Abstract
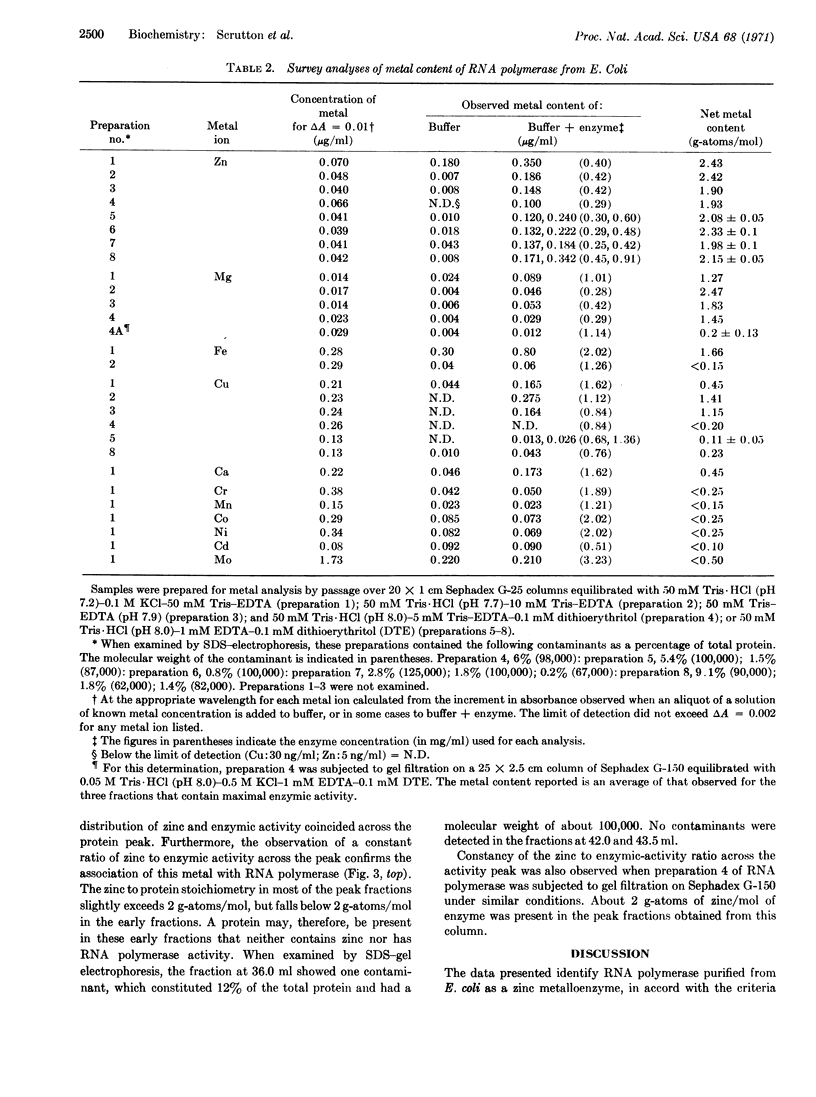
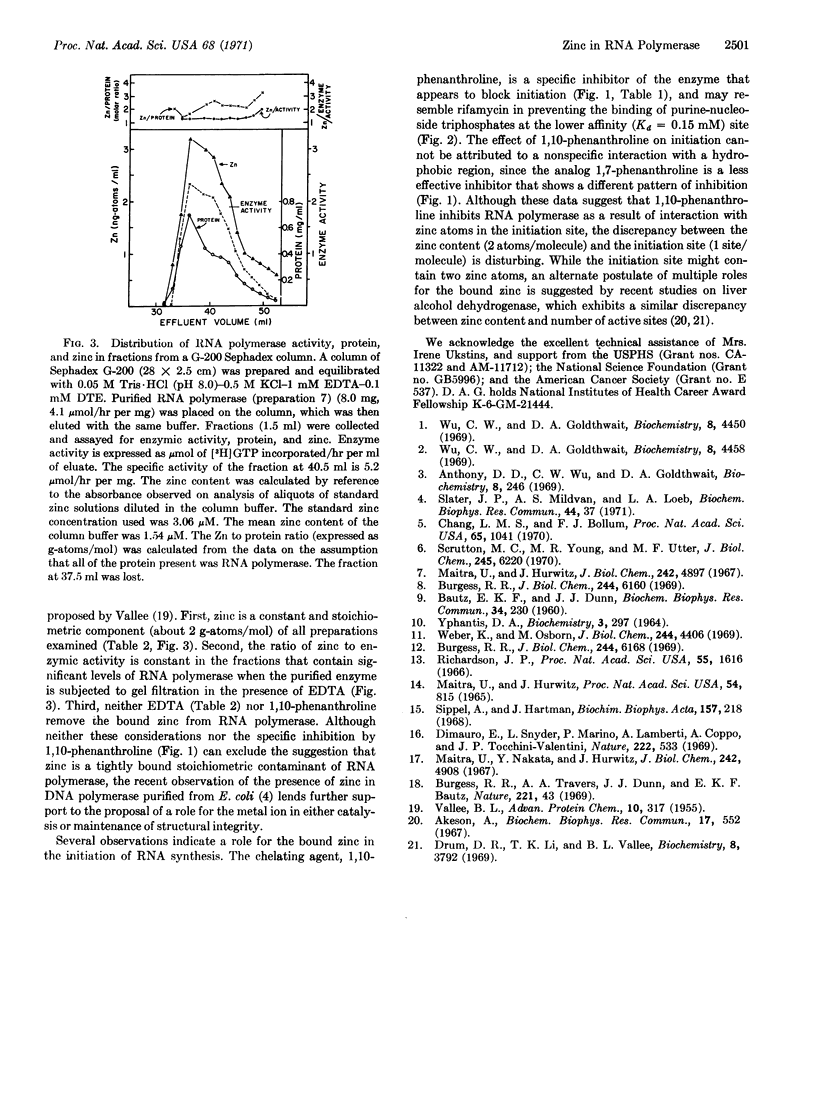
Highly purified preparations of the DNA-dependent RNA polymerase obtained from Escherichia coli contain about 2 g-atoms of tightly bound zinc per mol (molecular weight 370,000) of enzyme. When the purified enzyme is fractionated on Sephadex G-150 or G-200, correlation is observed between the zinc and enzymic activity. Although some of the preparations examined also contain iron, copper, and magnesium, the content of these metal ions show no consistent correlation with RNA polymerase activity.
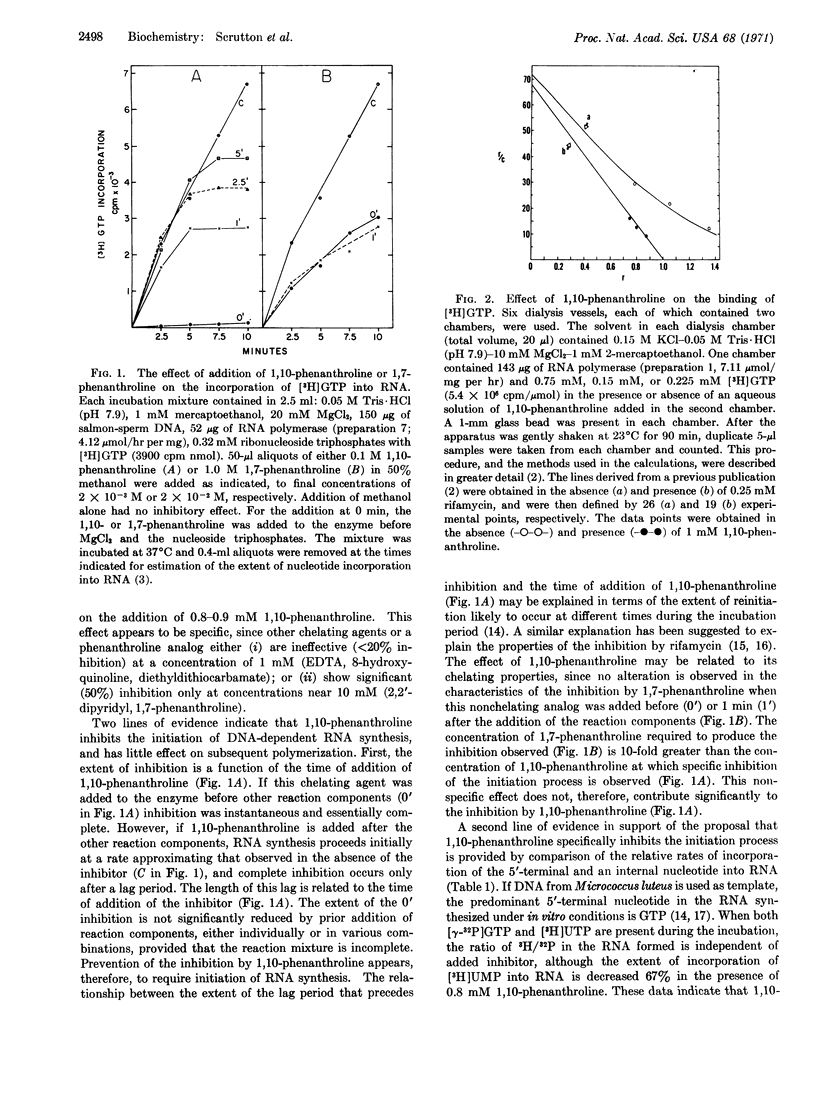
Initiation of RNA synthesis is specifically inhibited by 1,10-phenanthroline. Less-effective inhibition is observed for other chelating agents or for a nonchelating phenanthroline analog. The analog also exhibits a pattern of inhibition differing from that characteristic of 1,10-phenanthroline. Binding of purine nucleoside triphosphates at the lower-affinity (Kd = 0.15 mM) site may also be prevented by the addition of 1,10-phenanthroline. One or both of the bound zinc atoms may, therefore, participate in the initiation of RNA synthesis.
Keywords: 1,10-phenanthroline; 1,7-phenanthroline; divalent cations; metal-binding sites
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony D. D., Goldthwait D. A., Wu C. W. Studies with the ribonucleic acid polymerase. II. Kinetic aspects of initiation and polymerization. Biochemistry. 1969 Jan;8(1):246–256. doi: 10.1021/bi00829a035. [DOI] [PubMed] [Google Scholar]
- Bautz E. K., Dunn J. J. DNA-dependent RNA polymerase from phage T4 infected E. coli: an enzyme missing a factor required for transcription of T4 DNA. Biochem Biophys Res Commun. 1969 Jan 27;34(2):230–237. doi: 10.1016/0006-291x(69)90636-6. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Burgess R. R. Separation and characterization of the subunits of ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6168–6176. [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Doxynucleotide-polymerizing enzymes of calf thymus gland. IV. Inhibition of terminal deoxynucleotidyl transferase by metal ligands. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1041–1048. doi: 10.1073/pnas.65.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drum D. E., Li T. K., Vallee B. L. Zinc isotope exchange in horse liver alcohol dehydrogenase. Biochemistry. 1969 Sep;8(9):3792–3797. doi: 10.1021/bi00837a045. [DOI] [PubMed] [Google Scholar]
- Maitra U., Hurwitz H. The role of DNA in RNA synthesis, IX. Nucleoside triphosphate termini in RNA polymerase products. Proc Natl Acad Sci U S A. 1965 Sep;54(3):815–822. doi: 10.1073/pnas.54.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U., Hurwitz J. The role of deoxyribonucleic acid in ribonucleic acid synthesis. 13. Modified purification procedure and additional properties of ribonucleic acid polymerase from Escherichia coli W. J Biol Chem. 1967 Nov 10;242(21):4897–4907. [PubMed] [Google Scholar]
- Maitra U., Nakata Y., Hurwitz J. The role of deoxyribonucleic acid in ribonucleic acid synthesis. XIV. A study of the initiation of ribonucleic acid synthesis. J Biol Chem. 1967 Nov 10;242(21):4908–4918. [PubMed] [Google Scholar]
- Richardson J. P. Some physical properties of RNA polymerase. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1616–1623. doi: 10.1073/pnas.55.6.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrutton M. C., Young M. R., Utter M. F. Pyruvate carboxylase from baker's yeast. The presence of bound zinc. J Biol Chem. 1970 Nov 25;245(22):6220–6227. [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Slater J. P., Mildvan A. S., Loeb L. A. Zinc in DNA polymerases. Biochem Biophys Res Commun. 1971 Jul 2;44(1):37–43. doi: 10.1016/s0006-291x(71)80155-9. [DOI] [PubMed] [Google Scholar]
- VALLEE B. L. Zinc and metalloenzymes. Adv Protein Chem. 1955;10:317–384. doi: 10.1016/s0065-3233(08)60108-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wu C. W., Goldthwait D. A. Studies of nucleotide binding to the ribonucleic acid polymerase by a fluoresence technique. Biochemistry. 1969 Nov;8(11):4450–4458. doi: 10.1021/bi00839a034. [DOI] [PubMed] [Google Scholar]
- Wu C. W., Goldthwait D. A. Studies of nucleotide binding to the ribonucleic acid polymerase by equilibrium dialysis. Biochemistry. 1969 Nov;8(11):4458–4464. doi: 10.1021/bi00839a035. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]



