Abstract
Hepatocyte specific contrast agents including gadoxetic acid and gadobenate dimeglumine are very useful to diagnose various benign and malignant focal hepatic lesions and even helpful to estimate hepatic functional reservoir. The far delayed phase image referred to as the hepatobiliary phase makes the sensitivity of detection for malignant focal hepatic lesions increased, but specificity of malignant diseases, including hepatocellular carcinoma, metastasis and cholangiocarcinoma, characterization remained to be undetermined.
Keywords: Magnetic resonance imaging, Gadoxetic acid, Focal hepatic lesion, Hepatocellular carcinoma, Metastasis, Focal nodular hyperplasia
INTRODUCTION
Hepatic malignancies including hepatocellular carcinoma (HCC), cholangiocarcinoma and hepatic metastasis are usually encountered during practice. Ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) are the main liver imaging modalities. Ultrasound is very simple and safe for the patient, but it is operator-dependent and limited by air, bone and various types of artifacts. CT is a fast and generic imaging modality, but given ionizing radiation is an obstacle. In contrast, MRI is becoming the imaging method of choice for characterization of focal hepatic lesion.
Hepatocyte specific contrast agents including gadoxetic acid (Gd-EOB-DTPA, Primovist, Bayer, Germany) and gadobenate dimeglumin (Gd-BOPTA, MultiHance, Bracco, Italy) were introduced recently. These agents are taken up by normally functioning hepatocytes and then subsequently excreted in the biliary system and delayed scan after intravenous administration of these contrast agents (about 20 minutes to 1 hour later; called as hepatobiliary phase scan) help to distinguish between malignant and benign lesions by whether they contain normally functioning hepatocytes. Generally they are used for discrimination of HCC from benign lesions such as regenerative or dysplastic nodule and focal nodular hyperplasia (FNH) or FNH-like nodule and detection of hepatic metastasis.
In this review, I would like to describe the different types of hepatocyte specific contrast agent and their mechanism, imaging features and enhancing patterns of focal hepatic lesion on hepatobiliary phase, and the clinical use of hepatobiliary phase MR imaging.
Hepatocyte specific MR contrast agents
Most of gadolinium chelate MR contrast agents were excreted by the kidneys and a very small amount of contrast agent was eliminated via the hepatobiliary system. As an early formulation of hepatobiliary contrast agent, the manganese ion chelated to dipyridoxyl diphosphate is introduced.1 Both T1 and T2 shortening by manganese ion do not only result in high signal intensity of T1-weighted image but also low signal intensity on T2-weighted images. Hepatic enhancement begins at 1 minute following intravenous administration, reaching a peak at 20 minutes and lasting for around 4 hours. Bile duct enhancement appears at about 5 minutes after injection. Unfortunately, there are some limitations in manganese agents: a lack of dynamic phase imaging and potential of manganese toxicity.2
There are two available hepatocyte specific MR contrast agent in Korea; Gd-EOB-DTPA and Gd-BOPTA (Table 1). Two agents are second generation gadolinium chelates and combine the properties of a conventional extracellular gadolinium agent with shortened T1 relaxation time. Like manganese chelate, some percentages of these two agents are also taken into hepatocytes and eliminated via biliary tract. Gd-BOPTA is salified with 2 molecules of meglumine, and it is eliminated roughly 96% of the injected dose excreted renally via glomerular filtration; the remaining 3-5% taken up by functioning hepatocytes is excreted in the bile via the hepatobiliary pathway, leading to a marked and long-lasting enhancement of normal liver parenchyma.3 The enhancement of the liver parenchyma begins at 30 minutes after administration, with maximum contrast between normal and abnormal tissue occurring between 40 and 120 minutes and the hepatobiliary phase seen at 1-4 hours. In contrast, Gd-EOB-DTPA exploits the carrier for the uptake of bilirubin.4 Likewise Gd-BOPTA, this agent also acts initially as an extracellular contrast agent. Approximately 50% of the injected dose of Gd-EOB-DTPA is taken up by hepatocytes and eliminated through the hepatobiliary system. In healthy control, maximum contrast between parenchyma and focal hepatic lesions is seen at about 20 minutes following injection and lasts for approximately 2 hours.
Table 1.
Comparison of the characteristics of liver specific MR contrast agents

Looking at the mechanism of cellular uptake of Gd-BOPTA and Gd-EOB-DTPA, it is specifically taken up by hepatocytes which have the cloned organic anion transporting polypeptides (OATPs), and excreted via multidrug resistance-associated proteins (MRPs) to bile canaliculi (MRP2=apical transporter) or sinusoidal space (MRP3, MRP4=basolateral transporters).5-7 Depending on the functional status of these transporters, absorption or excretion of gadolinium chelate could vary. Using the differences, the focal hepatic lesion which consists of deteriorated hepatocytes or non-hepatocytes such as metastatic malignant cell could be differentiated from the area of normally functioning hepatocytes on hepatobiliary phase MR imaging.
Although these gadolinium-based liver specific contrast agent is widely used for liver imaging because both dynamic and hepatobiliary phases imaging is possible at a time, the challenging problem of Gd-EOB-DTPA imaging is a poor image quality of early period (i.e. arterial phase) after administration of contrast agent. There are some possible causes of artifact. The first is a ringing artifact.8 This is derived from a steep concentration change by a small amount of gadolinium agent during data acquisition produces artifacts. Moreover, Gd-EOB-DTPA has higher relaxivity compared with other gadolinium agents. Although this artifact might be an inevitable event of dynamic MR imaging, especially during the arterial phase, we can try to reduce this phenomenon by selecting square matrix and slower injection rate. The second is acute transient dyspnea.9 Although the cause of this phenomenon is not known, it is described as a temporary, self-limiting effect lasting for roughly 10-20 seconds. It causes a sort of motion artifact that interferes with detection and characterization of focal hepatic lesion. Therefore, radiologists and technicians should understand these problems and try to resolve them.
Imaging patterns on hepatobiliary phase images
As written above, gadolinium-based hepatocyte-specific MR contrast agents have the dynamic phase of contrast enhancement, which are similar to conventional extracellular contrast agents. After or during dynamic phase, parenchyma and lesions in the liver depict a contrast enhancement pattern which depends on the presence of functioning hepatocytes. After a certain period of time, the hyperintensity of the normal hepatic parenchyma by the hepatocellular uptake is observed. This imaging phase was referred to as the "hepatobiliary" phase where uptake by the hepatocytes and excretion to the bile ductule have reached an optimal level for diagnosis.10 As noted above, hepatobiliary phase images are generally acquired 20-40 minutes after Gd-EOB-DTPA injection, or 1-2 hours after Gd-BOPTA injection because the contrast agent should be distributed into hepatocytes. In some patients with abnormal hepatic and renal function such as decompensated liver cirrhosis, delay time obtained hepatobiliary phase images should be elongated.11
Imaging patterns of hepatobiliary phase are divided into two categories: hypo-intensity and hyper-/iso-intensity. These patterns could be explained by OATP and MRP expression. In most HCC, the expression of OATP1B1/B3 is markedly decreased, and the MRP2 expression is conversely high. Thus influx of contrast agent is restricted and the excretion of contrast agent is enhanced, HCCs appear hypointense. In contrast, 5%-10% of HCCs are iso- or hyperintense relative to the liver. This has been related to low MRP2 expression or high MRP3 expression at the luminal membrane of pseudoglands.7
Hyper-/iso-intensity means that contrast agent influx into the hepatocytes which compose the lesion, i.e. preservation of OATP function. Because regenerative or low-grade dysplastic nodules consist of functioning hepatocytes and they have a normal biliary excretory system, hepatobiliay phase imaging shows iso-intense. However, malformed bile duct in FNH,12 abnormal biliary excretion by decreased MRP2 expression, and pseudoglandular type of HCC7 lead to an accumulation of contrast agent in the lesion, and hepatobiliary phase imaging shows hyper-intense.
Clinical usefulness of Liver MRI using liver specific contrast agents
Hepatocellular carcinoma
HCC is a malignant tumor originating from hepatocytes, almost occurs in the setting of cirrhosis and chronic liver disease. The classic HCC appearance on enhanced MRI is arterial enhancement, subsequent "wash-out" during portal venous or equilibrium phases, with or without delayed enhancing fibrous capsule which consists of atrophied cirrhotic nodule by compression of HCC and incorporation of cirrhotic scar (Fig. 1). It is believed that gradual gain of tumoral arterial blood supply via unpaired artery and loss of portal venous blood supply, occurring by a multistep carcinogenesis. As well as arterial enhancement, the presence of "wash-out" and enhanced fibrous capsule are highly specific features of HCC using extracellular contrast agents.13
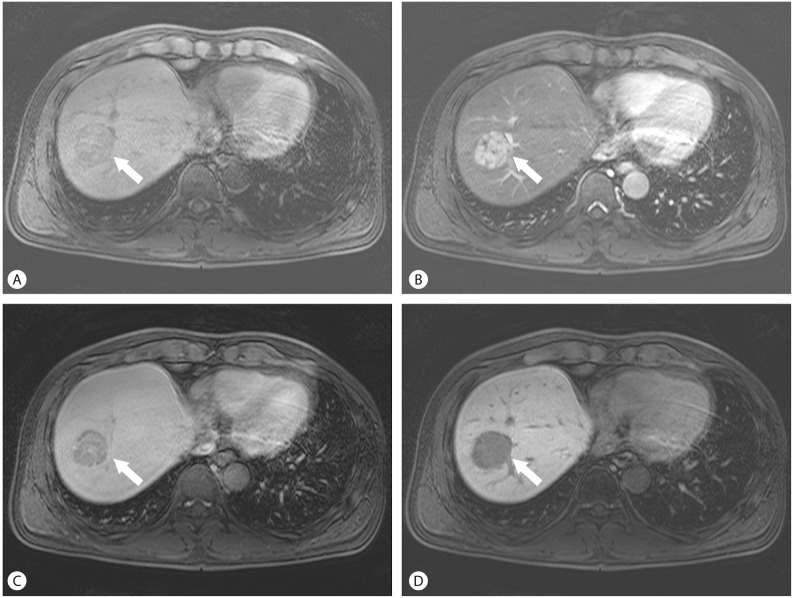
Figure 1.
Hepatocellular carcinoma (HCC). On the non-contrast T1 weighted image, a heterogeneously hypointense mass (arrow) is seen in the right liver (A). It has arterial enhancement (B), and subsequent "wash-out" during the equilibrium phase, with or without a delayed enhancing fibrous capsule (C). On the hepatobiliary phase image, hypointensity of the mass is obvious (D).
Hepatobiliary phase imaging improves sensitivity and specificity for diagnosis of HCC. In a multicenter study,14 liver imaging with Gd-EOB-DTPA resulted in lower false positive findings and a higher detection rate of small HCC lesion in comparison to CT. Because most HCCs are hypointense on hepatobiliary phase, Gd-EOB-DTPA can play a possible role in differentiating arterial pseudolesions such as arterioportal shunts from small HCC. However, it remains controversial, with reports of paradoxical enhancement of HCC,5 nonretention by dysplastic nodules and confluent fibrosis.15,16 Nevertheless, the hepatobiliary phase imaging is used for differentiation of nodules in cirrhotic liver and histologic grading of HCCs.10
Cholangiocarcinoma
Cholangiocarcinoma is an aggressive malignant tumor arising from the bile duct, and about 25% of the disease originates from intrahepatic bile duct. On MRI, T2 weighted image shows variable signal intensity in the tumor due to concurrent coagulative necrosis and desmoplastic fibrosis. The contrast enhancement pattern after MR contrast agent depends on the histological components of the tumor. Due to neoangiogenesis in the periphery of the tumor, strong arterial enhancement and portal venous wash-out is often observed (Fig. 2).10 Therefore, the initial peripheral rim enhancement of cholangiocarcinoma should not be mistaken for interrupted peripheral enhancement of hemangioma.17 Approximately 10-15 minutes after contrast injection the desmoplastic fibrous components of the tumor demonstrate mild enhancement. However, there is significant uptake of contrast into surrounding liver parenchyma at this time, and hepatobiliary phase image shows relatively hypointensity of the tumor. Combined hepatocellular and cholangiocarcinoma is a rare form of the hepatic malignancy, and its clinical and imaging features are similar either to HCC and cholangiocarcinoma. According to a recent study, rim enhancement of combined tumor during early arterial phase and target appearance of cholangiocarcinoma on hepatobiliary phase helps to differentiate between two malignancies.18
Figure 2.
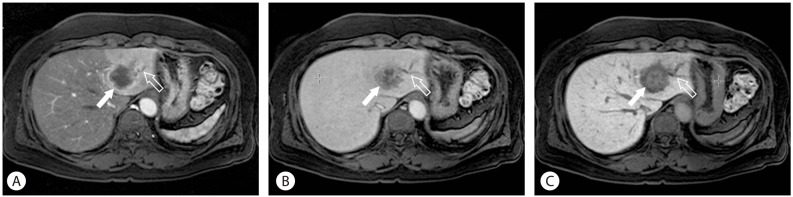
Cholangiocarcinoma. Due to neoangiogenesis in the periphery of the tumor, strong arterial enhancement (arrow) and ductal dilatation (open arrow) are observed (A). On the equilibrium phase, the center of the mass is gradually enhanced because of the desmoplastic fibrous components of the tumor (arrow) (B). The hepatobiliary phase image also shows the enhancement of fibrous component. However, because there is significant uptake of contrast into surrounding liver parenchyma, the mass is relatively hypointensity (arrow) (C).
Hepatic metastasis
Contrast enhanced liver MR imaging is very useful when previous imaging with ultrasound or CT remains unclear to diagnosis liver metastasis. Using both extracellular and liver specific contrast agents, similar imaging features are demonstrated during the dynamic study: typical peripheral rim enhancement and central hypointensity due to necrosis.10 Because the metastatic lesions deplete hepatocytes, the lesions are significantly hypointense on the hepatobiliary phase without exception. Liver specific MR contrast agent increases sensitivity in the detection of metastases by exploiting the liver to lesion contrast caused by the hyperintense background liver parenchyma. However, as with other malignant focal hepatic diseases, specificity of metastasis characterization remained to be undetermined because benign hepatic lesions such as cysts and hemangioma also appear hypointense on hepatobiliary phase image.
Focal nodular hyperplasia and hepatic adenoma
The representative benign lesions of hepatocellular origin are hepatic adenoma and FNH. Although they occurs in young women, imaging features of MR are different each other. FNH has a quite typical imaging feature. It is a completely benign lesion which does not need any specific treatment including surgical resection. It consists of normal hepatocytes with abnormal nodular architecture, malformed vessels and abnormal biliary proliferation. Multiplicity is relatively high (about 20-25%), and it is associated with oral contraceptives or pregnancy. The typical features of FNH are a predominantly hepatic arterial supply (it is the most important feature that is exploited to diagnose FNH), their composition of functioning hepatocytes, and the presence of malformed biliary structures.10 The majority of FNH contain a central scar. If a central scar is present, the fibrous tissue of the central scar accumulates contrast agents circulating in the blood pool, during the later phases. With hepatobiliary contrast agents the central scar also enhances, but the surrounding hepatocytes within the FNH accumulate higher concentration of contrast agents and the central scar will appear relatively hypointense (Fig. 3).12
Figure 3.
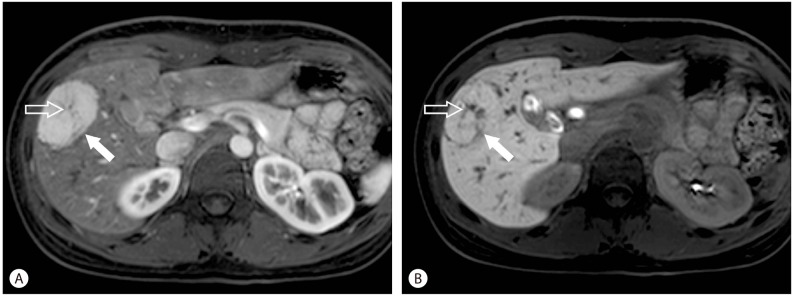
Focal nodular hyperplasia (FNH). The typical features of FNH are a predominantly hepatic arterial supply (arrow), and a central scar (open arrow) containing a vascular pedicle (A). With hepatobiliary contrast agents, the surrounding hepatocytes within the FNH accumulate higher concentration of contrast agents (arrow) and the central scar will appear relatively hypointense (open arrow) (B).
Hepatic adenoma is also benign tumor of hepatocellular origin. Component cells are arranged in cords without acinar architecture, separated by thin walled vascular channels which are perfused primarily by branches of the hepatic artery. The absence of bile ducts within the tumor is a key feature used by pathologists to differentiate from FNH. Because of a risk of hemorrhage and malignant transformation, it is often treated by surgical resection. Hepatic adenoma is also a typically hypervascular lesion detected during the late arterial phase. The enhancement pattern is highly variable on dynamic enhancement studies. Unlikel FNH, because the altered cellular structure in hepatic adenoma results in an abnormal cell membrane transport system,19 hypointensity or isointensity on hepatobiliary imaging is characteristic of hepatic adenoma (Fig. 4).12
Figure 4.
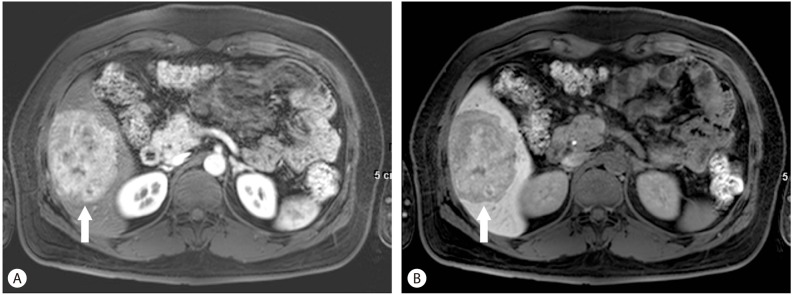
Hepatic adenoma. Hepatic adenoma is also a typically hypervascular lesion detected during the late arterial phase (arrow) (A). However, unlikel FNH, the altered cellular structure in the mass results in hypointensity on hepatobiliary imaging (B).
Hemangiomas
Hepatic hemangiomas are the most common benign focal hepatic lesions. Its incidence is known by 2-20% in the population. In general, MRI features of the hemangioma are round or lobular margins, "light bulb"-like T2 hyperintensity, and characteristic enhancement pattern: peripheral, nodular, and centripetal enhancement. Imaging features on hepatobiliary phase are not specific: most of them are hypointense, mirroring the signal intensity of the portal veins. This imaging appearance has been referred to as "pseudo-wash-out" which is expected given the lack of hepatocytes (Fig. 5). Despite these imaging appearances on hepatobiliary phase, hepatocyte-specific contrast agent is not the best choice for suspected hemangiomas.17
Figure 5.
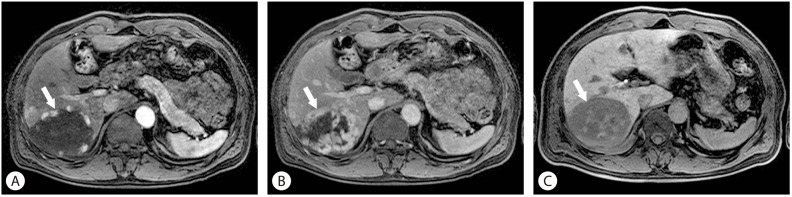
Hemangioma. The characteristic enhancement pattern of hemangioma is peripheral, nodular, and centripetal enhancement (arrow) (A, B). Imaging features on hepatobiliary phase are hypointense, mirroring the signal intensity of the portal veins referred to as "pseudo-wash-out" (C).
Diffuse liver disease
In the normally functioning hepatocytes, the hepatocyte specific MR contrast agent will be taken into the hepatocytes and produce T1 shortening in the area that contains contrast agent. Conversely, if the hepatocytes do not function normally or they are replaced by other cells such as metastatic tumor cells, the contrast agent will not be taken up and the signal intensity of the area will be lower than that of the normal liver. Therefore, the enhancement by liver-specific contrast agent depends on the underlying liver function: liver perfusion, vascular permeability, extracellular diffusion, and hepatocyte transporter expression. Thus, peak enhancement of the cirrhotic liver may be diminished and delayed.11,20
Estimation of liver function by measuring the enhancement of the liver or ratio of hepatic enhancement to splenic enhancement with Gd-EOB-DTPA MRI may be an alternative to indocyanine green clearance in the workup before deciding major hepatic resection.21 Hepatobiliary contrast agent can only be used for the preliminary staging of hepatic fibrosis, but a study have demonstrated that Gd-EOB-DTPA MRI is more reliable than diffusion weighted imaging for staging hepatic fibrosis.22 Nevertheless, pharmacokinetics analyses of Gd-EOB-DTPA MRI enhancement have several issues,20 including the lack of linearity between the signal intensity and the concentration of contrast agent.
CONCLUSION
Because gadolinium-based hepatocyte-specific MR contrast agents have dual properties for dynamic and hepatobiliary imaging, they are very useful to detect and characterize various benign and malignant focal hepatic lesions. Moreover they can show functional information and are helpful to estimate hepatic functional reservoir. If we can overcome several pitfalls such as ringing artifact and acute transient dyspnea during arterial phase and can develop imaging techniques for higher spatial and temporal resolution, the usefulness of hepatocyte-specific MR contrast agents will be more increased.
Abbreviations
- CT
Computed tomography
- FNH
Focal nodular hyperplasia
- Gd-BOPTA
Gadobenate dimeglumine
- Gd-EOB-DTPA
Gadoxetic acid
- HCC
Hepatocellular carcinoma
- MRI
Magnetic resonance imaging
- MRP
Multidrug resistance-associated protein
- OATP
Organic anion transporting polypeptide
Footnotes
The authors have no conflicts to disclose.
References
- 1.Burke C, Alexander Grant L, Goh V, Griffin N. The role of hepatocyte-specific contrast agents in hepatobiliary magnetic resonance imaging. Semin Ultrasound CT MR. 2013;34:44–53. doi: 10.1053/j.sult.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Reimer P, Schneider G, Schima W. Hepatobiliary contrast agents for contrast-enhanced MRI of the liver: properties, clinical development and applications. Eur Radiol. 2004;14:559–578. doi: 10.1007/s00330-004-2236-1. [DOI] [PubMed] [Google Scholar]
- 3.Kirchin MA, Pirovano GP, Spinazzi A. Gadobenate dimeglumine (Gd-BOPTA). An overview. Invest Radiol. 1998;33:798–809. doi: 10.1097/00004424-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Schuhmann-Giampieri G, Schmitt-Willich H, Frenzel T, Schitt-Willich H. Biliary excretion and pharmacokinetics of a gadolinium chelate used as a liver-specific contrast agent for magnetic resonance imaging in the rat. J Pharm Sci. 1993;82:799–803. doi: 10.1002/jps.2600820809. [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Jeong WK, Kim Y, Kim MY, Kim J, Pyo JY, et al. Hepatocellular carcinoma composed of two different histologic types: imaging features on gadoxetic acid-enhanced liver MRI. Clin Mol Hepatol. 2013;19:92–96. doi: 10.3350/cmh.2013.19.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastor CM, Planchamp C, Pochon S, Lorusso V, Montet X, Mayer J, et al. Kinetics of gadobenate dimeglumine in isolated perfused rat liver: MR imaging evaluation. Radiology. 2003;229:119–125. doi: 10.1148/radiol.2291020726. [DOI] [PubMed] [Google Scholar]
- 7.Tsuboyama T, Onishi H, Kim T, Akita H, Hori M, Tatsumi M, et al. Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging--correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology. 2010;255:824–833. doi: 10.1148/radiol.10091557. [DOI] [PubMed] [Google Scholar]
- 8.Tanimoto A, Higuchi N, Ueno A. Reduction of ringing artifacts in the arterial phase of gadoxetic acid-enhanced dynamic MR imaging. Magn Reson Med Sci. 2012;11:91–97. doi: 10.2463/mrms.11.91. [DOI] [PubMed] [Google Scholar]
- 9.Davenport MS, Viglianti BL, Al-Hawary MM, Caoili EM, Kaza RK, Liu PS, et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013;266:452–461. doi: 10.1148/radiol.12120826. [DOI] [PubMed] [Google Scholar]
- 10.Frydrychowicz A, Lubner MG, Brown JJ, Merkle EM, Nagle SK, Rofsky NM, et al. Hepatobiliary MR imaging with gadolinium-based contrast agents. J Magn Reson Imaging. 2012;35:492–511. doi: 10.1002/jmri.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong WK, Byun JH, Lee SS, Won HJ, Kim KW, Shin YM, et al. Gadobenate dimeglumine-enhanced liver MR imaging in cirrhotic patients: quantitative and qualitative comparison of 1-hour and 3-hour delayed images. J Magn Reson Imaging. 2011;33:889–897. doi: 10.1002/jmri.22492. [DOI] [PubMed] [Google Scholar]
- 12.Grazioli L, Bondioni MP, Haradome H, Motosugi U, Tinti R, Frittoli B, et al. Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology. 2012;262:520–529. doi: 10.1148/radiol.11101742. [DOI] [PubMed] [Google Scholar]
- 13.Khan AS, Hussain HK, Johnson TD, Weadock WJ, Pelletier SJ, Marrero JA. Value of delayed hypointensity and delayed enhancing rim in magnetic resonance imaging diagnosis of small hepatocellular carcinoma in the cirrhotic liver. J Magn Reson Imaging. 2010;32:360–366. doi: 10.1002/jmri.22271. [DOI] [PubMed] [Google Scholar]
- 14.Hammerstingl R, Huppertz A, Breuer J, Balzer T, Blakeborough A, Carter R, et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008;18:457–467. doi: 10.1007/s00330-007-0716-9. [DOI] [PubMed] [Google Scholar]
- 15.Golfieri R, Grazioli L, Orlando E, Dormi A, Lucidi V, Corcioni B, et al. Which is the best MRI marker of malignancy for atypical cirrhotic nodules: hypointensity in hepatobiliary phase alone or combined with other features? Classification after Gd-EOB-DTPA administration. J Magn Reson Imaging. 2012;36:648–657. doi: 10.1002/jmri.23685. [DOI] [PubMed] [Google Scholar]
- 16.Park YS, Lee CH, Kim BH, Lee J, Choi JW, Kim KA, et al. Using Gd-EOB-DTPA-enhanced 3-T MRI for the differentiation of infiltrative hepatocellular carcinoma and focal confluent fibrosis in liver cirrhosis. Magn Reson Imaging. 2013;31:1137–1142. doi: 10.1016/j.mri.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Fowler KJ, Brown JJ, Narra VR. Magnetic resonance imaging of focal liver lesions: approach to imaging diagnosis. Hepatology. 2011;54:2227–2237. doi: 10.1002/hep.24679. [DOI] [PubMed] [Google Scholar]
- 18.Hwang J, Kim YK, Park MJ, Lee MH, Kim SH, Lee WJ, et al. Differentiating combined hepatocellular and cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma using gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2012;36:881–889. doi: 10.1002/jmri.23728. [DOI] [PubMed] [Google Scholar]
- 19.Vander Borght S, Libbrecht L, Blokzijl H, Faber KN, Moshage H, Aerts R, et al. Diagnostic and pathogenetic implications of the expression of hepatic transporters in focal lesions occurring in normal liver. J Pathol. 2005;207:471–482. doi: 10.1002/path.1852. [DOI] [PubMed] [Google Scholar]
- 20.Van Beers BE, Pastor CM, Hussain HK. Primovist, Eovist: what to expect. J Hepatol. 2012;57:421–429. doi: 10.1016/j.jhep.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Yamada A, Hara T, Li F, Fujinaga Y, Ueda K, Kadoya M, et al. Quantitative evaluation of liver function with use of gadoxetate disodium-enhanced MR imaging. Radiology. 2011;260:727–733. doi: 10.1148/radiol.11100586. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe H, Kanematsu M, Goshima S, Kondo H, Onozuka M, Moriyama N, et al. Staging hepatic fibrosis: comparison of gadoxetate disodium-enhanced and diffusion-weighted MR imaging-preliminary observations. Radiology. 2011;259:142–150. doi: 10.1148/radiol.10100621. [DOI] [PubMed] [Google Scholar]