Abstract
A water-soluble ionizing radiation mitigator would have considerable advantages for the management of acute and chronic effects of ionizing radiation. We report that a novel oxetanyl sulfoxide (MMS350) is effective both as a protector and a mitigator of clonal mouse bone marrow stromal cell lines in vitro, and is an effective in vivo mitigator when administered 24 h after 9.5 Gy (LD100/30) total-body irradiation of C57BL/6NHsd mice, significantly improving survival (P =0.0097). Furthermore, MMS350 (400 μM) added weekly to drinking water after 20 Gy thoracic irradiation significantly decreased: expression of pulmonary inflammatory and profibrotic gene transcripts and proteins; migration into the lungs of bone marrow origin luciferase+/GFP+ (luc+/GFP+) fibroblast progenitors (in both luc+ marrow chimeric and luc+ stromal cell line injected mouse models) and decreased radiation-induced pulmonary fibrosis (P < 0.0001). This nontoxic and orally administered small molecule may be an effective therapeutic in clinical radiotherapy and as a counter measure against the acute and chronic effects of ionizing radiation.
INTRODUCTION
Radiation-induced pulmonary fibrosis remains a major complication of radiotherapy for thoracic malignancies, particularly non-small cell lung cancer (1, 2). The clinical picture of radiation-induced pulmonary fibrosis is one of replacement of alveolar and distant bronchiolar anatomic structures with myofibroblasts (3, 4), shown recently to be comprised of both endogenous pulmonary fibroblasts and bone marrow origin migratory cells (5, 6). Elucidation of the mechanism of radiation-induced pulmonary fibrosis involves the discovery of those factors, which stimulate proliferation of resident lung fibroblasts and those controlling the migration into the lungs of marrow origin fibroblast progenitors. In the C57BL/6J mouse model, these two processes, which are involved in radiation-induced pulmonary fibrosis, are initiated after a latent period of at least 150 days, following the initial 14 days of an acute radiation-induced pneumonitis phase (3).
Mouse models for radiation-induced pulmonary fibrosis emphasize the separate and independent processes of acute radiation pneumonitis from late pulmonary fibrosis. Both the fibrosis-prone C57BL/6J mouse and fibrosis resistant C3H/HeJ mice demonstrate a similar acute pulmonary radiation reaction (7, 8). Similarities in histopathology between radiation-induced pulmonary fibrosis and lung fibrosis associated with bleomycin chemotherapy (9), idiopathic pulmonary fibrosis and sclerodermal lung (10, 11) suggest a common role for a late onset inflammatory response accompanied by elevated biomarkers of oxidative stress (12–16); however, published clinical trials of treatment of pulmonary fibrosis have shown incomplete effectiveness of antioxidant therapies such as N-acetylcysteine or amifostine (10, 11). A better understanding of the molecular pathophysiology of radiation-induced pulmonary fibrosis might lead to the identification of critical pathways and new targets for small molecule therapeutics that could have applications in a variety of clinical settings.
Recent studies of radiation-induced pulmonary fibrosis in the C57BL/6J mouse model have clearly defined the latent period following acute pneumonitis (4–6, 17). During the latent period, pulmonary histology appears normal, but is then followed by fibrosis, which is heralded by elevation of both protective enzymes such as MnSOD and profibrotic cytokines including TGF-β (4, 17). Understanding the molecular and cellular events during the latent period that led up to the initiation of fibrosis, is a major challenge. Specific molecular targets for prevention of fibrosis have not been identified, consequently limiting the discovery of therapeutic drugs to ameliorate this highly problematic late radiation-induced complication.
The development of a water-soluble small molecule mitigator that is suitable for oral administration would greatly contribute to both protection of normal tissue during clinical radiotherapy and efficiency of deployment of radiation counter measures (18–21). Effective small molecule radiation mitigators include the GS-nitroxides (18–20), triphenylphosphonium conjugated Imidazole Fatty Acids (22), phospho-inositol-3 kinase inhibitors (23), and a variety of other small molecules, which inhibit ionizing radiation-induced cell death (24). Delivery of some of these small molecules at 24 h or later after total-body irradiation has proven effective in animal models of the hematopoietic syndrome (21). GS-nitroxides have proven effective in radiation protection in both total-body irradiation (25) and organ-specific protection of the esophagus (26) from ionizing irradiation.
A challenge for the development of small molecule radiation mitigators has been the design and implementation of a nontoxic and reliable delivery system. The insolubility of many new small molecule radiation mitigators has necessitated their administration by intravenous, intraperitoneal or other systemic routes (18, 22), coupled with delivery formulations that require liposomal or other vehicles, some of which have been unsuitable for oral administration (26). We now report a novel water-soluble radiation mitigator (MMS350) (27), which when delivered in drinking water over several weeks, reduces late radiation-induced biomarker elevations and both marrow stromal cell mediated and overall pulmonary fibrosis in C57BL/6NHsd mice.
MATERIALS AND METHODS
Mouse Total-Body and Thoracic Irradiation
C57BL/6NHsd mice were obtained from Harlan Sprague Dawley and C57BL6-Luc+GFP+ mice were obtained from Steve Thorne, University of Pittsburgh Cancer Institute and housed 5 per cage according to Institutional IACUC protocols. Total-body irradiation and transplantation of luciferase+ (luc+) marrow was performed as published (5, 6).
For lung irradiation, the thoracic cavity was irradiated with shielding of the head and neck region and abdomen and lower body parts according to published methods (5). Animals received 20 Gy single-fraction thoracic irradiation and were then maintained according to IACUC directed laboratory conditions. Mice were sacrificed at serial time points after thoracic irradiation including preirradiation, days 2, 7, 14, 21, 25, 28, 50, 75, 100, 110, 125, 150 and 200 post-irradiation. Lungs were removed and representative lung lobes were tested by RT-PCR for levels of detectable message for inflammatory cytokines, redox sensitive promoters, and antioxidants such as MnSOD. Lung tissue was also removed at select time points for histology according to published methods (28, 29).
In Vitro Radiation Survival Curves
The murine C57BL/6NHsd bone marrow stromal cell line (6, 17) and its use in clonogenic radiation survival curves has been described (6, 17).
Cell Line and Animal Irradiation
Cell lines were irradiated at a dose rate of 70 cGy/min using a JL Shepherd Mark1 Model 68 cesium irradiator (San Fernando, CA) according to published methods (6). Clonogenic survival curve assays with bone marrow stromal cell lines were carried out according to published methods (6). Mice were irradiated whole body with 9.5 Gy of gamma rays from a JL Shepherd Mark 1 Model 68 irradiator or thoracically with 20 Gy of 6 MV X rays from a Varian Linear Accelerator (Varian Medical Systems, Inc., Palo Alto, CA) according to published methods (5, 18).
Small Molecule Radiation Protector and Mitigator Drugs
The syntheses of GS-nitroxide JP4-039 (18, 30) and the bis-oxetanyl sulfoxide MMS350 (27), a dimethylsulfoxide (DMSO) analog, have been described previously. Measurement of levels of gene transcripts for irradiation inducible transcription factors, growth factors, inflammatory cytokines, adhesion molecules and protective enzymes were determined by RT-PCR as follows.
RNA was extracted from mouse lung or purified cell populations using the TRIzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions, quantified using a spectrophotometer and stored at −80°C. Reverse transcription of 2 μg of total RNA to complementary DNA (cDNA) was accomplished using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol.
In subsequent steps, expression of specific RNA moieties included: GUSB (Gen-Bank: NM_010368.1); NF-κB (Gen-Bank: NM_199267.2); TNF-α (Gen-Bank: NM_013693.2); IFN-γ (Gen-Bank: NM_008337.3); Nrf2 (Gen-bank: NM_010902.3)(21); NF-κB (Gen-Bank: NM_008689.2); JUN (Gen-Bank: NM_010591.2); SP-1 (Gen-Bank: NM_013672.2); TGFβ1 (Gen-Bank: NM_011577.1); VEGFA (Gen-Bank: NM_001025250.3); IL-1α (Gen-Bank: NM_010554.4); FGF1 (Gen-Bank: NM_010197.3); IFN-γ (Gen-Bank: NM_008337.3); IL-6 (Gen-Bank: NM_031168.1); FAP (Gen-Bank: NM_007986.2); vWF (Gen-Bank: NM_011708.3); CTGF (Gen-Bank: NM_010217.2) (60); Myd88 (Gen-Bank: NM_010851.2); CCL13 (Gen-Bank: NM_018866.2); Toll Like Receptor TLR4 (Gen-Bank: NM_021297.2); MnSOD (Gen-Bank: NM_013671.3); IGFbp7 (Gen-Bank: NM_001159518.1); IL-12a (Gen-Bank: NM_001159424.1); and Collagen 1a (Gene-Bank: NM007742.3). Each was quantitated by real-time polymerase chain reaction (RT-PCR) as described in ref. (28). Ninety-six well plates were prepared with 10 μl of Taqman Gene Expression Master mix, 5 μl of RNase-free water, 1 μl of the corresponding Taqman Gene Expression probe, and 4 μl of cDNA (totaling 2 μg cDNA) using the Eppendorf epMotion 5070 automated pipetting system (Eppendorf, Westbury, NY). The cDNA was amplified with 40 cycles of 95°C (denaturation) for 15 s and 60°C (annealing and elongation) for 1 min using the Eppendorf Realplex2 Mastercycler.
Data for each gene transcript were normalized by calculating the differences (ΔCt) from the Ct-GUSB and Ct-Target genes. Subsequently, the relative increase or decrease in expression was calculated by comparing the reference gene with the target gene (ΔΔCt) and using the formula for relative expression (= 2ΔΔCt). Subsequently, (ΔΔCt) levels were compared and P values were calculated using the one-way ANOVA followed by Tukey’s multiple comparison tests. The results were presented as (10–104-fold) increase in RNA above baseline levels, which were adjusted to that for C57BL/6J/NHsd wild-type mice. The preirradiation baseline levels were used to determine the magnitude of decrease or elevation in mRNA detectable by RT-PCR.
Western Analysis for MnSOD, TGF-β, Nrf2 and NF-κB Protein Expression in Lung
To establish the level of protein expression, lung tissue was harvested and lysed in NP-400 buffer [50 mM Tris, pH 7.8, 10 mM ethylenediaminetetraacetic acid (EDTA), 150 mM NaC1, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1% NP-40 and a protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN)]. Protein samples were separated in 15% polyacrylamide gels by electrophoresis and transferred to nitrocellulose membranes. Primary anti-MnSOD antibody (Novus Biologicals, Littleton, CO) or α-tubulin (Sigma Aldrich, St. Louis, MO) antibody was used. Horseradish peroxidase anti-rabbit or anti-mouse secondary antibody (Promega, Pittsburgh, PA) was then applied and membranes developed with SuperSignal West Dura ECL (Thermo Scientific, Rockford, IL). Antibodies against MnSOD, TGF-β, NF-κB and Nrf2 were obtained from Santa Cruz Biochemical Laboratories, Santa Cruz, CA. For quantitation of levels of MnSOD, TGF-β, Nrf2 and NF-κB, band densities were quantified with ImageJ software (NIH; http://rsbweb.nih.gov/ij/).
Live Imaging of Luciferase Positive Cells in Marrow and Lungs Chimeric Marrow Model
C57BL/6NHsd mouse-luc+ marrow chimeric mice were prepared with 10 Gy total-body irradiation; followed by i.v injection of 1 × 106 luc+ marrow cells 24 h later (5). Chimerism was documented by bioluminescence imaging as reported (44). Subgroups of these chimeric mice were irradiated with 16 Gy to the pulmonary cavity (5).
Marrow Stromal Cell Injection and Live Imaging Model
After 20 Gy thoracic irradiation nonchimeric mice were injected intraperitoneally (i.p.) according to published methods (6) at each of several time points with 1 × 106 or 2 × 106 luc+ bone marrow stromal cells from a luc+ stromal cell line. In preliminary studies, attempted i.v. injection of these cells in numbers over 1 × 105 cells was lethal due to clumping and emboli. Starting 2 days after cell line injection, mice were imaged at serial time points after injection of D-luciferin (Gold Biotechnology, St. Louis, MO) using a Xenogen IVIS Imaging System 200 Series and the bioluminescent signal for each mouse was quantitated. As controls for thoracic irradiation, other C57BL/6NHsd mice were irradiated with 20 Gy to the right hind limb and injected i.p. with luc+ bone marrow stromal cells in the same cell numbers and at the same time points used for the thoracic irradiation experiment. Animals were imaged for luc+ cell migration into irradiated hind limbs using methods identical to those used for irradiated pulmonary groups. The bioluminescence of the mice treated with MMS350 in the drinking water was compared to mice on regular drinking water using a Student’s t test.
Pulmonary Histopathology
Lungs from irradiated mice and from control unirradiated mice were removed, fixed in 2% paraformaldehyde and cryoprotected in 30% sucrose prior to freezing. Frozen sections were stained with H&E, Masson’s trichrome (for collagen) and CD45 (leukocyte common antigen). For CD45 immunostaining, sections were incubated with a monoclonal rat anti-mouse CD45 primary antibody (BD Pharmingen, San Jose, CA), followed by a goat anti-rat Alexa Fluor 555 secondary antibody (Invitrogen, Grand Island, NY). Light microscopic quantitation of percentage of fibrosis (organizing alveolitis) in H&E stained sections was performed. Quantitation of percentage of fibrosis in lung sections was performed using ImageJ software (42). For each lung sample section, the total lung area was determined using manual outlining. Next the total area of the lung that was fibrotic was determined, measurements of total area versus fibrotic areas were summed and the percentage of fibrosis obtained for each lung sample. For each animal, 2 slides with 2 whole-lung sections (fields)/slide were scored, blinded, by two observers, for a total of 4 fields/animal. Percentage of fibrosis was scored in 4 animals for the acute phase and latent period time points and in 12 animals for the late fibrotic phase time points. Results are reported as mean ± SEM. The two-sided two-sample t test was used to compare treatment groups.
Radiation Mitigator Drug Administration
For total-body irradiation mitigation experiments, mice were injected i.p. 24 h after irradiation with MMS350 in phosphate buffered saline (PBS) (27) or JP4-039 (GS-nitroxide) at 20 mg/kg (25) in 10% cremphor/10% ethanol/80% water according to published methods (18). For long-term administration of MMS350, the drug was added to the water bottles and the bottles changed every 7 days.
To determine the daily consumed dose of MMS350, 3 methods of calculation were used: First, the water bottles were weighed daily for 7 days divided by the number of mice per cage, and the amount of water consumed by each mouse in that cage determined based on the density of water of 1 g/ml. Based on this method, pilot experiments with 10 mice per group followed for 7 days, were carried out with several conditions. Nonirradiated C57BL/6NHsd mice consumed 6.0 ± 0.3 ml of water per day. Minimally less water (5.5 ± 0.8 ml) was consumed daily by 20 Gy thoracic-irradiated mice, and 5.4 ± 0.5 ml was consumed by mice daily administered MMS350 in drinking water before 20 Gy thoracic irradiation, P =0.4926 and 0.2847, respectively, compared to nonirradiated control mice. Second, we used a recently published method (40) indicating that a hydrated mouse drinks at least 0.098 ml/g body weight. Third, another report (41) indicated that a mouse drinks 1.5 ml/10 g body weight. By these second and third calculations, a normal adult C57BL/6NHsd mouse of 25 g would drink 2.45 ml to 3.75 ml per day. We then determined the range of possible levels of water intake based on the three methods and estimated the amount of drug consumed per mouse per day. The dose of MMS350 in each water bottle was 400 μM. Based on the range of minimal water amount required for survival and measured volumes of water reduced in the bottles per mouse/day, we calculated a consumption of 2.45 ml to 6.0 ml per day per mouse. Therefore, the total MMS350 consumed by each animal daily ranged from 213 μg to 522 μg per day.
MMS350 Stability Assays
A sample of 4 mg of MMS350 was dissolved in 1 mL of water (Sigma-Aldrich Chromasolv) and monitored for stability at room temperature. Chemical analysis was performed at 3, 5, 7 and 14 days by liquid chromatography-mass spectrometry analysis (LC/MS) on a ThermoScientific Exactive Orbitrap instrument using a Waters XBridge BEH C18 column (2.5 um particle size, 2.1 × 50 mm ID × length; gradient 3:92:5, acetonitrile:water:methanol, to 93:2:5, acetonitrile:water:methanol over 4 min, with a 2 min wash, 1 min reverse gradient and 3 min equilibration; acetonitrile and water contain 0.1% formic acid additive; ionization was in HESI positive mode). All analyses indicated >99.9% purity of MMS350, and no decomposition was detected. Independently, neat MMS350 was kept at room temperature for 6 months and no decomposition was detected by LC/MS analysis. Therefore, the drug MMS350 was stable in drinking water for 7 days and the consumed dose by mice ranged from 213–522 μg/mouse/day.
Statistical Methods
The in vivo mouse survival data are presented as Kaplan-Meier curves and compared with two-sided log-rank tests (28). The in vitro radiation survival curves were analyzed with the linear-quadratic model and the single-hit multi-target model were compared using D0 (final slope representing multiple-event killing) and Ñ (extrapolation number measuring width of the shoulder on the radiation survival curve) (28, 43). Results for D0 and Ñ are presented as the mean ± standard error of the mean (SEM) from multiple measurements and compared with the two-sided two-sample t test (43).
Tissue gene expression was measured by the RT-PCR (28) method. The data were normalized by calculating the differences (ΔCt) from the Ct-GUSB and Ct-Target genes. The relative increase or decrease in expression was calculated by comparing the reference gene with the target gene (ΔΔCt) and using the formula for relative expression (= 2ΔΔCt). Groups were compared in terms of relative expression. In the experiment for time course of induction and duration of elevation of mRNA expression in 20 Gy irradiated lung, gene expression at various time points after irradiation was compared to preirradiation at day 0. Testing the effect of MMS350 treatment in irradiation induced transcripts for genes associated with pulmonary fibrosis, was carried out by comparing each day to the day 0 result, and comparing groups with and without MMS350 on each day after irradiation. All comparisons were done with the two-sided two-sample t test.
In bone marrow luc+ stromal cell line injection and live imaging experiments, bioluminescence in the lungs of live mice treated with MMS350 in the drinking water was compared to mice given regular drinking water. In pulmonary histopathology experiments, the percentage of lung fibrosis in each lung specimen was compared between irradiated and unirradiated control groups. For quantitation of MMS350 effects on reduced expression of radiation-induced proteins (NF-κB, Nrf2, MnSOD and TGF-β) in the lungs of 20 Gy irradiated mice by Western blot, the level of protein expression was analyzed by comparing each day to the day 0 time point, and comparing treated mice with MMS350 in drinking water to those untreated and sacrificed on the same day after irradiation. All these comparisons were done with the two-sided two-sample t test. Every experiment was carried out 3 times. Numbers of mice per experiment are shown in the figure legends.
In all the above exploratory studies, we did not adjust P values for multiple tests.
RESULTS
MMS350 is a Water-Soluble Radiation Protector and Mitigator
The structure and synthesis of MMS350 (27) is shown in Fig. 1. MMS350 was protective and mitigative for C57BL/6 bone marrow stromal cells in vitro (Fig. 2) and was mitigative against radiation-induced death in C57BL/6NHsd mice irradiated with 9.5 Gy total-body irradiation (Fig. 3). The effects were comparable to those obtained from using the GS-nitroxide JP4-039 (18) (Fig. 3).
FIG. 1.
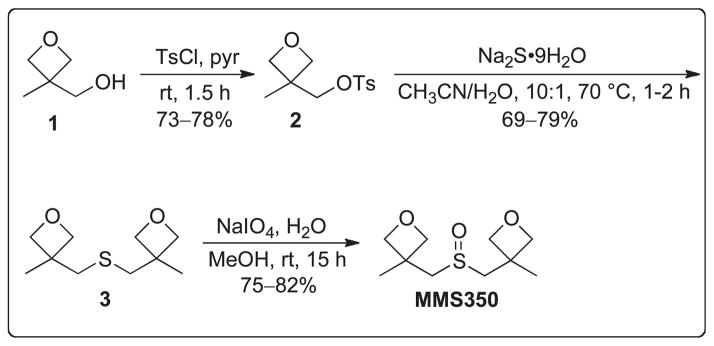
Chemical synthesis and molecular structure of MMS350. Commercially available alcohol 1 was converted to tosylate 2. Dimerization of tosylate 2 using disodium sulfide nonahydrate followed by oxidation with sodium periodate afforded the bifunctional dimethyl sulfoxide MMS350.
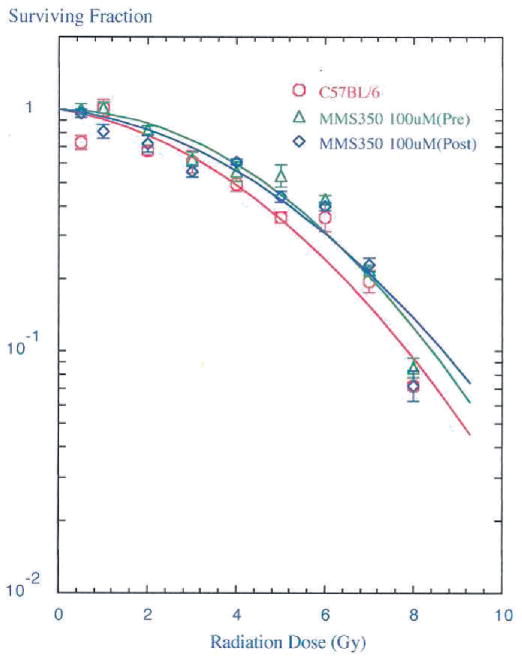
FIG. 2.
In vitro radioprotection and mitigation of bone marrow stromal cells. In vitro survival curves were performed using a bone marrow stromal cell line derived from C57BL/6NHsd mice. Cells were incubated in 100 μM MMS350 for 1 h before irradiation or MMS350 (100 μM) was added to the cells 10 min after irradiation. Cells were irradiated to doses ranging from 0–8 Gy, plated in 4-well tissue culture plates, incubated for 7 days at 37°C in a humidified CO2 incubator, and stained with crystal violet. Colonies of greater than 50 cells were counted and analyzed using a linear quadratic model or single-hit, multi-target model. Cells incubated in MMS350 before irradiation were more resistant to radiation as seen by an increased shoulder on the survival curve (ñ =15.8 ± 2.9 compared to 5.8 ± 1.1 for control cells, P = 0.0039). Cells administered MMS350 after irradiation were also more radioresistant as demonstrated by an increased D0 (2.4 ± 0.3 compared to 1.9 ± 0.1 for control cells, P = 0.0444).
FIG. 3.
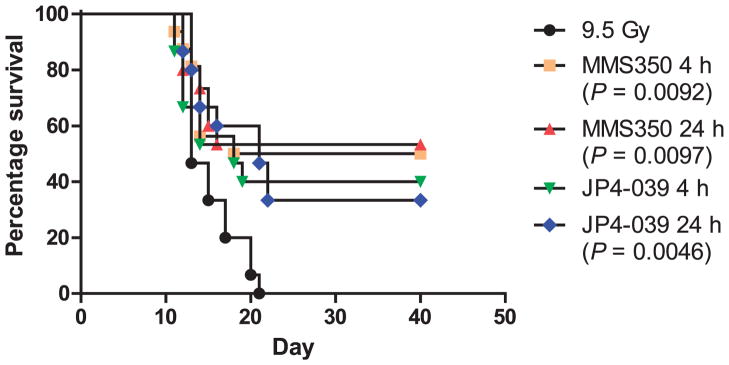
In vivo mitigation of total-body irradiation by MMS350. MMS350 was dissolved in water at a concentration of 2 mg/ml. C57BL/ 6NHsd mice (n =15/group) were total-body irradiated with 9.5 Gy and i.p. injected with 20 mg/kg of MMS350 or JP4-039 at 10 mg/kg (18) at 4 or 24 h after irradiation. The mice were watched for the development of the hematopoietic syndrome at which time the mice were sacrificed. Mice administered MMS350 at either 4 or 24 h after irradiation had significantly increased survival (P =0.0092 and 0.0097, respectively) compared to the 9.5 Gy irradiated control mice. [(●) 9.5 Gy alone; (■) MMS350 4 h; (▲) MMS350 24 h; (▼) JP4-039 4 h; (◆) JP4-039 24 h].
Thoracic Irradiated Luciferase Bone Marrow Chimeric Mice Demonstrate Pulmonary Migration of Marrow Cells During the Onset of Radiation-Induced Pulmonary Fibrosis
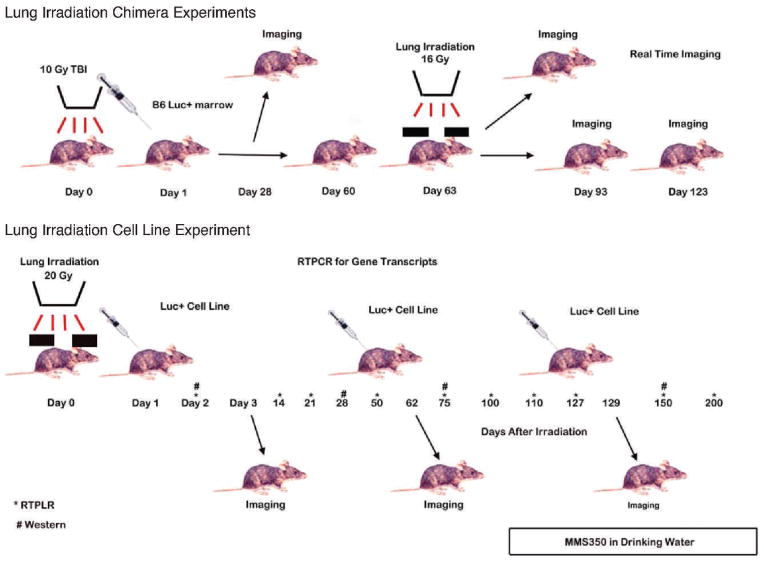
We tested the hypothesis that serial time live imaging of luc+ marrow chimeric mice would identify the timing of marrow migration to the lungs and the onset of pulmonary fibrosis. Luc+ chimeric mice were prepared by total-body irradiation according to procedures described in the Materials and Methods. A schematic of the experimental plan is shown in Fig. 4. At day 63 post-luc+ bone marrow injections, the thoracic cavity of chimeric mice were irradiated with 16 Gy, according to published methods (5).
FIG. 4.
Flow diagram describing sequence of events in chimeric and cell line injection experiments in thoracic-irradiated mice.
As shown in Fig. 5 (see Supplementary Figs. S1–S3; http://10.1667/RR3233.1.S1), TBI, luc+ bone marrow chimeric mice had marrow cavity restricted bioluminescence (day 28) but no pulmonary concentration of luc+ cells until 60 days after thoracic irradiation (day 123, post-TBI). These results were confirmed in multiple experiments (see Supplementary Figs. S1–S3; http://10.1667/RR3233.1.S1). The results confirm and extend prior published experiments with GFP+ marrow transplanted mice that show marrow cell migration to the lungs at the time of onset of pulmonary fibrosis (5, 6).
FIG. 5.
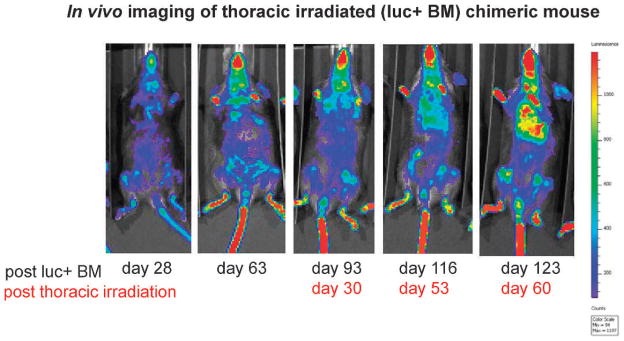
Pulmonary migration of luc+ marrow cells to irradiated lungs of luc+ chimeric mice. Groups of 30 C57BL6NHsd mice were given 10 Gy TBI then 24 h later 1 × 106 luc+ bone marrow cells (i.v.). Chimerism was confirmed by bioluminescent imaging of marrow at days 28 and 63 as described (44). At day 63, subgroups received an additional 16 Gy thoracic irradiation. Total-body irradiated, luc+ bone marrow chimeric mice demonstrated marrow cavity specific bioluminescence (day 28) and no specific pulmonary concentration of luc+ cells until 60 days after thoracic irradiation (day 123 post luc+ cells). Mice were serially imaged for luciferase, as described in the Materials and Methods section. A representative mouse is shown in Fig. 5 (see Supplementary Figs. S1–S3; http://dx.doi.org/10.1667/RR3233.1.S1).
MMS350 Administration Decreases Luciferase+ Bone Marrow Stromal Cell Migration to Irradiated Lung
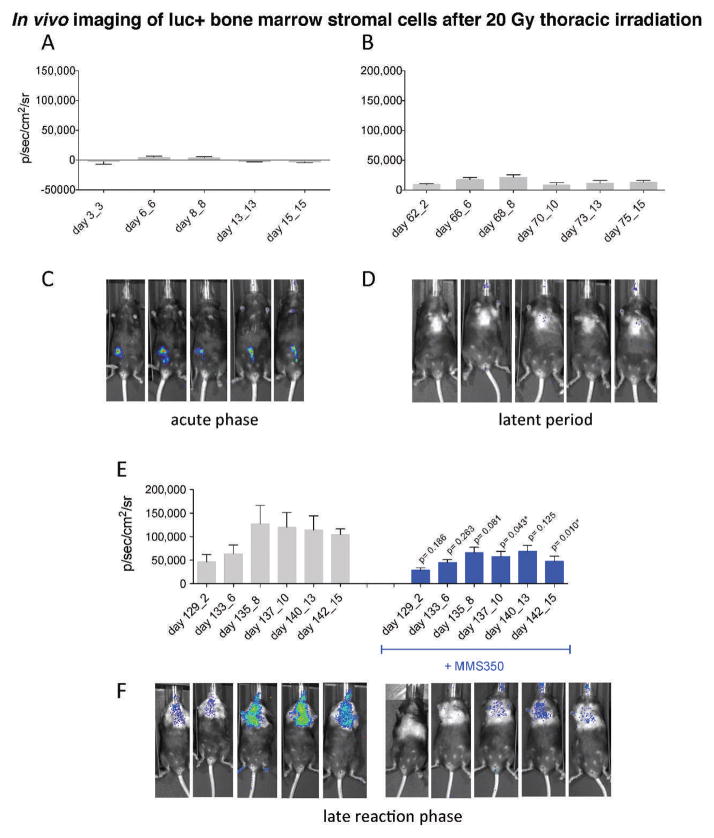
In a second established experimental model of marrow stromal cell line migration to the irradiated lung (5), C57BL/6NHsd mice were irradiated with 20 Gy to the thoracic cavity and then held for varying intervals. At day 3, 60 or 127, subgroups of mice received i.p. injections of 1 × 106 cells (Fig. 6) from a luc+ bone marrow stromal cell line that was established from C57BL6-luc+ GFP+ marrow using published methods (5). As shown in Fig. 6 (see Supplementary Figs. S4–S7; http://10.1667/RR3233.1.S1), there was no significant accumulation of luc+ cells in the lungs of mice injected during the acute phase radiation response at day 3 (Fig. 6A and C) or day 60 during the latent period (Fig. 6B and D). In marked contrast, mice injected at day 127 during the onset of lung fibrosis (organizing/alveolitis) showed significant migration of luc+ cells to the lungs (Fig. 6E and F). The specificity of the migration to the lung was confirmed in another experiment using mice whose hind leg was irradiated and held for equal intervals before i.p. injection of the same luc+ cell line. No cell migration to the irradiated limb was observed (Fig. 7). For all experiments, i.p. bone marrow stromal cell line injection was used, because i.v. injections caused clumping and rapid deaths (within minutes of injection) from pulmonary emboli. The results were similar and more prominent in mice receiving a higher cell number of injected bone marrow stromal cells, which migrated from the peritoneal cavity to the lungs (see Supplementary Figs. S4–S5; http://10.1667/RR3233.1.S1). Mice receiving MMS350 in drinking water beginning at day 100 showed less luc+ cell migration to the lungs (Fig. 6C and F; see Supplementary Figs. S6–S7; http://10.1667/RR3233.1.S1).
FIG. 6.
Pulmonary migration of luc+ stromal cells to lungs of irradiated mice is reduced by MMS350. C57BL/6NHsd mice (n = 30/group) were irradiated with 20 Gy to the pulmonary cavity. The mice were shielded so that only the pulmonary cavity was irradiated. On day 100 after irradiation, half of the mice were placed on 400 μM MMS350 in drinking water. In subgroups, at days 3 (5 mice), 60 (5 mice) or 127 (15 mice) after irradiation, mice were injected i.p. with 1 × 106 cells of a bone marrow stromal cell line derived from a luciferase+/GFP+ mouse. Bioluminescence was measured by imaging as described (44). In vivo imaging revealed little or no migration of luc+ bone marrow stromal cells to the lungs of 20 Gy thoracic irradiated mice on days 3–15 after irradiation (panels A and C) or days 60–75 after irradiation (panels B and E). By 129 days after 20 Gy thoracic irradiation, migration of luc+ bone marrow stromal cells to the lungs was significant (panels E and F). The graphs (panels A–C) represent mean bioluminescence + SEM for each time point after irradiation and day post luc+ cell injection. P values are for the comparison between mice that received MMS350 and those that did not (panel E). Serial bioluminescence imaging of a corresponding representative mouse (panel D–F) is shown for the time points and treatment groups indicated in the graphs. Mice administered MMS350 in drinking water displayed a significant decrease in pulmonary migration of luc+ bone marrow stromal cells compared to control irradiated mice (panels C and F).
FIG. 7.
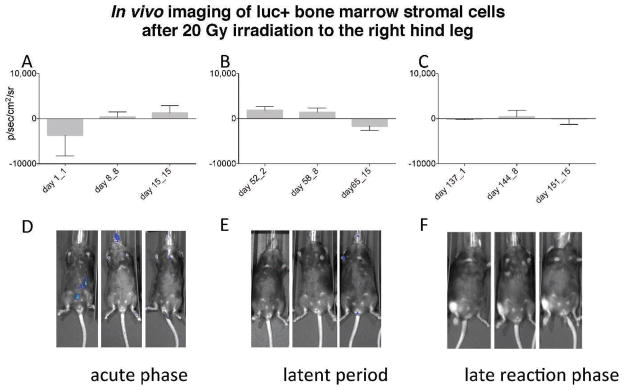
In vivo serial imaging of luc+ bone marrow stromal cells after 20 Gy irradiation to the right hind leg. C57BL/6NHsd mice (n = 15) were irradiated with 20 Gy to the right hind leg. The mice were divided into three groups (5 mice/group) with the first group injected with 1 × 106 luc+ bone marrow stromal cells 24 h after irradiation, the second group injected on day 50 after irradiation and the third group injected 136 days after irradiation. The graphs (panels A–C) represent mean bioluminescence + SEM for each time point after irradiation and day after luc+ cell injection carried out according to published methods (44). A corresponding representative mouse from each group (panels D–F) is shown for the time points indicated in the graphs. In vivo imaging revealed no migration of 1 × 106 luc+ bone marrow stromal cells on days 1–15 (panels A and D); days 52–65 (panels B and E); or days 137–152 (panels C and F) after 20 Gy irradiation to the right hind leg.
Irradiation Induced Elevation of Pulmonary Gene Transcripts and Proteins in Whole Lung
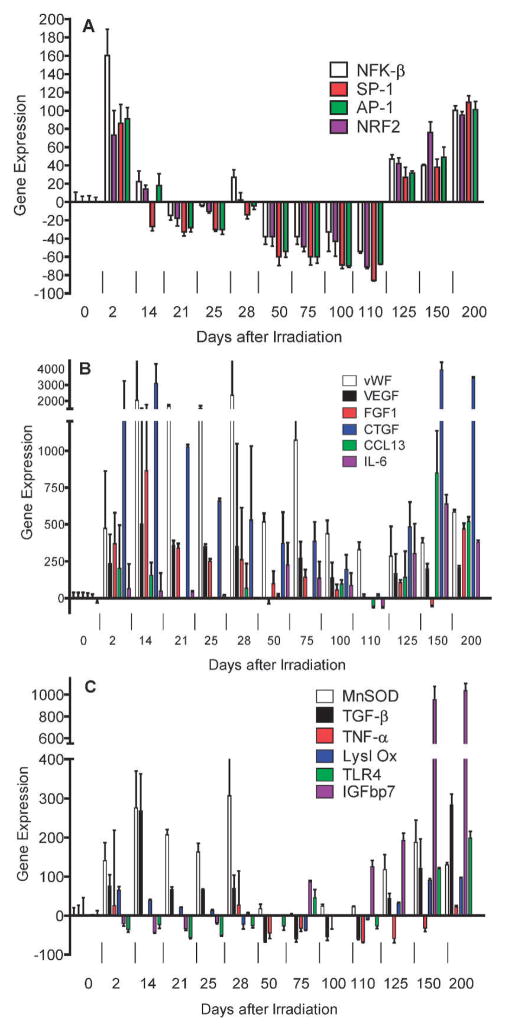
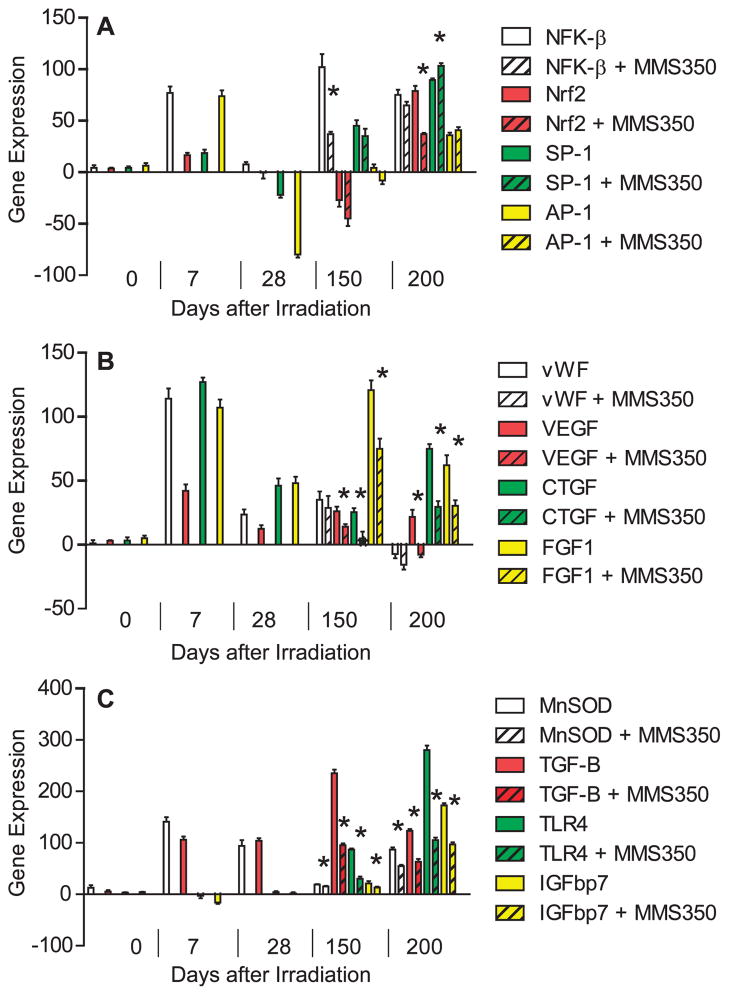
To elucidate the mechanism by which late post-thoracic irradiation time-specific bone marrow stromal cell migration to the irradiated lungs was observed, we measured the relative levels of specific gene transcripts in whole lung at serial time points over 200 days after 20 Gy irradiation. We first addressed detection of three categories of gene transcripts that have been associated with ionizing irradiation effects on the lung. The acute lung radiation response has been associated with a pneumonitis-related histopathology including alveolar cell and endothelial cell swelling, alveolar space transudates and infiltration with inflammatory cells (3, 4, 7, 8). Resolution of the acute phase in C57BL/6 mice is followed by a latent period during which the histopathology of the lung returns to normal (4, 17). As shown in Fig. 8 and Table 1, elevated levels of RNA transcripts associated with the acute pulmonary radiation-induced reaction between days 1 and 14 included those for transcription factors associated with oxidative stress (Nrf2), and DNA damage (NF-κB), AP-1, SP-1 (Fig. 8A), endothelial cell specific genes vWF, VEGF (Fig. 8B), growth factors IL-6, CTGF, FGF-1 (Fig. 8B), and downstream response elements including the protective enzyme marker (MnSOD) and the cytokine (TGF-β) (Fig. 8C). Transcripts for acute phase associated gene activation products including the protective enzyme MnSOD decreased after day 14 and remained low during the latent period between days 28 and 120, but increased again at day 125 along with new markers IGFbp7 and TLR4 (Fig. 8C).
FIG. 8.
Radiation induction of mRNA by RT-PCR for acute phase, latent period and late fibrotic period transcript genes. C57BL/6NHsd mice (n = 30) were irradiated with 20 Gy to the pulmonary cavity. The mice were sacrificed on various days after irradiation and then the lungs were removed, immediately frozen on dry ice, and mRNA was extracted and real time RT-PCR was performed using primers specific for promoters associated with: Panel A: Pulmonary early response genes, NF-κB (NFK-β) (white), NRF2 (purple), SP-1 (red), AP-1 (green); Panel B: endothelial cell specific markers, vWF (white), VEGF (black), FGF1 (red), CCL13 (green), CTGF (blue), IL-6 (purple); or Panel C: genes associated with development of pulmonary fibrosis, MnSOD (white), TGF-β (black), TNF-α (red), Lysl Ox (blue), IGFbp7 (purple), TLR4 (green).
TABLE 1.
Time Course of Induction and Duration of Elevation of mRNA Expression in 20 Gy Irradiated Whole-Mouse Lung
| Day after irradiation
|
|||||
|---|---|---|---|---|---|
| mRNA | 0 | 2 | 50 | 150 | 200 |
| NF-κβ | 0.0 + 10.6 | 160.0 + 29.0 | −38.6 + 8.1 | 139.1 + 1.0 | 99.4 + 11.8 |
| P | 0.0005 | 0.0106 | <0.0001 | 0.0001 | |
| Nrf2 | 0.0 + 6.1 | 73.0 + 27.0 | −38.2 + 10.5 | −25.2 + 11.5 | −5.6 + 7.9 |
| P | 0.0267 | <0.0001 | 0.0584 | 0.5889 | |
| vWF | 0.0 + 39.1 | 474.1 + 388.5 | 520 + 55.4 | 279.0 + 30.7 | 484.8 + 26.8 |
| P | 0.0177 | <0.0001 | <0.0001 | <0.0001 | |
| VEGF | 0.0 + 37.5 | 255.2 + 195.5 | −12.2 + 28.9 | 90.1 + 34.8 | 104.3 + 25.1 |
| P | 0.0145 | 0.05377 | 0.0033 | 0.0004 | |
| CTGF | 0.0 + 28.0 | 1720.9 + 1788.0 | 319.5 + 211.5 | 3841.2 + 47.0 | 3312.8 + 135.0 |
| P | 0.1068 | 0.3437 | 0.0051 | <0.0001 | |
| TNF-α | 0.0 + 1.0 | 8.7 + 4.7 | 5.0 + 14.1 | 116.3 + 4.0 | 126.8 + 60.7 |
| P | 0.0127 | 0.7033 | 0.0003 | 0.0006 | |
| MnSOD | 0.0 + 20.1 | 140.6 + 46.1 | 17.9 + 11.2 | 89.8 + 55.9 | 31.5 + 11.4 |
| P | 0.0186 | 0.2808 | 0.0077 | 0.023 | |
| TGF-β | 0.0 + 26.5 | 76.4 + 28.1 | −63.3 + 3.6 | 21.4 + 49.0 | 185.4 + 53.6 |
| P | 0.0300 | 0.0058 | 0.5634 | 0.0002 | |
| TLR4 | 0.0 + 4.9 | −29.0 + 3.1 | −24.0 + 2.5 | 32.5 + 5.3 | 111.9 + 35.4 |
| P | 0.0009 | 0.0017 | 0.0001 | 0.0009 | |
| Lysl Oxidase | 0.0 + 4.0 | 57.7 + 8.9 | −38.5 + 4.0 | 94.0 + 10.1 | 110.0 + 25.7 |
| P | 0.0074 | 0.0019 | 0.0001 | 0.0159 | |
| IGFbp7 | 0.0 + 2.5 | −76.0 + 6.0 | 10.5 + 2.5 | 890.7 + 167.8 | 1026.3 + 70.1 |
| p | 0.0063 | 0.2929 | 0.0026 | 0.0015 | |
Notes. C57BL/6NHsd mice were irradiated with 20 Gy to the pulmonary cavity. The mice were sacrificed at various times after irradiation, the lungs were removed, and mRNA was extracted. RT-PCR was performed to measure gene expression. Shown above is the gene expression in fold difference level compared to baseline level at 0, 2, 50, 100, 150 and 200 days after irradiation with comparisons made to day 0 to demonstrate increased gene expression after irradiation. Results at additional time points for all genes tested are shown in Supplementary Table S1; http://dx.doi.org/10.1667/RR3233.1.S1. Significant P values below 0.05 are bold.
During the latent period (days 14–110), a different pattern of gene transcript expression was detected, and included endothelial cell specific genes. As shown in Fig. 8B, endothelial cell associated gene transcripts remained elevated during the latent period including (vWF, VEGF, FGF1) as well as CTGF and IL-6. Therefore, some endothelial cell associated transcripts remained elevated after initial induction during the acute phase and well into the latent period distinct from the pattern observed with the transcripts NF-κB, Nrf2, SP-1, AP-1 (Fig. 8A) and inflammatory associated TGF-β, TNF-α and MnSOD (Fig. 8C). These results establish a pattern of elevated endothelial cell associated gene transcripts during the latent period in the lungs of thoracic irradiated C57BL/6NHsd mice during the time when no histopathologic changes have been identifiable (4, 8, 17).
The late, fibrotic phase of irradiation induced pulmonary damage in C57BL/6HNsd mice was associated with a secondary elevation of some of the gene products identified during the acute phase (MnSOD, TGF-β) (4, 17), but new gene transcripts after day 120 and extending out to day 200, including IGFbp7 and TLR4 (Fig. 8C).
MMS350 Administration Reduces Expression of Gene Transcripts and Proteins Associated with Late Radiation-Induced Fibrosis
We tested the hypothesis that administration of MMS350 continuously in drinking water might reduce both transcription of genes and proteins associated with radiation-induced pulmonary fibrosis. In a repeat experiment, MMS350 at 400 μM was administered in drinking water continuously beginning at day 100 after thoracic irradiation with 20 Gy. Mice receiving MMS350 showed decreased expression at 150 and 200 days after irradiation of several of the late fibrosis phase mRNA transcripts, including MnSOD, TGF-β, TLR4, IGFbp7, VEGF, CTGF and FGF1 (Fig. 9; see Supplementary Table S2: http://dx.doi/10.1667/RR3233.1.S1), including those for both endothelial cell markers, and cytokine and inflammatory markers (Fig. 9). At 200 days after irradiation, mice treated with MMS350 showed decreased levels of gene transcripts for Nrf2, endothelial cell genes VEGF, CTGF, and FGF1, and fibrosis associated transcripts for MnSOD, TGF-β, TLR4 and IGFbp7 (statistical evaluation is shown in Supplementary Table S2: http://dx.doi/10.1667/RR3233.1.S1). These results establish that MMS350 treatment decreases levels of radiation-induced expression of several RNA transcripts associated with late pulmonary fibrosis. Decreased transcript levels in MMS350 treated mouse lungs correlated with decreases in protein levels for TGF-β and Nrf2 (Fig. 10; see Supplementary Table 3: http://dx.doi/10.1667/RR3233.1.S1).
FIG. 9.
MMS350 decreases radiation-induced transcripts of genes associated with pulmonary fibrosis (organizing alveolitis). C57BL/6/NHsd female mice (n =30) were irradiated with 20 Gy to the thoracic cavity with a subset of mice placed on MMS350 in the drinking water on day 100 after irradiation. The mice were sacrificed on day 0, 7, 28, 150 and 200 after irradiation, then the lungs perfused with PBS, removed and frozen on dry ice. RNA was extracted and RT-PCR was performed using primers specific for promoters associated with: Panel A: Pulmonary early response genes, NF-κB (NFK-β), Nrf2, SP-1, AP-1; panel B: endothelial cell specific markers, vWF, VEGF, FGF1, CTGF; or panel C: genes associated with development of pulmonary fibrosis, MnSOD, TGF-β, IGFbp7, TLR4. Significant differences (P < 0.05) in gene expression between irradiated control mice and MMS350 treated mice are indicated by the (*) symbol.
FIG. 10.
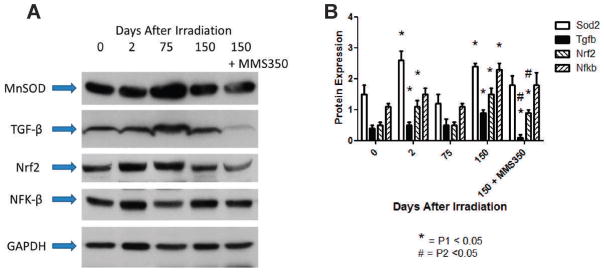
MMS350 reduces radiation-induced proteins in lungs of mice irradiated 20 Gy. C57BL/6NHsd female mice (n =30) were irradiated with 20 Gy to the thoracic cavity and some were placed on drinking water containing MMS350 at 100 days after irradiation. Mice (5/time) were sacrificed at day 0, 2, 75, 150 and 200, the lungs were perfused, removed and frozen in dry ice. Panel A: Lungs were then homogenized, and protein analyzed by Western analysis as described in the Materials and Methods. GAPDH was used as a loading control. Panel B: Quantitative presentation of data from densitometry of the bands [NF-κB (NFK-β), Nrf2, MnSOD and TGF-β] was performed to quantitate protein expression (see Supplementary Table S3; http://dx.doi.org/10.1667/RR3233.1.S1). Mice administered MMS350 daily in drinking water beginning at day 100 had significantly decreased TGF-β and Nrf2 protein at 150 days after irradiation. P1 compares results at the indicated day after irradiation to day 0; P2 compares results with MMS350 treated to irradiation control at that day.
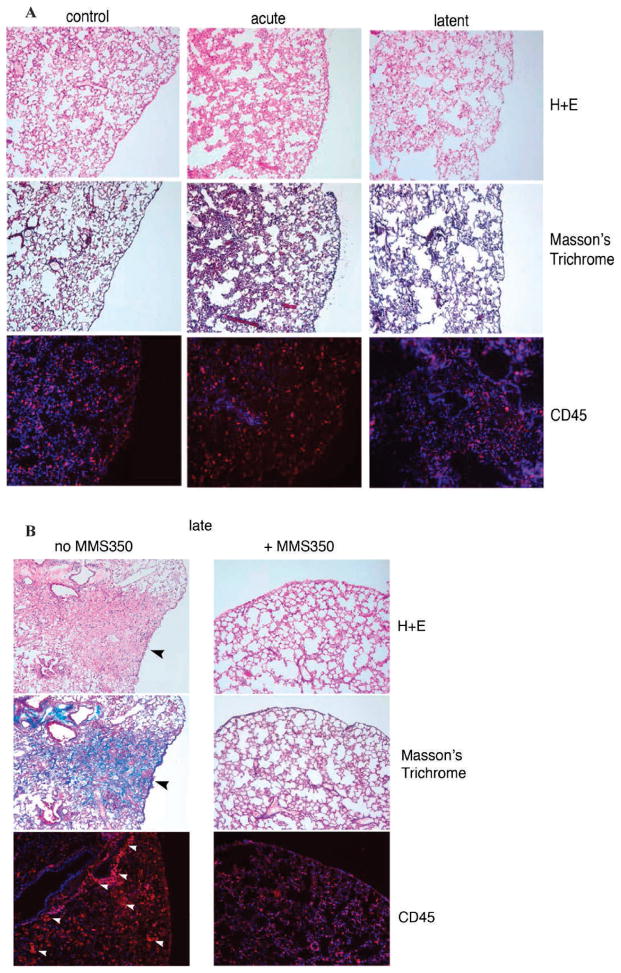
MMS350 Reduces Histopathologically Detectable Fibrosis in Lungs of Thoracic Irradiated Mice
We next determined whether the MMS350-mediated reduction in both marrow stromal cell migration to the irradiated lungs and radiation-induced gene transcripts reduced the level of histopathologically detectable fibrosis. We compared the lung histology of mice placed on MMS350 in the drinking water weekly with that of irradiated controls. We quantitated the level of fibrosis, collagen deposition and inflammatory cell accumulation in the lungs of mice at several time points. Light microscopic quantitation of the percentage of fibrosis in H&E stained lung sections from the acute phase, latent period and late fibrotic time points was performed. As shown in Fig. 11, there was a significant reduction in late pulmonary fibrosis (day 200) in mice administered MMS350 compared to irradiated controls (P < 0.0001). There was no detectable fibrosis in the lungs of acute phase or latent period lung specimens (Fig. 12A). In contrast, there was significant collagen deposition detected by Masson’s trichrome stain of irradiated lungs during the fibrosis phase at day 200 (Fig. 12B). The level of fibrosis at day 200 was decreased in MMS350 treated mice (Fig. 12B). The number and distribution of CD45+ inflammatory cells in the lungs of MMS350 treated mice was also decreased compared to irradiated controls (Fig. 12B). No detectable differences in these parameters were observed in the lungs of acute phase or latent period irradiated lung compared to unirradiated control lungs (Fig. 12A).
FIG. 11.
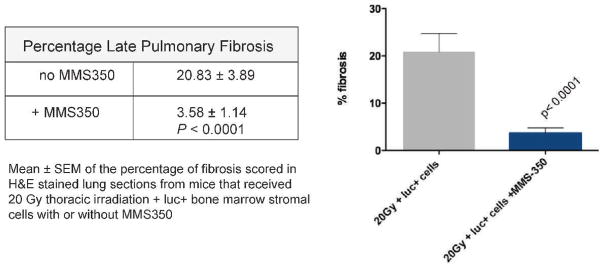
MMS350 in drinking water decreases late pulmonary fibrosis in thoracic irradiated C57BL/6NHsd mice. Twenty Gy irradiated pulmonary control mice (n =30) and a subgroup of mice treated with MMS350 in the drinking water daily beginning at day 100 (n =15) were injected with luc+ bone marrow cells at day 120 after irradiation. At 200 days after irradiation, lungs were removed and frozen sections were stained with hematoxylin and eosin (H&E). Light microscopic quantitation of percentage of fibrosis in H&E stained sections was performed using ImageJ software as described in the Materials and Methods. For each animal, 2 slides with 2 fields/slide were scored, blinded, for a total of 4 fields/animal. Results are reported as mean ± SEM. Irradiated control mice had significantly more fibrosis than mice treated with MMS350 in their drinking water (P < 0.0001).
FIG. 12.
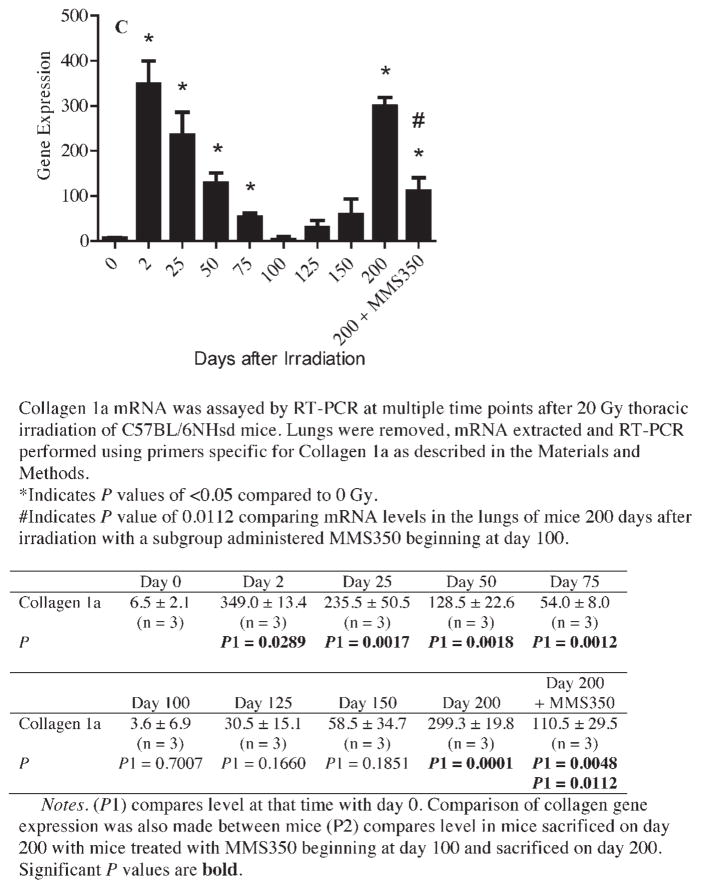
MMS350 decreases late pulmonary fibrosis, collagen deposition and accumulation of CD45+ inflammatory cells in lungs of thoracic irradiated C57BL/6NHsd mice. Frozen sections (n =5/group) from unirradiated control (day 0), irradiated acute phase (day 14), latent period (day 50) (panel A) and late phase (day 200) (panel B) reaction phase lungs were stained with H&E, Masson’s trichrome (for collagen) and CD45. No fibrosis by H&E or increased collagen by Masson’s trichrome was observed in control, acute or latent phase lung tissue (panel A) and immunostaining for CD45 (red) showed similar low level of rare and distribution of CD45+ cells (panel A). H&E staining showed a significant decrease in late pulmonary fibrosis in mice maintained on MMS350 compared to mice that received no MMS350. Panel B: Increased collagen (blue) in Masson’s trichrome stained sections (black arrows) and increased number and peri-vascular distribution of CD45+ inflammatory cells (red) is also evident in mice that received no MMS350 (white arrows) (panel B). The lungs of MMS350 treated mice (panel B) were similar to control unirradiated, acute phase and latent period lungs. All images 10× magnification. Panel C: Collagen mRNA detected by RT-PCR at each of multiple time points after 20 Gy thoracic irradiation. Additional photos of day 120 and day 150 time points (n = 5/group) are shown in Supplementary Fig. S8; http://dx.doi.org/10.1667/RR3233.1.S1.
We next measured Collagen 1a gene transcript levels in lung specimen at 9 time points after thoracic irradiation with 20 Gy. Increased gene transcript expression for Collagen 1a (Fig. 12C) was detected at both acute time points and at the time of collagen deposition (Fig. 12C). The increased mRNA expression for Collagen 1a during days 2–50 decreased during the latent phase (days 50–150). During the fibrotic phase (day 200), there was significantly increased Collagen 1a mRNA. Mice treated with MMS350 had significantly decreased levels of Collagen 1a mRNA at day 200 (Fig. 12C).
DISCUSSION
The current results establish that a novel water-soluble oxetanyl sulfoxide has potent radiation mitigation properties against both total-body radiation-induced hematopoietic syndrome and late thoracic radiation-induced pulmonary fibrosis. When administered in a single dose 24 h after total-body irradiation, MMS350 (27) was an effective radiation mitigator against the LD100/30 dose of total-body irradiation in C57BL/6J/NTac mice and was an effective radiation protector and mitigator for cells in vitro. Radiation mitigation was comparable to levels observed with other small molecule radiation protectors and mitigator drugs that are not water soluble (18, 22–23).
Administration of MMS350 in drinking water decreased the magnitude of late radiation-induced pulmonary fibrosis in 20 Gy thoracic-irradiated mice and decreased the pulmonary migration (homing) of luc+ bone marrow stromal cells in both marrow chimeric and cell line injected mouse models. The present data with serial imaging of luc+ cells in live mice (44) confirm and extend previous publications establishing the marrow origin of cells contributing to radiation pulmonary fibrosis (5, 6). In the present study, we did not track or quantitate proliferation of luc+ cells in the lungs after i.p. cell injection. The increased bioluminescence over several weeks suggests that proliferation may have occurred. Lung-specific homing of luc+ cells was confirmed by imaging other organs in these animals at the time of sacrifice. No detectable bioluminescence was observed in heart, thymus, ribs, liver, intestine, spine or skin. Previously reported studies with BrdU labeling of stromal cell line injected, lung-irradiated mice have confirmed that cell division does occur after homing of GFP+ bone marrow cells (5). In cell line injected mice, lung specific accumulation could have followed i.p. injection due to lymphatic system travel of cells to the lungs; however, marrow chimeric mice in which blood born migration was likely, also showed the same lung specific accumulation of luc+ cells.
Circulating bone marrow stromal cells (mesenchymal stem cells, mesenchymal stromal cells) have multiple roles with respect to the lung damage response (5–8) including transfer of mitochondria to inflammation damaged pulmonary epithelial cells (36), and repopulation of irradiation damaged marrow sites (37, 38). We observed marrow stromal cell homing to the lungs during the late fibrosis period by imaging luc+ bone marrow stromal cells in live animals. Bioluminescence was observed only in the lung. The current studies do not rule out the possibility of homing of smaller numbers of cells to bone marrow (37) or other organs including liver and spleen. Regulatory signals from myofibroblasts in the damaged liver have been known to regulate fibrosis (39).
Evaluation of lung specimens during the fibrotic phase of the pulmonary radiation response demonstrated increased Collagen 1a by RT-PCR, Masson’s trichrome staining and fibrosis by scoring for percentage of fibrosis in overall lung sections. There was also a significant increase in CD45+ inflammatory cells in the lungs during the fibrotic phase. Both overall levels of fibrosis and inflammatory cell accumulation in lungs were significantly decreased by MMS350 treatment starting 100 days after thoracic irradiation. Thus, MMS350 decreased the overall level of radiation-induced pulmonary fibrosis, accumulation of inflammatory cells and bone marrow origin stromal cell migration to the lungs. These results provide evidence that MMS350 is an anti-fibrotic and anti-inflammatory agent, as well as an inhibitor of cell migration into irradiated lungs. Thus, MMS350 has potential applications not only for mitigation of acute total-body irradiation effects, but also for late radiation-induced inflammatory and fibrotic effects.
We used real-time RT-PCR analysis of the molecular and cellular biomarkers during the acute phase (4, 7, 8, 33–35) and the chronic phase (7, 8) of pulmonary radiation-induced damage and the latent period. Endothelial cell associated gene transcripts for vWF, VEGF, CCL3, IL6 and CTGF were elevated during the latent period and maintained during the time of fibrosis. Administration of MMS350 in drinking water during the latent period after thoracic irradiation also reduced the levels of several biomarkers of late radiation-induced pulmonary fibrosis. There were also downregulated transcripts of genes that we determined to be associated with the late pulmonary fibrotic reaction, including TGF-β and TLR4 (12, 13, 31, 32). Furthermore, MMS350 modulated the radiation-induced elevation of both mRNA transcripts and proteins, including some specific to pulmonary endothelial cells including VEGF.
We do not know how the gene transcript signature relates to the two components of lung fibrosis: (1) proliferation of endogenous lung fibroblast progenitors, and (2) migration of marrow stromal cells to the lungs at day 200. The evidence points to TLR4 and IGFbp7 as potentially relevant late gene expression targets. Studies of the other 8 TLR family transcripts are in progress to determine if endogenous lung fibroblasts and migratory marrow origin cells are stimulated by TLR4. Cytokine and chemokine receptor elevations have been shown to occur in radiation-induced pulmonary fibrosis (45). It is not yet known whether lung fibrosis – resistant C3H/HeJ mice (7, 8) demonstrate the same pattern and time course of radiation-induced upregulation of gene products by RT-PCR, and bone marrow derived stromal cell homing to the lungs. Whether there is a pulmonary radioprotective effect of MMS350 in other mouse strains is also not known. Experiments blocking the heart during thoracic irradiation are in progress with larger numbers of mice to test whether MMS350 treatment improves overall survival after thoracic irradiation.
The time of migration to the lungs of luciferase+ marrow stromal cells was associated with late elevations of transcripts for TLR4, TGFβ, IGFb7 and MnSOD and these elevations were inhibited by administration of MMS350 in drinking water. The present discovery of continuous elevation during the latent period of vWF and VEGF as well as other endothelial cell markers indicates that endothelial cells in the irradiated lung demonstrate persistent elevation in gene expression after ionizing irradiation and after histopathologic markers of the acute response have subsided.
Mobilization and homing of bone marrow stromal cells to the lung occurred specifically during the late phase after lung irradiation at 120–200 days and was associated with upregulation of TLR4, IGFb7, MnSOD and TGF-β. The present studies did not address the questions of whether a minimum pulmonary radiation dose is necessary for late homing of stromal cells to the lung, whether homing occurs after fractionated or partial lung irradiation, nor did the studies address if the mouse genotype specifically correlates to gene transcripts involved in late pulmonary fibrosis (7, 8, 15, 16).
The mechanism of action of MMS350 as a radiation mitigator and anti-fibrotic agent is not yet known but it may involve its antioxidant properties. Irradiated cells in culture treated with MMS350 showed decreases in the following: radiation-induced DNA strand breaks by neutral Comet assay, apoptosis by Apotag® assay, antioxidant stores by Trolox equivalent units assay and modulation of radiation-induced G1/S- and G2/M-cell cycle arrest. Studies to determine whether MMS350 acts at the level of mitochondrial mediated apoptosis or through direct effects on nuclear DNA repair or a combination of these pathways are in progress.
The water solubility and ease of administration of MMS350 in drinking water makes long-term administration practical. The availability of mice with genetically altered radiation-damage and cytokine response pathways should facilitate the investigation of the mechanism of MMS350 mediated mitigation. Preliminary data indicate that a toxic level of orally delivered MMS350 has not yet been reached. Further studies will be required to determine which factor(s) signals stromal cell migration from the marrow to the lung to cause pulmonary radiation fibrosis.
Supplementary Material
Acknowledgments
Supported by NIH Grants CA-R01-CA119927 and NIAID U19-A1068021. This project used the UPCI Animal Facility that is supported in part by award P30CA047904.
Footnotes
Editor’s note. The online version of this article (DOI: 10.1667/ RR3233.1) contains supplementary information that is available to all authorized users.
References
- 1.Rengan R, Maity AM, Stevenson JP, Hahn Stephen M. New strategies in non-small cell lung cancer: Improving outcomes in chemoradiotherapy for locally advanced disease. Clinical Can Res. 2011;17:4192. doi: 10.1158/1078-0432.CCR-10-2760. [DOI] [PubMed] [Google Scholar]
- 2.Mazeron R, Etienne-Mastroianni B, Perol D, Arpin D, Vincent M, Faichero L, et al. Predictive factors of late radiation fibrosis: a prospective study in non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2010;77:38–43. doi: 10.1016/j.ijrobp.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Rubin P, Casarett GW. Clinical Radiation Pathology. Philadelphia, PA: WB Saunders; 1968. [DOI] [PubMed] [Google Scholar]
- 4.Epperly MW, Travis EL, Sikora C, Greenberger JS. Magnesium superoxide dismutase (MnSOD) plasmid/liposome pulmonary radioprotective gene therapy: Modulation of irradiation-induced mRNA for IL-1, TNF-α, and TGF-β correlates with delay of organizing alveolitis/fibrosis. Biol Blood Marrow Transplantation. 1999;5:204–214. doi: 10.1053/bbmt.1999.v5.pm10465100. [DOI] [PubMed] [Google Scholar]
- 5.Epperly MW, Sikora CA, Defilippi S, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Resp Molecular Cell Biol. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 6.Epperly MW, Franicola D, Zhang X, Nie S, Wang H, Bahnson A, et al. Decreased irradiation pulmonary fibrosis in Smad3−/− marrow chimeric mice correlates to reduced bone marrow stromal cell migration in vitro. In Vivo. 2006;20:573–582. [PubMed] [Google Scholar]
- 7.Franko AJ, Sharplin J. Development of fibrosis after lung irradiation in relation to inflammation and lung function in a mouse strain prone to fibrosis. Radiat Res. 1994;140:347–355. [PubMed] [Google Scholar]
- 8.Dieto CL, Travis EL. Fibroblast radiosensitivity in vitro and lung fibrosis in vivo: comparison between a fibrosis-prone and fibrosis-resistant mouse strain. Radiat Res. 1996;146:61–67. [PubMed] [Google Scholar]
- 9.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 11.Costabel U. Emerging potential treatments: new hope for idiopathic pulmonary fibrosis patients? European Respiratory Review. 2011;20(121):201–207. doi: 10.1183/09059180.00002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson EM, Williams A, Yauk CL, Vincent R. Overexpression of tumor necrosis factor-α in the lungs alters immune response, matrix remodeling, and repair and maintenance pathways. Am J Pathol. 2012:180-1413-1430. doi: 10.1016/j.ajpath.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Gan L, Xue J-X, Li X, Liu D-S, Ge Y, Ni P-Y, et al. Blockade of lysophosphatidic acid receptors LPAR1/3 ameliorates lung fibrosis induced by irradiation. Biochem Biophy Res Comm. 2011;409:7–13. doi: 10.1016/j.bbrc.2011.04.084. [DOI] [PubMed] [Google Scholar]
- 14.Yang H-Z, Wang J-P, Mi S, Liu H-Z, Cui B, Yan H-M, et al. TLR4 activity is required in the resolution of pulmonary inflammation and fibrosis after acute and chronic lung injury. Am J Pathol. 2012;180:275–292. doi: 10.1016/j.ajpath.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Travis EL, Rachakonda G, Zhou X, Korhonen K, Sekhar KR, Biswas S, et al. Nrf2 deficiency reduces life span of mice administered thoracic irradiation. Free Rad Biol Med. 2011;51:1175–1183. doi: 10.1016/j.freeradbiomed.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kikuchi N, Ishii Y, Morishima Y, Yageta Y, Haraguchi N, Itoh K, et al. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respiratory Research. 2010;11:31–39. doi: 10.1186/1465-9921-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epperly MW, Sikora CA, DeFilippi SJ, Gretton JE, Bar-Sagi D, Carlos T, et al. Pulmonary irradiation-induced expression of VCAM-1 and ICAM-1 is decreased by MnSOD-PL gene therapy. Biol Blood Bone Marrow Transplant. 2002;8:175–187. doi: 10.1053/bbmt.2002.v8.pm12014807. [DOI] [PubMed] [Google Scholar]
- 18.Rwigema J-CM, Beck B, Wang W, Doemling A, Epperly MW, Shields D, et al. Two strategies for the development of mitochondrial-targeted small molecule radiation damage mitigators. Int J Radiat Oncol Biol Phys. 2011;80:860–868. doi: 10.1016/j.ijrobp.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fink MP, Macias CA, Xiao J, Tyurina YY, Delude RL, Greenberger JS, et al. Hemigramicidin-TEMPO Conjugates: Novel mitochondria-targeted anti-oxidants. Biochem Pharmacol. 2007;74:801–809. doi: 10.1016/j.bcp.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Jiang J, Belikova NA, Xiao J, Zhao Q, Greenberger JS, Wipf P, et al. A mitochondria-targeted nitroxide/hemi-gramicidin S conjugate protects mouse embryonic cells against γ –irradiation. Int J Radiat Oncol Biol Phys. 2008;70:816–825. doi: 10.1016/j.ijrobp.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, et al. Models for evaluating agents intended for the prophylaxis, mitigation, and treatment of radiation injuries. Radiat Res; Report of an NCI Workshop; December 3–4, 2003; 2004. pp. 711–728. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson J, Kapralov AA, Yanamala N, Pearce L, Peterson J, Tyurina Y, et al. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation induced death. Nature Communications. 2011;2:497. doi: 10.1038/ncomms1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zellefrow C, Sharlow ER, Reese C, Shun T, Kagan V, Epperly M, et al. Development and deployment of an siRNA assay to identify druggable targets for radiation mitigation. Radiat Res. 2012;178:150–159. doi: 10.1667/rr2810.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu M, St Clair DK. Regulation of superoxide dismutase genes: implications in disease. Free Radical Biol Med. 2009;47:344–356. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goff JP, Epperly MW, Shields D, Wipf P, Dixon T, Greenberger JS. Radiobiologic effects of GS-nitroxide (JP4-039) in the hematopoietic syndrome. In Vivo. 2011;25:315–324. [PMC free article] [PubMed] [Google Scholar]
- 26.Epperly MW, Rwigema J-CM, Li S, Gao X, Wipf P, Goff J, et al. Intraesophageal administration of GS-nitroxide (JP4-039) protects against ionizing irradiation-induced esophagitis. In Vivo. 2010;24:811–821. [PMC free article] [PubMed] [Google Scholar]
- 27.Sprachman MM, Wipf P. A bifunctional dimethylsulfoxide substitute enhances the aqueous solubility of small organic molecules. Assay Drug Dev Technol. 2012;10:269–277. doi: 10.1089/adt.2011.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajagopalan MS, Stone B, Rwigema J-C, Salimi U, Epperly MW, Goff J, et al. Intraesophageal manganese superoxide dismutase-plasmid liposomes ameliorates novel total body and thoracic irradiation sensitivity of homologous deletion recombinant negative nitric oxide synthase-1 (NOS−/−) mice. Radiat Res. 2010;174:297–312. doi: 10.1667/RR2019.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epperly MW, Guo HL, Jefferson M, Wong S, Gretton J, Bernarding M, et al. Cell phenotype specific duration of expression of epitope-tagged HA-MnSOD in cells of the murine lung following intratracheal plasmid liposome gene therapy. Gene Therapy. 2003;10:163–171. doi: 10.1038/sj.gt.3301852. [DOI] [PubMed] [Google Scholar]
- 30.Frantz M-C, Pierce JM, Li K, Wan Q, Johnson M, Wipf P. Large-scale asymmetric synthesis of the bioprotective agent JP4-039 and analogs. Org Lett. 2011;13:2318–2321. doi: 10.1021/ol200567p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brouty-Boye D, Pottin-Clemenceau C, Doucet C, Jasmin C, Azzarone B. Chemokines and CD40 expression in human fibroblasts. Eur J Immunol. 2000;30:914–919. doi: 10.1002/1521-4141(200003)30:3<914::AID-IMMU914>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 32.Paun A, Fox J, Balloy V, Chignard M, Qureshi ST, Haston CK. Combined Tir2 and Tir4 deficiency increases radiation-induced pulmonary fibrosis in mice. Int J Radiat Oncol Biol Phys. 2010;77:1198–1205. doi: 10.1016/j.ijrobp.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 33.Cho H-Y, Reddy SPM, Yamamoto M, Kleeberger SR. The transcription factor Nrf2 protects against pulmonary fibrosis. FASEBJ. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 34.Reddy NM, Potteti HR, Mariani TJ, Biswal S, Reddy SP. Conditional deletion of Nrf2 in airway epithelium exacerbates acute lung injury and impairs the resolution of inflammation. Am J Respir Cell Mol Biol. 2011;45:1161–1168. doi: 10.1165/rcmb.2011-0144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald JT, Kim K, Norris AJ, Vlashi E, Phillips TM, Lagadec C, et al. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010;70:8886–8892. doi: 10.1158/0008-5472.CAN-10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature Med. 2012;18:759–768. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anklesaria P, Kase KR, Glowacki J, Holland CH, Sakakeeny MA, Wright JH, et al. Engraftment of a clonal bone marrow stromal cell line in vivo stimulates hematopoietic recovery from total body irradiation. Proc Natl Acad Sci, USA. 1987;84:7681–7685. doi: 10.1073/pnas.84.21.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anklesaria P, FitzGerald TJ, Kase K, Ohara A, Bentley S, Greenberger JS. Improved hematopoiesis in anemic S1/S1d mice by therapeutic transplantation of a hematopoietic microenvironment. Blood. 1989;74:1144–1152. [PubMed] [Google Scholar]
- 39.Kisseleva T, Cong M, Paik YH, Scholten D, Jiang C, Benner C, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci, USA. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox JG. The Mouse in Biomedical Research. In: Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL, editors. Normative Biology, Husbandry, and Models. 2. Philadelphia, PA: Elsevier Press; 2007. p. 75. [Google Scholar]
- 41.American Association of Laboratory Animal Sciences. The Laboratory Mouse Handbook. Memphis, TN: Hedrich Hans, Ed; 2006. p. 22. [Google Scholar]
- 42.Rasband WS. ImageJ, US. National Institutes of Health; Bethesda, Maryland, USA: 1997–2012. http://imagej.nih.gov/ij/ [Google Scholar]
- 43.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 6. New York: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 44.Cao Y-A, Bachmann MH, Beilhack A, Yang Y, Tanaka M, Swijinenburg R-J, et al. Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation. Transplantation. 2005;80:134–139. doi: 10.1097/01.tp.0000164347.50559.a3. [DOI] [PubMed] [Google Scholar]
- 45.Johnston CJ, Williams JP, Okunieff P, Finkelstein Radiation-induced pulmonary fibrosis: Examination of chemokine and chemokine receptor families. Radiat Res. 2002;157(3):256–265. doi: 10.1667/0033-7587(2002)157[0256:ripfeo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.