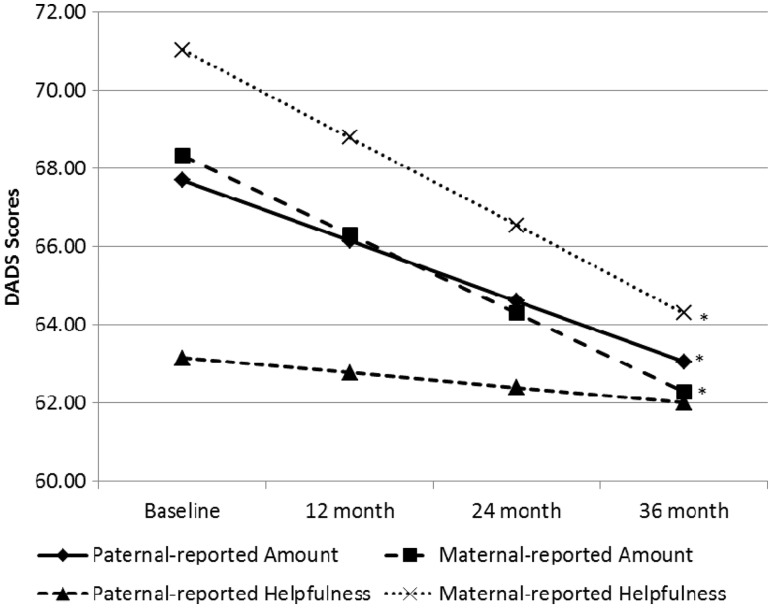
The unconditional growth curves illustrating changes in DADS scores from baseline to 36 months appear in Figure 1, and mean scores at each time point are summarized in Table I. Supporting hypotheses, both maternal and paternal reports of the amount of fathers’ involvement, significantly decreased over the 3-year observation period (F(1,128) = 14.79; F(1,111) = 12.95, respectively, p < .01). Maternal ratings decreased at a rate of 2.0 U per year, and paternal ratings decreased at a rate of 1.5 U per year. Whereas maternal perceptions of the level of fathers’ contributions decreased over time as hypothesized (F(1,131) = 23.6, p < .01), at a rate of 2.2 U per year, paternal ratings of their own level of contribution did not demonstrate significant change (F(1,112) = 0.84, p = .36), contrary to hypotheses.
Figure 1.
Unconditional growth models illustrating change in maternal and paternal reports of DADS Amount and Helpfulness scores across 3 years. Note. * = significant change over time (p < .05).
Table I.
Paternal Involvement, Adherence, and Glycemic Control Over 3 Years, M ± SD (n)
| Variables | Baseline | Year 1 | Year 2 | Year 3 |
|---|---|---|---|---|
| DADS scores | ||||
| Maternal-A | 68.5 ± 22.2 (135) | 65.8 ± 21.7 (128) | 63.6 ± 21.4 (122) | 62.2 ± 21.8 (126) |
| Paternal-A | 68.9 ± 16.6 (134) | 64.8 ± 16.2 (119) | 65.3 ± 17.6 (111) | 63.3 ± 15.7 (109) |
| Maternal-H | 71.4 ± 18.0 (135) | 68.5 ± 17.3 (128) | 66.0 ± 15.7 (122) | 65.2 ± 17.3 (126) |
| Paternal-H | 63.2 ± 14.0 (134) | 63.3 ± 13.5 (119) | 62.3 ± 15.4 (111) | 62.1 ± 13.8 (109) |
| DSMP Scores | ||||
| Youth | 61.5 ± 8.1 (136) | 61.2 ± 7.8 (131) | 59.0 ± 8.9 (127) | 57.6 ± 9.1 (129) |
| Maternal | 66.0 ± 8.4 (136) | 63.4 ± 9.2 (128) | 61.2 ± 9.4 (123) | 57.3 ± 10.0 (127) |
| Paternal | 65.2 ± 8.8 (133) | 62.9 ± 9.0 (113) | 60.1 ± 9.5 (110) | 57.1 ± 10.0 (109) |
| BGM frequency | 5.35 ± 1.78 (134) | 4.87 ± 1.95 (129) | 5.03 ± 2.13 (126) | 4.82 ± 2.07 (126) |
| A1c | 7.9% ± 1.2% (134) | 8.2% ± 1.4% (129) | 8.3% ± 1.4% (127) | 8.6% ± 1.6% (129) |
Note. Sample size varies due to missing data on specific measures from individual respondents.
M = mean; SD = standard deviation; n = sample size; DADS = Dads’ Active Disease Support scale; A = Amount scale; H = Helpfulness scale; DSMP = Diabetes Self-Management Profile; BGM frequency = mean daily blood glucose monitoring frequency from blood glucose meters; A1c = hemoglobin A1c.
Regression Analyses
Tables II, III, and IV summarize the results of the regression models that resulted in at least one significant effect between a DADS score and a diabetes outcome (in all significant cases, youth- or maternal-reported DSMP scores).
Table II.
Unstandardized Results for Significant Bidirectional Regression Models Between Youth-Report DSMP and Paternal-Report DADS-Amount Scores
| Independent Variables | DSMP (Y) slope |
DADS-A (P) slope |
||||
|---|---|---|---|---|---|---|
| Est. | 95% CI | p | Est. | 95% CI | p | |
| Baseline covariates | ||||||
| Site 13 | 0.108 | −0.123, 0.339 | .36 | 3.19 | −1.10, 7.48 | .15 |
| Site 12 | −0.200 | −0.34, −0.06 | .004 | −1.29 | −3.68, 1.10 | .29 |
| Primary caregiver education | −0.10 | −0.20, 0.01 | .07 | −0.08 | −1.89, 1.72 | .93 |
| Household composition | 0.033 | −0.187, 0.253 | .77 | 5.72 | 1.92, 9.52 | .003 |
| Tanner stage | −0.001 | −0.066, 0.064 | .97 | 1.28 | 0.08, 2.48 | .04 |
| Gender | −0.060 | −0.160, 0.040 | .24 | −0.15 | −1.95, 1.66 | .87 |
| Race | −0.018 | −0.161, 0.125 | .80 | −3.48 | −6.24, −0.72 | .01 |
| Age | −0.032 | −0.093, 0.029 | .31 | 0.04 | −1.04, 1.12 | .95 |
| Diabetes duration | −0.006 | −0.026, 0.014 | .58 | −0.13 | −0.50, 0.24 | .50 |
| Insulin regimen | −0.005 | −0.119, 0.109 | .93 | 2.34 | 0.24, 4.44 | .03 |
| Predictor variables | ||||||
| DSMP (Y) intercept | 0.006 | −0.010, 0.022 | .46 | −0.22 | −0.02, −0.42 | .03 |
| DADS-A (P) intercept | 0.002 | −0.002, 0.006 | .30 | 0.01 | −0.06, 0.08 | .81 |
Note. Significant results (p < 0.05) in bold.
Est. = unstandardized estimate; 95% CI = 95% confidence interval; DADS-A (P) = DADS-Amount (paternal report); DSMP(Y) = Diabetes Self-Management Profile (youth report); Site 12 = Cincinnati versus Wilmington site; Site 13 = Cincinnati versus Miami site.
Table III.
Unstandardized Results for Significant Bidirectional Regression Models Between Maternal-Report DSMP and Paternal-Report DADS-Amount Scores
| Independent Variables | DSMP (M) slope |
DADS-A (P) slope |
||||
|---|---|---|---|---|---|---|
| Est. | 95% CI | p | Est. | 95% CI | p | |
| Baseline covariates | ||||||
| Site 13 | −0.036 | −0.257, 0.185 | .75 | 3.56 | −0.76, 7.88 | .11 |
| Site 12 | −0.073 | −0.193, 0.047 | .23 | −1.96 | −4.28, 0.36 | .10 |
| Primary caregiver education | −0.064 | −0.152, 0.024 | .16 | −0.50 | −2.20, 1.19 | .56 |
| Household composition | −0.039 | −0.247, 0.169 | .71 | 5.49 | 1.67, 9.31 | .005 |
| Tanner stage | 0.025 | −0.036, 0.086 | .43 | 1.23 | 0.04, 2.42 | .04 |
| Gender | −0.059 | −0.153, 0.035 | .22 | −0.24 | −2.05, 1.56 | .79 |
| Race | 0.055 | −0.082, 0.192 | .43 | −3.49 | −6.28, −0.70 | .01 |
| Age | 0.004 | −0.051, 0.059 | .88 | −0.44 | −1.50, 0.63 | .42 |
| Diabetes duration | 0.003 | −0.017, 0.023 | .75 | −0.14 | −0.51, 0.23 | .47 |
| Insulin regimen | −0.088 | −0.196, 0.020 | .11 | 2.33 | 0.19, 4.47 | .03 |
| Predictor variables | ||||||
| DSMP (M) intercept | 0.004 | −0.006, 0.014 | .46 | −0.15 | −0.296, −0.002 | <.05 |
| DADS-A (P) intercept | −0.001 | −0.005, 0.003 | .74 | .02 | −0.049, 0.093 | .55 |
Note. Significant results (p < 0.05) in bold.
Est. = unstandardized estimate; 95% CI = 95% confidence interval; DADS-A (P) = DADS-Amount (paternal report); DSMP(Y) = Diabetes Self-Management Profile (youth report); DSMP (M) = Diabetes Self-Management Profile (maternal report); Site 12 = Cincinnati versus Wilmington site; Site 13 = Cincinnati versus Miami site.
Table IV.
Unstandardized Results for Significant Bidirectional Regression Models Between Maternal-Report DSMP and Maternal-Report DADS-Helpfulness Scores
| Independent Variables | DSMP (M) Slope |
DADS-H (M) Slope |
||||
|---|---|---|---|---|---|---|
| Est. | 95% CI | p | Est. | 95% CI | p | |
| Baseline covariates | ||||||
| Site 13 | −0.012 | −0.239, 0.215 | .92 | −2.95 | −7.36, 1.46 | .19 |
| Site 12 | −0.087 | −0.210, 0.036 | .16 | 2.16 | −0.21, 4.53 | .08 |
| Primary caregiver education | −0.069 | −0.159, 0.021 | .13 | −0.09 | −1.82, 1.64 | .92 |
| Household composition | 0.041 | −0.182, 0.264 | .72 | −0.45 | −4.80, 3.90 | .84 |
| Tanner stage | 0.019 | −0.044, 0.082 | .56 | 0.81 | −0.42, 2.04 | .20 |
| Gender | −0.077 | −0.175, 0.021 | .12 | 0.39 | −1.51, 2.29 | .69 |
| Race | 0.065 | −0.074, 0.204 | .36 | −0.18 | −2.86, 2.50 | .90 |
| Age | 0.004 | −0.053, 0.061 | .88 | −0.06 | −1.16, 1.04 | .92 |
| Diabetes duration | 0.002 | −0.018, 0.022 | .85 | 0.02 | −0.35, 0.39 | .91 |
| Insulin regimen | −0.082 | −0.188, 0.024 | .13 | 0.37 | −1.70, 2.44 | .73 |
| Predictor variables: | ||||||
| DSMP (M) intercept | 0.007 | −0.005, 0.019 | .22 | −0.16 | −0.35, 0.03 | .10 |
| DADS-H (M) intercept | −0.005 | −0.009, −0.001 | .02 | −0.03 | −0.22, 0.16 | .47 |
Note. Significant results (p < 0.05) in bold.
Est. = unstandardized estimate; 95% CI = 95% confidence interval; DADS-H (M) = DADS-Helpfulness (maternal report); DSMP (M) = Diabetes Self-Management Profile (maternal report); Site 12 = Cincinnati versus Wilmington site; Site 13 = Cincinnati versus Miami site.
After controlling for all measured demographic and clinical covariates, lower youth- and maternal-reported DSMP intercepts each significantly predicted an increasing slope in fathers’ self-reported DADS-Amount scores (Tables II and III, respectively), as hypothesized (b = −0.22, −0.15, respectively, p < .05). That is, lower self-management scores at baseline predicted faster increases in fathers’ amount of involvement over the subsequent 3 years. For both youth and maternal reports, significant covariates included having more people in the home (b = 5.72, 5.49, p < .01), higher Tanner stage (b = 1.28, 1.23, p < 0.05), non-Hispanic Caucasian ethnicity (b = −3.48, −3.49, p < .05), and insulin delivery via CSII (b = 2.34, 2.33, p < .05). The overall model fit for youth-reported DSMP and fathers’ self-reported DADS-Amount scores was (pseudo) R2 = 0.62, χ2(12) = 34.1, p ≤ .001 and (pseudo) R2 = 1.0, χ2(12) = 30.0, p = .003, respectively. Similarly, the overall model fit for maternal-reported DSMP and fathers’ self-reported DADS-Amount scores was (pseudo) R2 = 0.38, χ2(12) = 20.0, p = .07 and (pseudo) R2 = 1.0, χ2(12) = 29.5, p = .003, respectively. Contrary to hypotheses, the paternal DADS-Amount intercept did not predict the DSMP slope, meaning the baseline amount of paternal involvement was unrelated to how quickly self-management changed over time.
In addition, a lower intercept on the paternal self-reported DADS-Helpfulness scale predicted an increasing slope in maternal-reported DSMP scores (b = −0.01, p < .05), with no significant covariates (Table IV). That is, lower levels of fathers’ perceived level of contribution at baseline predicted faster increases in youths’ self-management over time. The overall model fit for maternal-reported DSMP and maternal-reported paternal level of contribution was (pseudo) R2 = 0.54, χ2(12) = 25.3, p = .01 and (pseudo) R2 = 0.36, χ2(12) = 14.4, p = .28, respectively. The direction of this finding was opposite to hypotheses. In addition, the bidirectional link between the DSMP intercept and the slope of paternal-reported scores on the DADS-Helpfulness scale was not significant, as maternal ratings of self-management did not predict an increase or decrease in fathers’ level of contribution over time.
Contrary to hypotheses, the above-reported associations were not evident with maternal-reported DADS scores or paternal-reported DSMP scores, and there were no significant associations between DADS scores from either reporter and BGM frequency or A1c.
Discussion
The aims of this study were to (a) document the trajectories of fathers’ contributions to early adolescents’ diabetes care, and (b) evaluate associations between fathers’ involvement and key indices of diabetes management and control. A small yet steady decrease in fathers’ involvement in youths’ diabetes self-management was evident over 3 years across the transition to adolescence in this sample, expanding on previous evidence of decreasing involvement of mothers (Palmer et al., 2004) and parents in general (Anderson, Ho, Brackett, Finkelstein, & Laffel, 1997; Schilling, Knafl, & Grey, 2006) during this period. These data also extend previous findings by demonstrating that different aspects of paternal involvement predict and are predicted by youths’ diabetes self-management trajectories. Both the amount of fathers’ involvement and the level of their contributions were intricately related to the way their children managed their diabetes across the transition to adolescence.
Paternal involvement was in the medium range (i.e., involved in needed tasks around 50% of the time, made coping slightly easier) initially. Although the absolute values significantly decreased over 3 years, the relative amount and level of fathers’ contributions remained in this same range, which was similar to that reported for the standardization sample across a range of chronic conditions (Wysocki & Gavin, 2004). Although the overall involvement decreased modestly in the current sample, it is possible that the ways in which fathers were involved shifted. For example, although fathers’ direct involvement in management tasks (e.g., insulin administration, BGM) may decrease as their children become more autonomous, indirect or supportive involvement (e.g., monitoring) may remain steady or increase. Indeed, parents’ collaboration and monitoring is seen as more developmentally appropriate for young adolescents than direct behavioral involvement in diabetes tasks and is consistently associated with better outcomes (Leonard, Garwick, & Adwan, 2005; Palmer et al., 2011; Wiebe et al., 2005). The current findings suggest that similar patterns may be applicable to fathers as well as mothers.
Despite overall decreases in paternal involvement over time, some fathers may increase their involvement as the need arises. In this sample, the associations between mothers’ and youths’ lower ratings of self-management and the increasing slope of paternal involvement suggest that fathers of youth with suboptimal self-management may become more involved in daily diabetes care over time. In contrast, fathers’ higher initial scores on the self-reported DADS-Helpfulness scale predicted deteriorating maternal-rated youth self-management slopes. It is possible that this unexpected association may reflect processes that were already underway at baseline. As suggested by the findings of Hansen et al. (2012) that elevated pediatric parenting stress is linked with more paternal involvement, fathers with children at greater risk for poor diabetes outcomes (e.g., those with chronically elevated A1c levels) may feel compelled or be asked to be more involved in diabetes management. If fathers had already ramped up their level of contribution when their children appeared to be on a downward trajectory of self-management, their relatively higher levels of contribution at baseline may not have been sufficient to slow or reverse the trajectory. It is also possible that fathers’ involvement may have been perceived as intrusive, a phenomenon known as “miscarried helping” that predicts poorer outcomes (Harris et al., 2008; Weinger, O’Donnell, & Ritholz, 2001). On the other hand, fathers with children already on a good trajectory early in adolescence may not have determined a need to be highly involved and thus have lower baseline DADS ratings that predicted youth progression with good self-management.
Null associations between some aspects of paternal involvement and diabetes management and control are worthy of consideration. For example, all of the significant associations were unidirectional, despite evaluation of multiple hypothesized bidirectional links. These findings suggest that perceptions of the amount of paternal involvement may be more strongly impacted by youths’ self-management than vice versa, fathers’ level of contribution may have a bigger impact on subsequent patterns of self-management. This pattern recalls previous findings indicating that perception of a teen’s diabetes self-care competence is one factor that influences mothers’ transfer of responsibility to her adolescent child (Palmer et al., 2004). Alternatively, reactive changes in fathers’ or youths’ behaviors may occur more closely in time than the 12-month intervals of this study allowed, thereby obscuring true bidirectional associations. In addition, links between fathers’ self-reported scores on the DADS-Helpfulness scale and trajectories of BGM frequency or A1c were not evident. Despite their involvement, fathers may not perceive their contributions to be making an impact on their children’s diabetes management and control. Some fathers may support overall diabetes self-management more than manage specific tasks such as adherence to BGM. Indeed, the DADS measure includes a few questions that refer to direct disease management tasks, yet most other questions assess indirect or supportive activities such as paying medical bills, talking with teachers or coaches about diabetes management, and discussing the social–emotional impact of diabetes with the child and/or mother (Wysocki & Gavin, 2004). Although a wide range of potential ways for fathers to be involved is assessed, the lack of focus on direct disease management behaviors may have diluted associations with specific outcomes (e.g., adherence, glycemic control). Although BGM is often highlighted as a central task of diabetes management, fathers’ contributions to other parts of the regimen are likely beneficial and quite adaptive.
In addition to associations between the key study constructs, links with demographic and clinical covariates were also evident. For example, use of CSII regimens was linked with larger paternal involvement slopes, consistent with suggestions that fathers may be interested in and comfortable with these technologies (Sullivan-Bolyai, Knafl, Tamborlane, & Grey, 2004). Having more household members was also associated with faster increases in paternal amount of involvement. Although unmeasured, one possibility is that having more people in the home to share responsibility for general household chores may free up fathers’ time to become more active in diabetes management tasks. Moreover, fathers’ availability may increase as children get older, can take on more household responsibilities, and require less close parental monitoring. Contrary to mothers, who report transferring diabetes management responsibility to youth around the time of puberty (Palmer et al., 2004), fathers’ involvement increased more rapidly among youth with more advanced pubertal status. It may be that fathers’ contributions become particularly evident as mothers’ involvement declines, although research is needed to evaluate concurrent changes in each parent’s roles over this time. Finally, although factors such as limited access to resources (Swift, Chen, Hershberger, & Holmes, 2006) may be relevant to observed differences in paternal involvement by race, these findings may be an artifact of the relatively small subset of participating fathers who were from families of minority backgrounds (i.e., only 4 of the 136 participating fathers self-identified as Hispanic). As research related to fathers and their involvement in diabetes care advances, careful consideration of clinical and demographic factors will be essential.
There were several strengths of this study’s design. The relatively large sample of early adolescents with two caregivers provides a focused, prospective observation of youth and families during a specific, vulnerable developmental period. The rigorous data analyses evaluated both unidirectional and bidirectional, longitudinal change among three critical diabetes outcomes. Measurement of diabetes management and control captured both global and specific aspects of the construct and drew on both objective and subjective data from multiple reporters. An additional strength of this study is the assessment and inclusion of numerous contextual demographic and clinical characteristics as covariates in the analytic plan. Although these covariates may have detracted from some main effects between DADS scores and diabetes outcomes, their inclusion provides a more comprehensive view of which fathers are involved and in what ways.
A limitation of the study is the relatively high education and income level of families and youths’ relatively high diabetes treatment adherence and low mean A1c. Thus, the restricted range of diabetes management and control may have limited our ability to detect associations with paternal involvement, and results may not generalize to patients with type 1 diabetes from different medical or socioeconomic backgrounds. This is despite targeted over-recruitment of low-income and ethnic minority populations to maximize generalizability of findings, a design strength. Because this study focuses on two-parent families with fathers who were willing and able to participate in research, recruitment and retention were uneven across the sample and conclusions cannot be drawn about single fathers or families with less involved fathers. In addition, we were only able to report on trajectories of paternal involvement, as no parallel scale was available for mothers or others who participate in or support youths’ diabetes management. An important future research direction would be to collect data on both mothers’ and fathers’ roles in diabetes management and evaluate how they track together over time. Generally, a balanced approach to both measuring and describing the contributions of caregivers of both genders is advocated as research in this area continues to evolve. Finally, only mothers’ and fathers’ perspectives on paternal involvement were assessed. Particularly as youth take on more self-management responsibility in adolescence, their perspective on family involvement will be valuable.
These longitudinal results have implications for future clinical care and research. The associations between lower adherence and subsequent increases in paternal involvement identify a potential target of intervention. Specifically, for young adolescents who are demonstrating early signs of difficulty self-managing diabetes, it may be beneficial to include fathers in efforts to ramp up parental involvement before adherence problems worsen. For example, clinicians may strongly encourage fathers to join and participate in diabetes clinic visits. Additionally, clinicians may facilitate family conversations about diabetes management that identify specific ways for mothers, fathers, and other family members to support diabetes management and control (Kaugars et al., 2011). Established interventions that aim to promote maternal involvement (Wiebe et al., 2008) and enhance parental involvement more generally (Anderson et al., 1997; Wysocki et al., 2008) may need to be tailored to fit the experiences and strengths of fathers. These types of interventions may be especially applicable to families in which fathers are the primary or sole caregivers. Although the results of this study suggest that early adolescents with suboptimal adherence may be the first priority for intervention, this is but one step toward the broader goal to support a teamwork approach to diabetes management in all families during the vulnerable transition to adolescence.
Funding
National Institute of Diabetes and Digestive and Kidney Diseases (1R01-DK-069486 to D.D.).
Conflicts of interest: None declared.
Footnotes
1 The label of the DADS-Helpfulness scale is that used by the developers of the instrument (Wysocki & Gavin, 2004). We acknowledge that this scale label risks being interpreted as promoting gender stereotypes in which fathers are seen as supportive or secondary rather than as equal partners in caregiving. This implication is not intended and therefore throughout the manuscript we describe findings related to this scale as fathers’ level of contribution.
References
- Anderson B J, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. The Journal of Pediatrics. 1997;130:257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- Anderson B J, Holmbeck G, Iannotti R J, McKay S V, Lochrie A, Volkening L K, Laffel L. Dyadic measures of the parent-child relationship during the transition to adolescence and glycemic control in children with type 1 diabetes. Families, Systems, & Health. 2009;27:141–152. doi: 10.1037/a0015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg C A, Butler J M, Osborn P, King G, Palmer D L, Butner J, Murray M, Lindsay R, Donaldson D, Foster C, Swinyard M, Wiebe D J. Role of parental monitoring in understanding the benefits of parental acceptance on adolescent adherence and metabolic control of type 1 diabetes. Diabetes Care. 2008;31:678–683. doi: 10.2337/dc07-1678. [DOI] [PubMed] [Google Scholar]
- Berg C A, King P S, Butler J M, Pham P, Palmer D, Wiebe D J. Parental involvement and adolescents’ diabetes management: The mediating role of self-efficacy and externalizing and internalizing behaviors. Journal of Pediatric Psychology. 2011;36:329–339. doi: 10.1093/jpepsy/jsq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashiff C, Morrison S, Rowe J. Fathers of children and adolescents with diabetes: What do we know? Journal of Pediatric Nursing. 2008;23:101–119. doi: 10.1016/j.pedn.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Dashiff C J. Self- and dependent-care responsibility of adolescents with IDDM and their parents. Journal of Family Nursing. 2003;9:166–183. [Google Scholar]
- Drotar D, Ittenbach R, Rohan J M, Gupta R, Pendley J S, Delamater A. Diabetes management and glycemic control in youth with type 1 diabetes: Test of a predictive model. Journal of Behavioral Medicine. 2013;36:234–245. doi: 10.1007/s10865-012-9426-0. doi:10.1007/s10865-012-9426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin L, Wysocki T. Associations of paternal involvement in disease management with maternal and family outcomes in families of children with chronic illness. Journal of Pediatric Psychology. 2006;31:481–489. doi: 10.1093/jpepsy/jsj043. [DOI] [PubMed] [Google Scholar]
- Hansen J A, Weissbrod C, Schwartz D D, Taylor W P. Paternal involvement in pediatric type 1 diabetes: Fathers’ and mothers’ psychological functioning and disease management. Families, Systems, & Health. 2012;30:47–59. doi: 10.1037/a0027519. [DOI] [PubMed] [Google Scholar]
- Harris M A, Antal H, Oelbaum R, Buckloh L M, White N H, Wysocki T. Good intentions gone awry: Assessing parental “miscarried helping” in diabetes. Families, Systems, & Health. 2008;26:393–403. [Google Scholar]
- Harris M A, Wysocki T, Sadler M, Wilkinson K, Harvey L M, Buckloh L M, Mauras N, White N H. Validation of a structured interview for the assessment of diabetes self-management. Diabetes Care. 2000;23:1301–1304. doi: 10.2337/diacare.23.9.1301. [DOI] [PubMed] [Google Scholar]
- Helgeson V S, Snyder P R, Seltman H, Escobar O, Becker D, Siminerio L. Trajectories of glycemic control over early to middle adolescence. Journal of Pediatric Psychology. 2010;35:1161–1167. doi: 10.1093/jpepsy/jsq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M E, Rohan J M, Carle A C, Pendley J S, Delamater A, Drotar D. Fathers’ involvement in preadolescents’ diabetes adherence and glycemic control. Journal of Pediatric Psychology. 2011;36:911–922. doi: 10.1093/jpepsy/jsr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaser S. Family interaction in pediatric diabetes. Current Diabetes Reports. 2011;11:480–485. doi: 10.1007/s11892-011-0222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P S, Berg C A, Butner J, Drew L M, Foster C, Donaldson D, Murray M, Swinyard M, Wiebe D J. Longitudinal trajectories of metabolic control across adolescence: Associations with parental involvement, adolescents’ psychosocial maturity, and health care utilization. Journal of Adolescent Health. 2012;50:491–6. doi: 10.1016/j.jadohealth.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Goldston D, Obrosky D S, Iyengar S. Prevalence and predictors of pervasive noncompliance with medical treatment among youths with insulin-dependent diabetes mellitus. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:1112–1119. doi: 10.1097/00004583-199211000-00020. [DOI] [PubMed] [Google Scholar]
- McNally K, Rohan J, Pendley J S, Delamater A, Drotar D. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159–1162. doi: 10.2337/dc09-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B J, Garwick A, Adwan J Z. Adolescents’ perceptions of parental roles and involvement in diabetes management. Journal of Pediatric Nursing. 2005;20:405–414. doi: 10.1016/j.pedn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Palmer D L, Berg C A, Butler J, Fortenberry K, Murray M, Lindsay R, Donaldson D, Swinyard M, Foster C, Wiebe D J. Mothers’, fathers’, and children’s perceptions of parental diabetes responsibility in adolescence: Examining the roles of age, pubertal status, and efficacy. Journal of Pediatric Psychology. 2009;34:195–204. doi: 10.1093/jpepsy/jsn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D L, Berg C A, Wiebe D J, Beveridge R M, Korbel C D, Upchurch R, Swinyard M T, Lindsay R, Donaldson D L. The role of autonomy and pubertal status in understanding age differences in maternal involvement in diabetes responsibility across adolescence. Journal of Pediatric Psychology. 2004;29:35–46. doi: 10.1093/jpepsy/jsh005. [DOI] [PubMed] [Google Scholar]
- Palmer D L, Osborn P, King P S, Berg C A, Butler J, Butner J, Horton D, Wiebe D J. The structure of parental involvement and relations to disease management for youth with type 1 diabetes. Journal of Pediatric Psychology. 2011;36:596–605. doi: 10.1093/jpepsy/jsq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares V, Lopez E, Fields S, Kamboukos D, Duhig A M. Are fathers involved in pediatric psychology research and treatment? Journal of Pediatric Psychology. 2005;30:631–643. doi: 10.1093/jpepsy/jsi050. [DOI] [PubMed] [Google Scholar]
- Rausch J R, Hood K K, Delamater A, Shroff Pendley J, Rohan J M, Reeves G, Dolan L, Drotar D. Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care. 2012;35:1219–1224. doi: 10.2337/dc11-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling L S, Knafl K A, Grey M. Changing patterns of self-management in youth with type 1 diabetes. Journal of Pediatric Nursing. 2006;21:412–424. doi: 10.1016/j.pedn.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Singer J D, Willett J B. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford Press; 2003. [Google Scholar]
- Sullivan-Bolyai S, Bova C, Lee M, Gruppuso P A. Mentoring fathers of children newly diagnosed with T1DM. The American Journal of Maternal/Child Nursing. 2011;36:224–231. doi: 10.1097/NMC.0b013e3182183bf5. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Knafl K A, Tamborlane W, Grey M. Parents’ reflections on managing their children’s diabetes with insulin pumps. Journal of Nursing Scholarship. 2004;36:316–323. doi: 10.1111/j.1547-5069.2004.04058.x. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Rosenberg R, Bayard M. Fathers’ reflections on parenting young children with type 1 diabetes. The American Journal of Maternal/Child Nursing. 2006;31:24–31. doi: 10.1097/00005721-200601000-00007. [DOI] [PubMed] [Google Scholar]
- Swift E E, Chen R, Hershberger A, Holmes C S. Demographic risk factors, mediators, and moderators in youths’ diabetes metabolic control. Annals of Behavioral Medicine. 2006;32:355–365. doi: 10.1207/s15324796abm3201_5. [DOI] [PubMed] [Google Scholar]
- Weinger K, O’Donnell K A, Ritholz M D. Adolescent views of diabetes-related parent conflict and support: A focus group analysis. Journal of Adolescent Health. 2001;29:330–336. doi: 10.1016/s1054-139x(01)00270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe D J, Berg C A, Fortenberry K T, Sirstins J, Lindsay R, Donaldson D, Murray M. Physician recommendations about maternal involvement in adolescent diabetes management. Diabetes Care. 2008;31:690–692. doi: 10.2337/dc07-1618. [DOI] [PubMed] [Google Scholar]
- Wiebe D J, Berg C A, Korbel C, Palmer D L, Beveridge R M, Upchurch R, Lindsay R, Swinyard M T, Donaldson D L. Children’s appraisals of maternal involvement in coping with diabetes: Enhancing our understanding of adherence, metabolic control, and quality of life across adolescence. Journal of Pediatric Psychology. 2005;30:167–178. doi: 10.1093/jpepsy/jsi004. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Gavin L. Psychometric properties of a new measure of fathers’ involvement in the management of pediatric chronic diseases. Journal of Pediatric Psychology. 2004;29:231–240. doi: 10.1093/jpepsy/jsh024. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Gavin L. Paternal involvement in the management of pediatric chronic diseases: Associations with adherence, quality of life, and health status. Journal of Pediatric Psychology. 2006;31:501–511. doi: 10.1093/jpepsy/jsj042. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris M A, Buckloh L M, Mertlich D, Lochrie A S, Taylor A, Sadler M, White N H. Randomized, controlled trial of behavioral family systems therapy for diabetes: Maintenance and generalization of effects on parent-adolescent communication. Behavior Therapy. 2008;39:33–46. doi: 10.1016/j.beth.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Nansel T R, Holmbeck G N, Chen R, Laffel L, Anderson B J, Weissberg-Benchell J, Steering Committee of the Family Management of Childhood Diabetes Study Collaborative involvement of primary and secondary caregivers: Associations with youths’ diabetes outcomes. Journal of Pediatric Psychology. 2009;34:869–881. doi: 10.1093/jpepsy/jsn136. [DOI] [PMC free article] [PubMed] [Google Scholar]