Abstract
This study aimed to assess the effects of different dialysate bicarbonate concentrations in correcting acid-base imbalance in 53 stable hemodialysis patients in a university-hemodialysis unit. Three different bicarbonate concentrations were assigned, i.e. 25 mEq/L in 10, 30 mEq/L in 30, and 35 mEq/L in 13 patients. Blood gas analyses from arterial line blood samples before and after dialysis in the mid-week were performed for the determination of pH and serum bicarbonate (HCO3-) concentration. The mean values of predialysis arterial HCO3- were mildly acidotic in all 3 groups, but not significantly different among them, whereas those of post-dialysis arterial HCO3- were alkalotic, especially in the group of 35 mEq/L as compared with the other two groups. The mean blood pH was not significantly different among the 3 groups. As expected, there was a positive correlation between pre-dialysis pH and post-dialysis pH (r=0.45, p=0.001), and pre-dialysis HCO3- and post-dialysis HCO3- (r=0.58, p=0.000), but with a negative correlation between pre-dialysis HCO3- and the increment of intradialytic HCO3- following hemodialysis (r=-0.46, p=0.001). In conclusion, this study shows that the impact of conventional dialysate bicarbonate concentrations ranging from 25 to 35 mEq/L is not quite different on the mild degree of predialysis acidemia, but the degree of postdialysis alkalemia is more prominent in higher bicarbonate concentrations. Base supply by hemodialysis alone does not seem to be the main factor to determine the predialysis acidosis in end-stage renal disease patients on chronic maintenance hemodialysis.
Keywords: Acid-base imbalance, Hemodialysis, Bicarbonate dialysate
Introduction
Metabolic acidosis adversely affects nutritional status and the musculoskeletal system as well as the cardiovascular system leading to worsening morbidity and mortality in patients in chronic renal failure1). Also, the experimental and clinical data showed the adverse effects of metabolic acidosis in maintenance dialysis patients regarding poor nutritional status due to its catabolic effects; which include increased protein breakdown, subnormal essential branched-chain amino acids, and abnormal bone metabolism2, 3). However, most of the recent epidemiologic studies have revealed an inverse correlation between metabolic acidosis and nutritional status, i.e. better nutritional status and lower relative risk for mortality and morbidity in hemodialysis patients with mild to moderate predialysis acidosis4, 5). Therefore, the optimal acidbase status in maintenance hemodialysis patients remains as an on-going debate6).
Though the method of hemodialysis in patients with end-stage renal disease (ESRD) is well known as a method of correction of metabolic acidosis by the supplement of bicarbonate, and NKF KDOQI (The National Kidney Foundation Kidney Disease Outcomes Quality Initiative) guideline recommends predialysis serum bicarbonate (HCO3-) concentration ≥22 mEq/L as a target to correct metabolic acidosis associated adverse effects7), the optimal target for the acid-base correction in hemodialysis by delivering base supply, i.e. bicarbonate buffer, is to maintain patients close to the physiological plasma bicarbonate range during the intradialytic as well as the interdialytic period. However, the variation in blood bicarbonate occurs in maintenance hemodialysis with the highest HCO3- just after dialysis and the lowest just before the next dialysis, the so-called "sawtooth pattern." In fact, mild to moderate predialysis metabolic acidosis with 22 mEq/L or less is very common in hemodialysis patients, which can be abolished by using high dialysate bicarbonate concentration (35-40 mEq/L), but only at the cost of postdialysis alkalosis8).
In general, the specific dialysate base concentration among the available commercial dialysate bicarbonate ranging from 25 to 40 mEq/L appears to be arbitrarily chosen without clear-cut guidelines for its selection so-far by physicians in charge of the dialysis unit. Therefore, there are two main issues to be resolved at present for maintaining the best acid-base status in maintenance hemodialysis patients. First, which one among varying dialysate bicarbonate solutions would be optimal as the standard concentration of bicarbonate in dialysate for maintaining or correcting acidbase status in maintenance hemodialysis patients. Second, which stage among predialysis, post dialysis, and interdialytic phase would be the optimal time for assessing the acid-base status in these patients?
To answer these two major unresolved questions, we assessed the acid-base status by arterial blood gas analysis at both predialysis and postdialysis stages in chronic maintenance hemodialysis patients allocated to one of 3 different conventional bicarbonate solutions, i.e. 25 mEq/L (low), 30 mEq/L (average), and 35 mEq/L (high).
Materials and Methods
1. Subjects
The study subjects were 53 stable ESRD patients receiving hemodialysis for 4 hours, three times weekly, for at least more than 3 months in Hanyang University Guri Hospital. Excluded from these 53 patients were patients having factors, which may have an influence on blood pH such as acute infections, respiratory diseases, oral bicarbonate supplementation, sevelamer as a phosphate binder, etc. At the time of this observational study, in January, 2006, as shown in Table 1, three different dialysate bicarbonate concentrations have been allocated to 53 subjects for mean duration of 64.4±47 months on maintenance hemodialysis. Of total 53 subjects, 10 patients on 25 mEq/L, 30 patients on 30 mEq/L, and 13 patients on 35 mEq/L of dialysate bicarbonate concentration has been used, respectively.
Table 1.
Baseline Characteristics and Prevalence of Metabolic Acidosis in Maintenance Hemodialysis Patients (n=53)
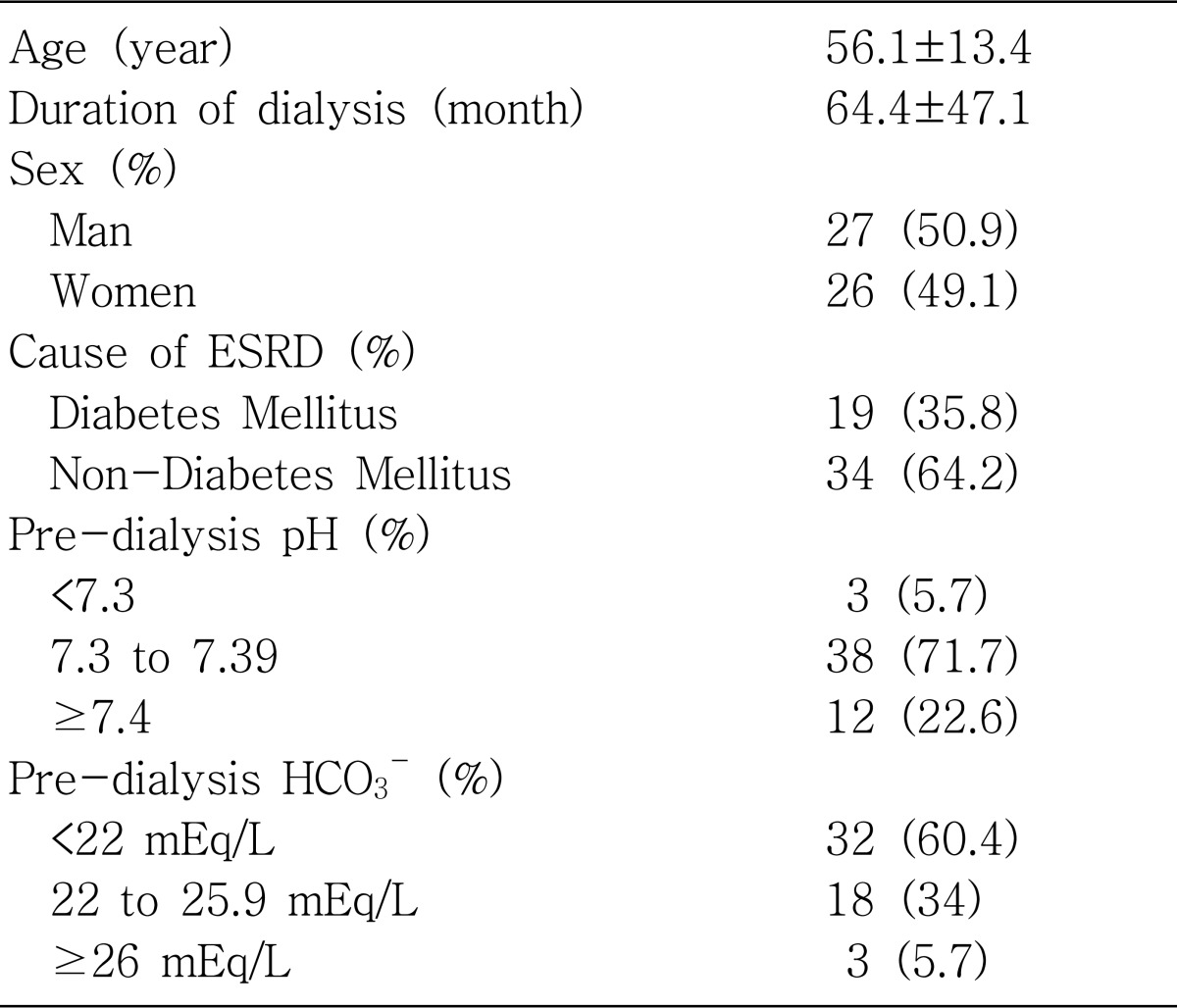
Values are expressed as mean±SD or number (%).
ESRD, End-stage renal disease
HCO3-; serum bicarbonate.
2. Laboratory data
In addition to monthly routine blood work-ups in our hemodialysis unit, arterialized blood samples were collected anaerobically in heparinized syringes at mid-week, either Wednesday (Monday-Wednesday-Friday schedule/week) or Thursday (Tuesday-Thursday-Saturday), from the arterio-venous vascular access immediately before (predialysis) and after dialysis (post-dialysis) after informing the patients of the blood samplings and getting its approval. These arterialized blood samples were analyzed for the determination of pH and HCO3- by blood gas analyzer using NOVA C.C.X (Nova Biomedical Product, USA).
3. Statistical analysis
All statistical analyses were performed by SPSS software, version 12.0. All data are presented as either mean±SD or percentage. The linear regression analysis was used for correlation between pre- and post-dialysis pH or pre- and post-dialysis HCO3- or pre-dialysis HCO3- and increment of intradialytic HCO3-. A statistical significant difference was defined by a p value less than 0.05.
Results
1. Baseline characteristics of the patients (n=53) with analysis of pre-dialysis pH and pre-dialysis HCO3- (Table 1)
The mean age of total 53 ESRD patients on maintenance hemodialysis was 56.1±13.4 years (27 males, 26 females). Nineteen (35.8%) of the 53 ESRD patients were diabetics.
The distribution of pre-dialysis pH revealed 5.7% (n=3) in pH less than 7.30, 71.7% (n=38) in pH between 7.30 to 7.39, and 22.6% (n=12) in pH at or more than 7.4, respectively. Therefore, the percentage of acidemic pH (pH level under 7.4) was 77%, whereas that of normal or alkalemic pH (pre-dialysis pH>7.4) was 23%. The distribution of pre-dialysis HCO3- was 60.4% (n=32) under 22 mEq/L, 34% (n=18) between 22 to 25.9 mEq/L, and 5.7% (n=3) at or more than 26 mEq/L, respectively. Therefore, the population of pre-dialysis moderate acidosis (<22 mEq/L) was 60%, whereas that of pre-dialysis mild acidosis (22 to 25.9 mEq/L) and even alkalosis (>26 mEq/L) was only 40%.
There was no statistically significant difference between sub-groups of age (<60 and >60), sex (male and female), and between diabetics and non-diabetics in both pre-dialysis pH and pre-dialysis HCO3-.
2. The changes of arterial pH and HCO3- by varying dialysate bicarbonate concentrations (25 mEq/L, 30 mEq/L, 35 mEq/L) before and after hemodialysis (Table 2)
Table 2.
Effects of Varying Dialysate Bicarbonate Concentrations (25 mEq/L, 30 mEq/L, 35 mEq/L) on Arterial pH and HCO3- Immediately before and after Hemodialysis
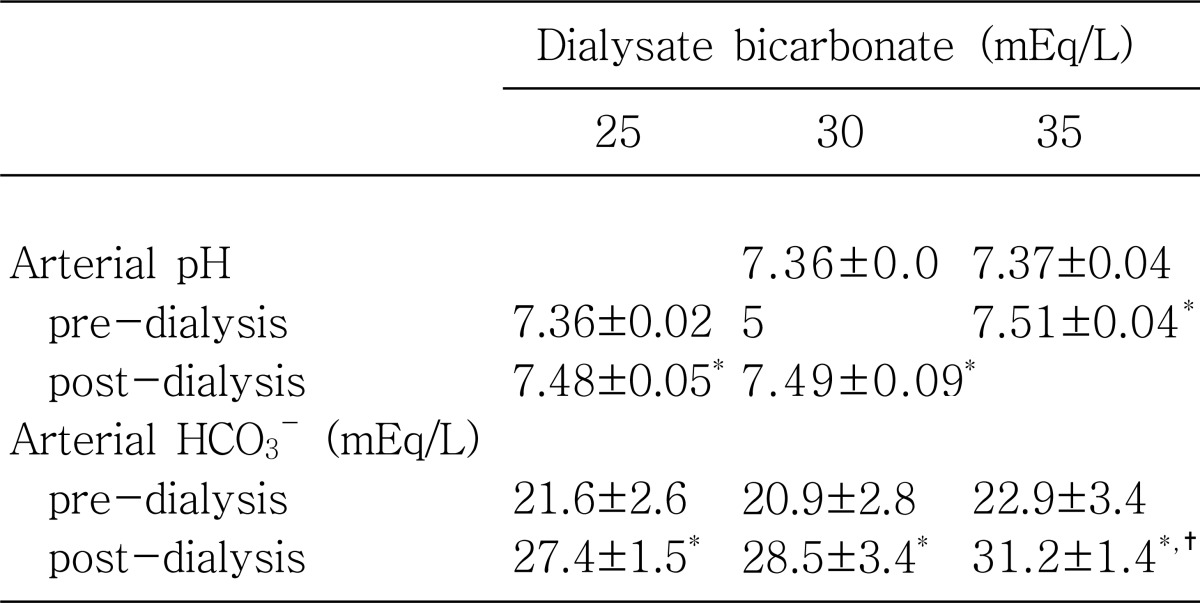
HCO3-, Serum bocarbonate.
*p value<0.05 between pre-dialysis vs post-dialysis.
†p value<0.05 between 35 mEq/L vs 30 mEq/L or 25 mEq/L.
Both, mean values of pre-dialysis pH and pre dialysis HCO3- were not significantly different among the 3 groups on 3 different dialysate bicarbonate concentrations: predialysis pH, 7.36±0.02 on 25 mEq/L vs 7.36±0.05 on 30 mEq/L vs 7.37±0.04 on 35 mEq/L (p=NS) and pre-dialysis HCO3-, 21.6±2.6 on 25 mEq/L vs 20.9±2.8 on 30 mEq/L vs 22.9±3.4 on 35 mEq/L (p=NS).
Immediately after hemodialysis, mean values of post-dialysis pH increased significantly toward alkalemia (pH>7.4) from those of pre-dialysis pH less than 7.4 in all 3 groups: post-dialysis pH, 7.48±0.05 on 25 mEq/L (p<0.05) on 25 mEq/L, 7.49±0.09 on 30 mEq/L (p<0.05) and 7.51±0.04 on 35 mEq/L (p<0.05). Similarly, mean values of post-dialysis HCO3- increased significantly toward alkalosis (>27 mEq/L) from those of pre-dialysis HCO3-, less than 24 mEq/L, in all 3 groups: post-dialysis HCO3-, 27.4±1.5 mEq/L on 25 mEq/L (p<0.05), 28.5±3.4 mEq/L on 30 mEq/L (p<0.05), and 31.2±1.4 mEq/L on 35 mEq/L (p<0.05). When the mean values of post-dialysis pH and post-dialysis HCO3- were compared among 3 groups, there were no significant differences, except for post-dialysis HCO3- on 35 mEq/L of dialysate bicarbonate concentration, 31.2±1.4 mEq/L, which was significantly higher than those of the other 2 groups with 25 mEq/L and 30 mEq/L (p<0.05).
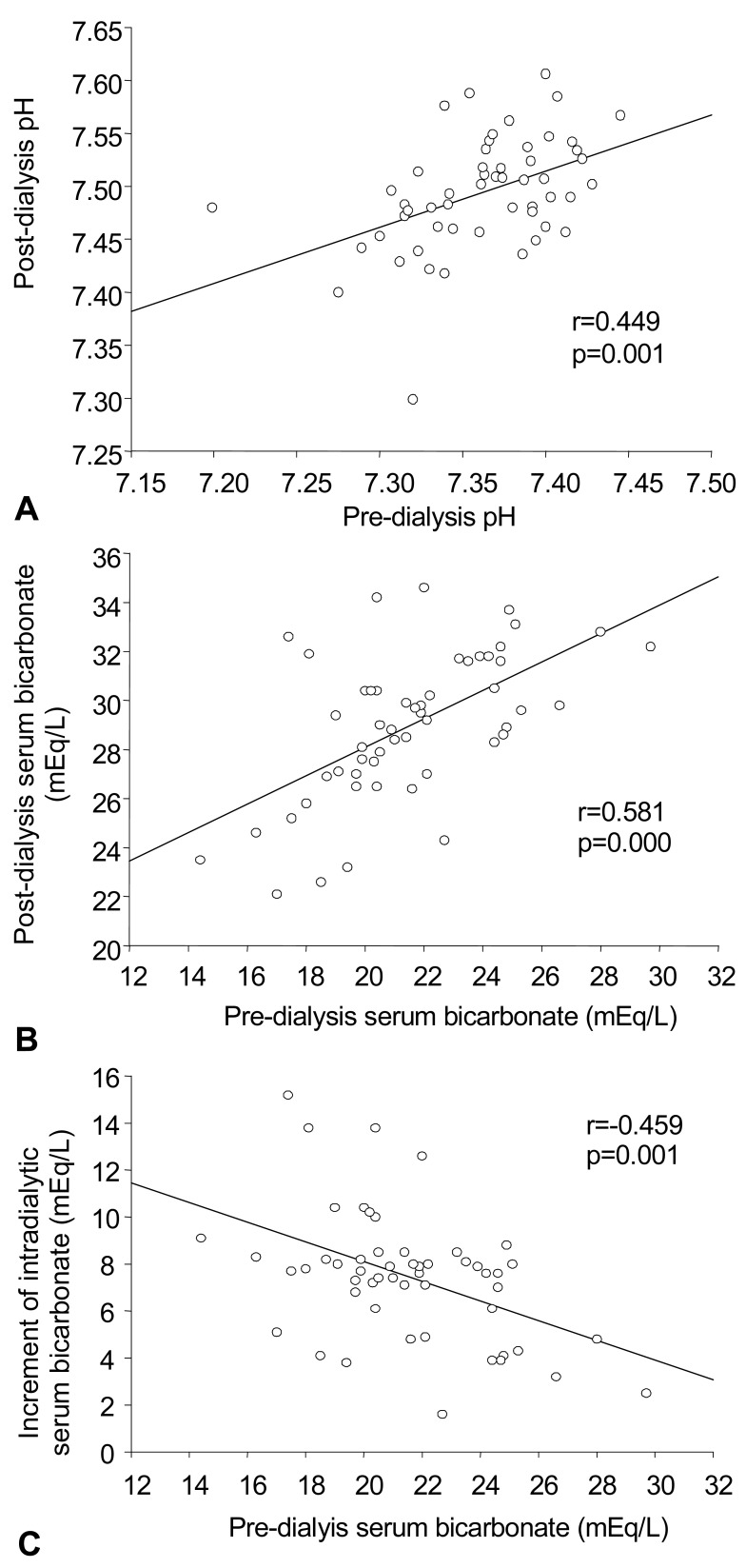
3. The correlation between pre-dialysis pH vs post-dialysis pH, pre-dialysis HCO3- vs post-dialysis HCO3-, and pre-dialysis HCO3- vs the increment of intradialytic HCO3- following hemodialysis (Fig. 1)
Fig. 1.
The correlations between A) pre-dialysis pH vs post-dialysis pH, B) pre-dialysis serum bicarbonate (HCO3-) vs post-dialysis HCO3-, and pre-dialysis HCO3- vs the increment of intradialytic HCO3- following hemodialysis.
pH between values seen before and after dialysis showed a positive relationship in all 53 subjects (r=0.45, p=0.001). Similarly, arterial HCO3- between values seen before and after dialysis also showed a positive relationship in all subjects (r=0.58, p=0.000). As expected, baseline arterial HCO3- before dialysis and the increment of arterial HCO3- after dialysis showed a negative relationship (r=-0.46, p=0.001).
Discussion
This study evaluated the impact of varying dialysate bicarbonate concentrations of 25 mEq/L, 30 mEq/L, and 35 mEq/L on acid-base status just before and after dialysis in 53 chronic maintenance hemodialysis patients. In addition, we tried to find out the optimal dialysate base (bicarbonate) concentration among commercially available various dialysate solutions in maintenance hemodialysis patients to correct the acid-base imbalance, which had been on continuous debate9). The main results of this study are summarized as follows: 1) the prevalence of metabolic acidosis in maintenance hemodialysis patients is high, when defined by pre-dialysis pH <7.4 (77%) and pre-dialysis HCO3- <22 mEq/L (60%) (Table 2), 2) predialysis acid-base status showing mild to moderate degree of metabolic acidosis is similar regardless of the amount of base (bicarbonate) delivery by hemodialysis using three different dialysate bicarbonate concentrations, which suggests the more important role of acid loading and generation or alkali regeneration during the interdialytic period (Table 1), and 3) post-dialysis acid-base status changes from pre-dialysis metabolic acidosis toward metabolic alkalosis in all three different kinds of dialysate bicarbonate concentration with more prominent alkalemia in higher dialysate bicarbonate concentration (Table 2).
It has been well recognized that the correction of metabolic acidosis is one of the major goals in hemodialysis treatment of chronic maintenance hemodialysis patients since metabolic acidosis, if left untreated, may lead to systemic detrimental effects such as malnutrition due to negative nitrogen balance and increased protein catabolism, anorexia, fatigue, alterations in bone metabolism and formation, secondary hyperparathyroidism, and decreased cardiovascular functions10). Therefore, the treatment of metabolic acidosis can be assumed to reverse its many side effects in chronic hemodialysis patients. But the level of bicarbonate concentration at which the correction of harmful effects of metabolic acidosis is seen has not been clearly defined so far6).
Among several preferred recommendations in the world, NKF KDOQI guideline recommends to preserve the predialysis HCO3- concentration at or above 22 mEq/L7). However, most of the maintenance hemodialysis patients can be found to have low pre-dialysis HCO3- concentration. In fact, the prevalence of metabolic acidosis based on pre-dialysis HCO3- less than 22 mEq/L in our study was more than half of the total patients (60%), which is higher than that of the hemodialysis (HEMO) study of 1,000 patients (50%) by Uribarri et al.4).
The clinical importance of metabolic acidosis in dialysis patients has not been clearly clarified due to contradictory findings between the clinical or experimental data of hemodialysis patients revealing previously well-known detrimental effects and the vast majorities of recent epidemiologic studies of them showing the pattern of reverse epidemiology5, 6). One of the recent epidemiologic studies, DOPPS (Dialysis Outcomes and Practice Pattern Study) shows that moderate Predialysis metabolic acidosis seems to be associated with better nutritional status and lower relative risk for mortality or hospitalization than is observed in patients with normal or severe pre-dialysis metabolic acidosis5). Furthermore, whether pre-dialysis bicarbonate level alone in maintenance hemodialysis patients can reflect their acid-base status has only been addressed in few studies8).
The delivered quantity of bicarbonate in dialysate solution during dialysis could be dependent by the concentration of bicarbonate, flow speed of solution, filtration rate, type of the machine and dynamics of bicarbonate. A formula to predict HCO3- concentration after dialysis was created as 15.87122 - 0.03889 (dialysis hours) - 0.0337 (fluid speed) + 0.01621 (injected dialysis solution) + 0.44359 (bicarbonate concentration before dialysis) + 0.04048 (body weight) + coefficient of used dialysis membrane11). However, the authors suggested that this calculation predicting HCO3- concentration can be more accurate when the hydrogen ion generation in the body besides base supply by dialysis can be estimated. In our study, since the predialysis acid-base status showing mild to moderate degree of metabolic acidosis is similar regardless of the amount of base (bicarbonate) delivery by hemodialysis using three different kinds of dialysate bicarbonate concentration (Table 1), it suggests that pre-dialysis metabolic acidosis is independent from bicarbonate supply in the intradialytic phase, and the base supply by dialysis does not seem to represent the main mechanism for the correction of acid-base imbalance by hemodialysis12). Diet, intestine, bone, and other intermediate metabolism including the effects of medications would be involved for acid-base balance in addition to the base delivery by dialysis in maintenance hemodialysis patients9). In fact, besides the base supply by dialysis, Soudan et al. reported that HCO3- concentration decreases by 1 mEq/per 0.2 g/kg/d of increased protein intake. HCO3- concentration decreases by 0.37 mEq/L per 2 g/d of sevelamer intake. It also decreases by 0.76 mEq/L per 0.2 of increased Kt/V and by 0.90 mEq/L per 2 g/d of calcium carbonate intake13).
In a study using 30 mEq/L of dialysate bicarbonate solution, the patients reached normal acid-base balance following dialysis but returned to a mild degree of acidosis before the next dialysis. However, when using 35 mEq/L of dialysate bicarbonate solution, patients did not show acidosis before dialysis but showed alkalosis following dialysis8). Another study divided 46 patients using 35 mEq/L of dialysate bicarbonate solution into group A (n=21) and group B (n=25). A lowevel bicarbonate (30 mEq/L) and high-level bicarbonate (40 mEq/L) dialysate solution were used alternatively to each group with an interval of 6 months, respectively. As a result, serum total carbon dioxide concentration showed a significant difference between the low-level and high-level of dialysate bicarbonate solutions. But, increased pH during dialysis was not meaningful before and after dialysis14). This phenomenon was explained by bicarbonate-transfer, achieving a new balanced situation. Thus, it has been suggested that even though the high-level of bicarbonate dialysis solution is used, progressive alkalosis will not occur and patients will be safe14, 15).
In this research, along with universal pre-dialysis metabolic acidosis, post-dialysis metabolic alkalosis immediately after dialysis was noted in all three different dialysate bicarbonate concentrations with more prominent alkalemia in higher dialysate bicarbonate concentration (Table 2). Therefore, it could be considered the time-averaged bicarbonate concentration may be within the normal range, but at the cost of post-dialysis metabolic alkalosis16). The risks of post-dialysis metabolic alkalosis include cardiac arrhythmias, respiratory suppression, hypotension, and muscle cramps as acute episodes, and vascular or metastatic calcifications as a chronic detrimental effect, particularly in the presence of elevated calcium-phosphorus product17).
The results of our study have limitations due to the observational retrospective analysis of cross-sectional study and because analysis was done on only pre- and post-dialysis arterial pH and HCO3-. To evaluate clear-cut advantages and disadvantages of either pre-dialysis metabolic acidosis or post-dialysis metabolic alkalosis shown in our results would require the prospective cross-over study on varying dialysate bicarbonate concentrations in which various parameters associated with acid-base imbalance can be assessed simultaneously.
In conclusion, the optimal dialysate among various dialysate bicarbonate concentrations ranging from 25 to 40 mEq/L could be selected for the need of acidbase correction of maintenance hemodialysis patients currently. However, the base supply by hemodialysis alone does not seem to be the main factor to determine the predialysis acidosis in ESRD patients on chronic maintenance hemodialysis. Therefore, besides dialysate bicarbonate concentration, other factors involved in acid-base metabolism in both interdialytic and intradialytic phases should be considered to maintain the best physiologic acid-base status in maintenance hemodialysis patients. Also, this study warrants for the particular attention to the ratio of benefits and risks of post-dialysis metabolic alkalosis when choosing high dialysate bicarbonate concentration for maintenance hemodialysis. Meantime, it would be prudent to choose a more tailored regimen of dialysate bicarbonate concentration for hemodialysis patients, which could avoid severe post-dialysis alkalemia.
References
- 1.Mehrotra R, Kopple JD, Wolfson M. Metabolic acidosis in maintenance dialysis patients: clinical considerations. Kidney Int Suppl. 2003;64(88):S13–S25. doi: 10.1046/j.1523-1755.2003.08802.x. [DOI] [PubMed] [Google Scholar]
- 2.Swendseid ME, Wang M, Vyhmeister I, Chan W, Siassi F, Tam CF, Kopple JD. Amino acid metabolism in the chronically uremic rat. Clin Nephrol. 1975;3:240–246. [PubMed] [Google Scholar]
- 3.Graham KA, Reaich D, Channon SM, Downie S, Gilmour E, Passlick-Deetjen J, Goodship TH. Correction of acidosis in CAPD decreases whole body protein degradation. Kidney Int. 1996;49:1396–1400. doi: 10.1038/ki.1996.196. [DOI] [PubMed] [Google Scholar]
- 4.Uribarri J, Levin NW, Delmez J, Depner TA, Ornt D, Owen W, Yan G. Association of acidosis and nutritional parameters in hemodialysis patients. Am J Kidney Dis. 1999;34:493–499. doi: 10.1016/s0272-6386(99)70077-6. [DOI] [PubMed] [Google Scholar]
- 5.Bommer J, Locatelli F, Satayathum S, Keen ML, Goodkin DA, Saito A, Akiba T, Port FK, Young EW. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44:661–671. [PubMed] [Google Scholar]
- 6.Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K. Association between serum bicarbonate and death in hemodialysis patients: Is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006;1:70–78. doi: 10.2215/CJN.00010505. [DOI] [PubMed] [Google Scholar]
- 7.Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001;37(Suppl 2):S66–S70. doi: 10.1053/ajkd.2001.20748. [DOI] [PubMed] [Google Scholar]
- 8.Rault R. Optimal dialysate bicarbonate during hemodialysis. ASAIO Trans. 1991;37:M372–M373. [PubMed] [Google Scholar]
- 9.Kovacic V, Roguljic L, Kovacic V. Metabolic acidosis of chronically hemodialyzed patients. Am J Nephrol. 2003;23:158–164. doi: 10.1159/000070205. [DOI] [PubMed] [Google Scholar]
- 10.Agroyannis B, Fourtounas C, Tzanatos H, Dalamangas A, Vlahakos DV. Relationship between interdialytic weight gain and acid-base status in hemodialysis by bicarbonate. Artif Organs. 2002;26:385–387. doi: 10.1046/j.1525-1594.2002.06883.x. [DOI] [PubMed] [Google Scholar]
- 11.Zucchelli P, Santoro A. Correction of acid-base balance by dialysis. Kidney Int Suppl. 1993;43(41):S179–S183. [PubMed] [Google Scholar]
- 12.Messa P, Mioni G, Maio GD, Ferrando C, Lamperi D, Famularo A, Paoletti E, Cannella G. Derangement of acid-base balance in uremia and under hemodialysis. J Nephrol. 2001;14(Suppl 4):S12–S21. [PubMed] [Google Scholar]
- 13.Soudan K, Ricanati ES, Leon JB, Sehgal AR. Determinants of metabolic acidosis among hemodialysis patients. Hemodial Int. 2006;10:209–214. doi: 10.1111/j.1542-4758.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 14.Williams AJ, Dittmer ID, McArley A, Clarke J. High bicarbonate dialysate in haemodialysis patients: effects on acidosis and nutritional status. Nephrol Dial Transplant. 1997;12:2633–2637. doi: 10.1093/ndt/12.12.2633. [DOI] [PubMed] [Google Scholar]
- 15.Oettingger CW, Oliver JC. Normalization of uremic acidosis in hemodialysis patients with a high bicarbonate dialysate. J Am Soc Nephrol. 1993;3:1804–1807. doi: 10.1681/ASN.V3111804. [DOI] [PubMed] [Google Scholar]
- 16.Graham KA, Hoenich NA, Goodship THJ. Pre and interdialytic acid-base balance in hemodialysis patients. Int J Artif Organs. 2001;24:192–196. [PubMed] [Google Scholar]
- 17.Kraut JA. Disturbances of acid-base balance and bone disease in end-stage renal disease. Semin Dial. 2000;13:261–266. doi: 10.1046/j.1525-139x.2000.00070.x. [DOI] [PubMed] [Google Scholar]