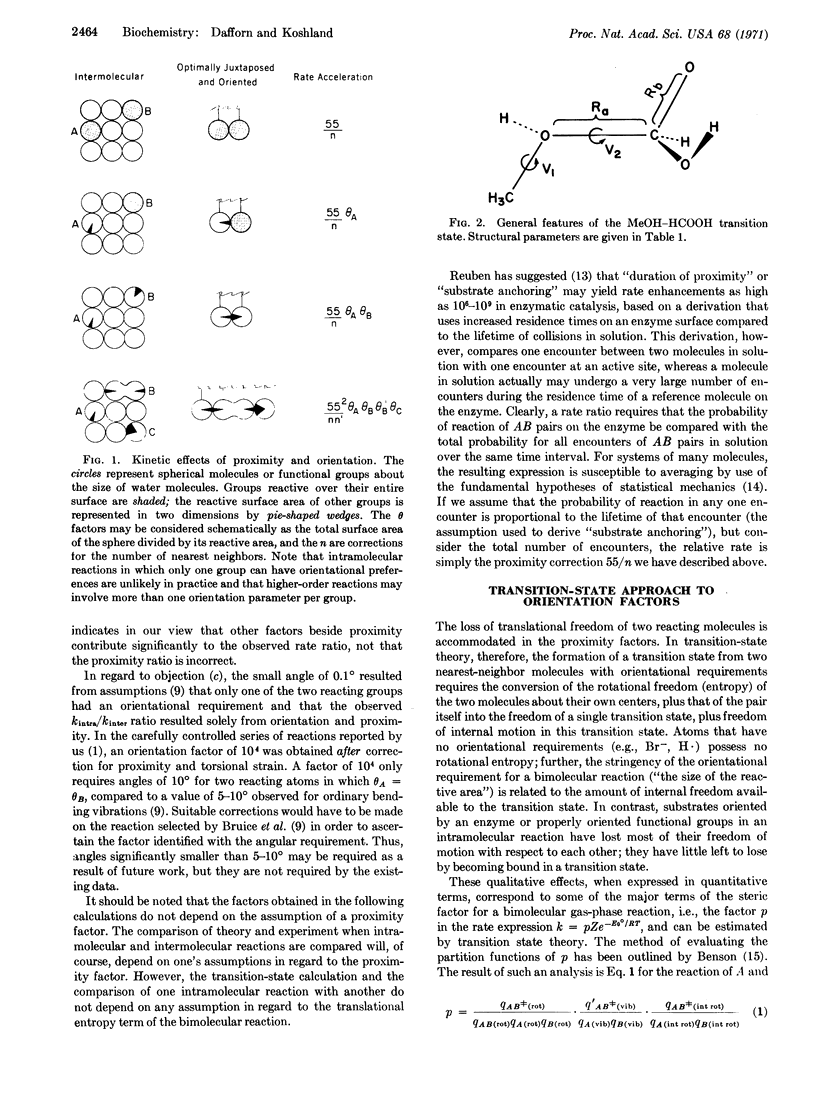
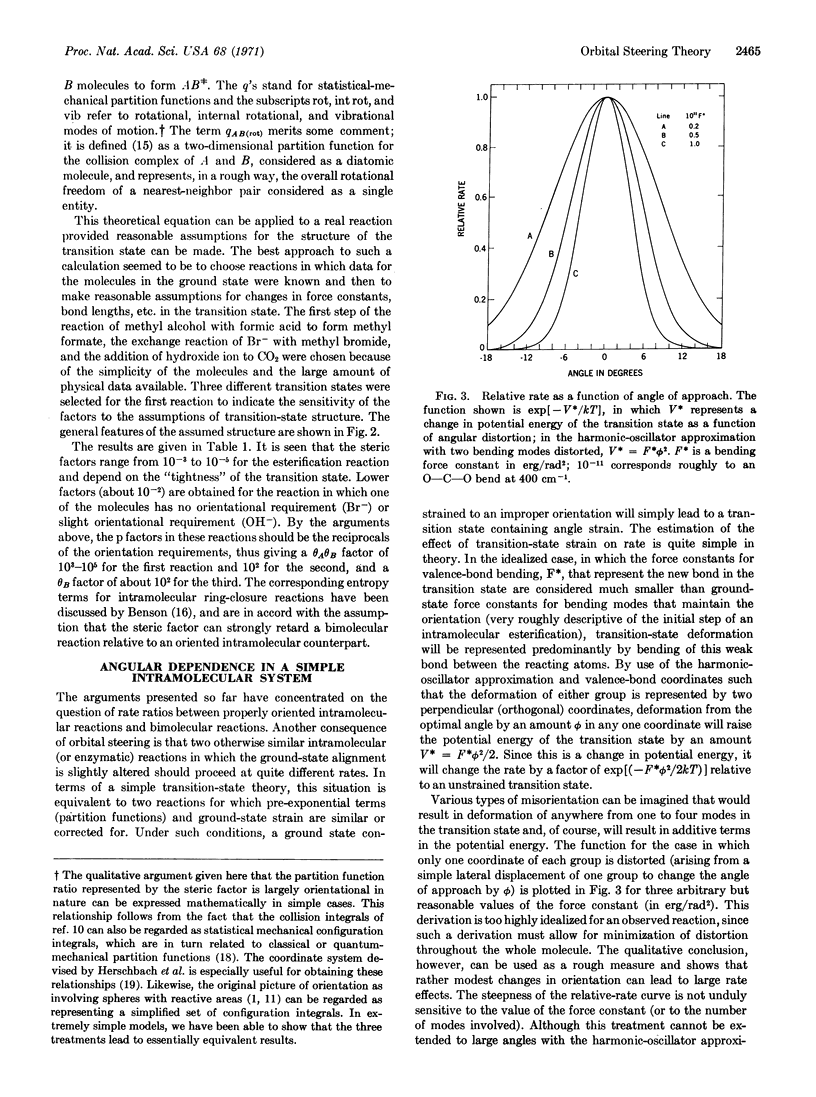
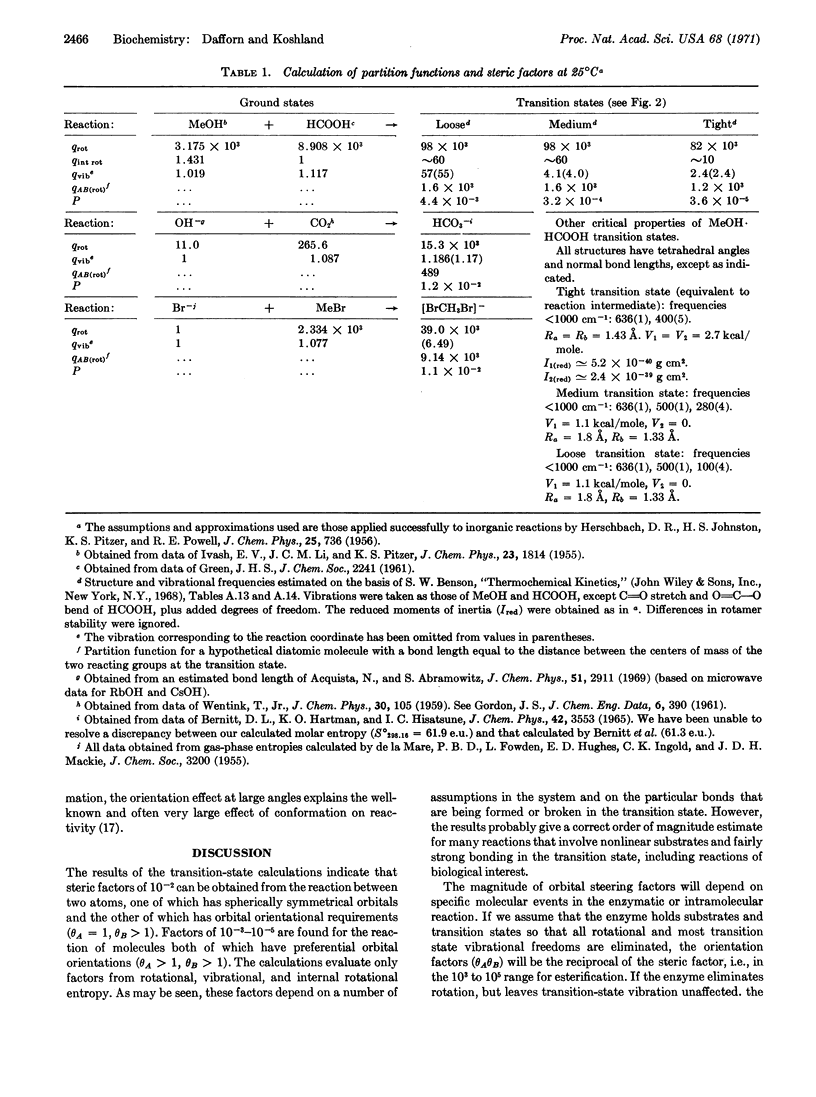
Abstract
A calculation based on transition-state theory leads to the conclusion that rate accelerations of 103-105 could be achieved in an optimally oriented reaction relative to a similar randomly oriented bimolecular reaction. This factor is obtained by the use of partition functions of simplified systems and is based on contributions to rotational and vibrational entropy from reasonable transition states. A simple harmonic oscillator calculation leads to a similar conclusion for series of intramolecular reactions. Although the uncertainty in theoretical calculations of this type is considerable, the results add support to the conclusion based on experimental studies that orientation factors can play a very significant role in the catalytic power of enzymes.
Keywords: enzyme, substrate orientation, catalytic efficiency, rate of reaction, entropy
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruice T. C., Brown A., Harris D. O. On the concept of orbital steering in catalytic reactions. Proc Natl Acad Sci U S A. 1971 Mar;68(3):658–661. doi: 10.1073/pnas.68.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer S. T., Kraut J., Robertus J. D., Wright H. T., Xuong N. H. Chymotrypsinogen: 2.5-angstrom crystal structure, comparison with alpha-chymotrypsin, and implications for zymogen activation. Biochemistry. 1970 Apr 28;9(9):1997–2009. doi: 10.1021/bi00811a022. [DOI] [PubMed] [Google Scholar]
- Henderson R. Structure of crystalline alpha-chymotrypsin. IV. The structure of indoleacryloyl-alpha-chyotrypsin and its relevance to the hydrolytic mechanism of the enzyme. J Mol Biol. 1970 Dec 14;54(2):341–354. doi: 10.1016/0022-2836(70)90434-1. [DOI] [PubMed] [Google Scholar]
- Neet K. E., Nanci A., Koshland D. E., Jr Properties of thiol-subtilisin. The consequences of converting the active serine residue to cysteine in a serine protease. J Biol Chem. 1968 Dec 25;243(24):6392–6401. [PubMed] [Google Scholar]
- Reuben J. Substrate anchoring and the catalytic power of enzymes. Proc Natl Acad Sci U S A. 1971 Mar;68(3):563–565. doi: 10.1073/pnas.68.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D. R., Koshland D. E. A source for the special catalytic power of enzymes: orbital steering. Proc Natl Acad Sci U S A. 1970 Jun;66(2):445–452. doi: 10.1073/pnas.66.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Blout E. R. Evidence for an extended active center in elastase. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1734–1740. doi: 10.1073/pnas.67.4.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]



