Abstract
Human ApoE4 accelerates memory decline in ageing and in Alzheimer's disease. Although intranasal insulin can improve cognition, this has little effect in ApoE4 subjects. To understand this ApoE genotype-dependent effect, we examined brain insulin signaling in huApoE3 and huApoE4 targeted replacement (TR) mice. At 32 weeks, lower insulin receptor substrate 1 (IRS1) at S636/639 and Akt phosphorylation at T308 were detected in fasting huApoE4 TR mice as compared to fasting huApoE3 TR mice. These changes in fasting huApoE4 TR mice were linked to lower brain glucose content and have no effect on plasma glucose level. However, at 72 weeks of age, these early changes were accompanied by reduction in IRS2 expression, IRS1 phosphorylation at Y608, Akt phosphorylation at S473, and MAPK (p38 and p44/42) activation in the fasting huApoE4 TR mice. The lower brain glucose was significantly associated with higher brain insulin in the aged huApoE4 TR mice. These results show that ApoE4 reduces brain insulin signaling and glucose level leading to higher insulin content.
Human apolipoprotein E (ApoE) is located on chromosome 19 encoding a 35 kDa protein1 that exists in 3 isoforms, E2, E3 and E42,3. These isoforms differ by amino acid substitutions at two positions (residues 112 and 158)4.
ApoE is synthesized in various organs1 and high expression is detected in the liver5 and in the brain6. In the peripheral tissues, ApoE is extensively studied as a group of lipid carrier molecules vital to the cholesterol homeostasis of the body5. But, emerging studies suggest that ApoE has other functions beyond cholesterol metabolism2,7.
Although ApoE is widely expressed in the brain6, little is known about the role of this protein in brain function2,8. However, inheriting the ApoE4 isoform is a strong genetic risk factor for Alzheimer's disease (AD)9,10. In AD, ApoE4 is linked to poor Aβ clearance11,12,13 and greater Aβ deposition14.
Positron emission tomography (PET) using [18F]flurodeoxyglucose (FDG-PET) shows reduced cerebral glucose metabolism in AD subjects15. Older asymptomatic ApoE4 carriers also show reductions in cerebral glucose metabolism10,16,17 in specific brain regions overlapping with those observed in AD subjects18,19.
While these reports are consistent with ApoE effects on cerebral glucose metabolism in AD, other studies also showed that the ApoE4 allele is associated with similar metabolic reductions in young subjects with little or no Aβ plaque20,21. To differentiate the contributions of Aβ and ApoE genotype to cerebral glucose metabolism, a recent study using FDG-PET and PET amyloid imaging shows that reduced cerebral glucose metabolism seen in older ApoE4 carriers is contributed by ApoE genotype and not due to aggregated Aβ22. These studies indicate that ApoE gene has other neural functions not related to Aβ23,24,25.
Human ApoE4 is known to accelerate memory decline in ageing and AD10,26,27,28. Although intranasal insulin can improve cognition29,30, this has little effect in ApoE4 elderly subjects31. To understand if this neuronal insulin function is due to ApoE genotype, we have conducted this study to compare the effect of this genetic polymorphism on brain insulin signaling in ageing huApoE3 and huApoE4 targeted replacement mice.
Results
Altered brain insulin receptor protein expression and phosphorylation in huApoE4 TR mice
Intranasal insulin has been shown to improve cognition29,30, but this has little effect in ApoE4 non-demented elderly subjects31. We therefore decided to determine if ApoE polymorphism can affect neuronal insulin signaling in ageing huApoE3 and huApoE4 targeted replacement (TR) mice.
As shown in figure 1A, lower phosphorylation of the insulin receptor substrate 1 (IRS1) at serine residue 636/639 (S636/639) was detected in the fasting huApoE4 TR mice as compared to fasting huApoE3 TR mice at 32 and 72 weeks of age. Densitometric analysis indicated a reduction of 59% and 50% IRS1 phosphorylation (S636/639) at 32 and 72 weeks of age respectively (Figure 1B). In aged (72 weeks) ApoE4 TR mice, IRS1 phosphorylation at tyrosine 608 (Y608) was reduced by 40% as compared to ApoE3 TR mice at similar age (Figures 1A and 1C).
Figure 1. Insulin receptor substrate proteins expression in huApoE TR mice.
(A) Western blot analysis of insulin receptor substrate-1 and -2 (IRS1 and IRS2), and phosphorylated IRS1 (S636/639 and Y608) levels in the brain of huApoE3 and huApoE4 TR mice at 32 and 72 weeks of age. β-actin was immunoblotted to ensure similar gel loading of the starting material in each sample. The blot is a representative of three independent experiments. Blot images were cropped for comparison. Densitometry analysis of phosphorylated IRS1 at (B) S636/639 and (C) Y608 relative to total IRS1, and (D) total IRS2 level relative to β-actin level, in 32 and 72 weeks old E3 (white bar) and E4 (grey bar) mice was performed using the NIH ImageJ software. Each value represents the mean ± SEM for individual mouse brain sample (n = 3 at each time point for each mouse line). Lower IRS1 phosphorylation at (S636/639) was detected in E4 TR mice at 32 and 72 weeks of age as compared to E3 TR mice of similar age. However, lower IRS1 phosphorylation at (Y608) was only detected in E4 TR mice at 72 weeks of age as compared to E3 TR mice of similar age. Lower total IRS2 level was detected in 72 weeks E4 TR mice as compared to E3 TR mice at similar age. (*p < 0.05; ** p < 0.04, using Student's t-test).
While total IRS1 expression was not altered between the two mouse lines (Figure 1A), total IRS2 level was lowered by 45% in 72 weeks old fasting huApoE4 mice (Figure 1A and 1D). However, we were unable to detect IRS2 phosphorylation at Ser-388 (S388) and Tyr-978 (Y978).
Lower MAPK activation in the brain of aged huApoE4 TR mice
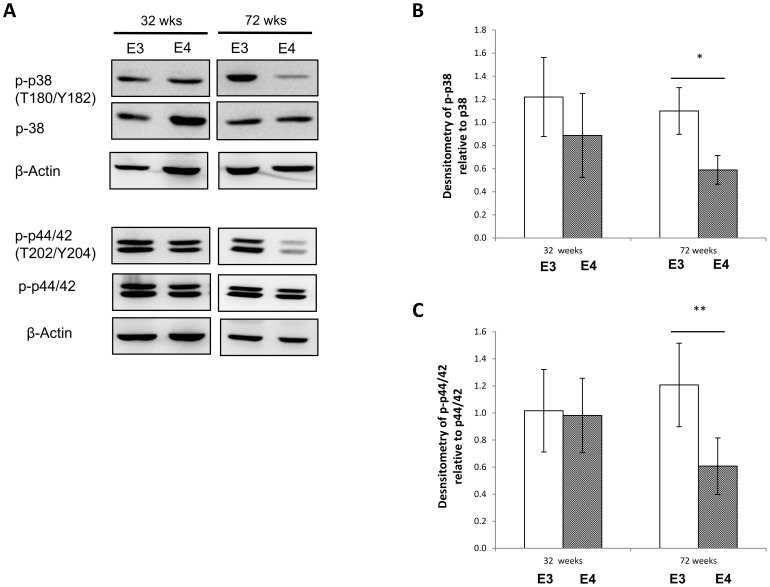
Altered IRS1 phosphorylation at S636/639 has been shown to affect MAPK activation32,33. We therefore examined the expression and phosphorylation of two major proteins in MAPK signaling; p38 and p44/42. As shown in figure 2, no changes in the expression and phosphorylation of p38 and p44/42 were detected in 32 weeks huApoE TR mice.
Figure 2. MAPK expression and phosphorylation in huApoE TR mice.
(A) Immunoblotting of total p38 and p44/42, phosphorylated p38 (T180/Y182) and phosphorylated p44/42 (T202/Y204) in huApoE3 and huApoE4 TR mice at 32 and 72 weeks of age. β-actin was immunoblotted to ensure similar gel loading of the starting material in each sample. The blot is a representative of three independent experiments. Blot images were cropped for comparison. Densitometry analysis of (B) phosphorylated p38(T180/Y182) level relative to total p38 and (D) phosphorylated p44/42(T202/Y204) level relative to total p44/42 level, in 32 and 72 weeks old E3 (white bar) and E4 (grey bar) mice was performed using the NIH ImageJ software. Each value represents the mean ± SEM for individual mouse brain sample (n = 3 at each time point for each mouse line). Lower p38 phosphorylation (T180/Y182) and p44/42 phosphorylated (T202/Y204) was detected in 72 weeks E4 TR mice as compared to E3 TR mice at similar age. (*p < 0.005; ** p < 0.02, using Student's t-test).
However, in the 72 weeks old mice, lower phosphorylation of p38 at threonine 180 and tyrosine 182 (T180/Y182), and p44/42 at threonine 202 and tyrosine 204 (T202/Y204) were detected in huApoE4 mice as compared to huApoE3 TR mice at similar age (Figure 2). The phosphorylation of both protein targets (p38 and p44/42) was reduced by almost 50% in the brain of aged huApoE4 TR mice.
Reduced Akt phosphorylation in the brain of huApoE4 TR mice
We next determined if the aberrant IRS expression and phosphorylation (Figure 1) will affect the downstream Akt protein expression and phosphorylation. Using immunoblotting, we did not detect any change in total Akt level between the fasting huApoE3 and huApoE4 TR mouse brain (Figure 3A and 3B). However, Akt phosphorylation at Serine-473 (S473) was reduced by 39% in the brain of 72 weeks old fasting huApoE4 TR mice as compared to the fasting huApoE3 mice of similar age (Figure 3A and 3C). In contrast, Akt phosphorylation at Threonine-308 (T308) was significantly reduced in both 32 and 72 weeks old fasting huApoE4 TR mouse brain as compared to fasting huApoE3 TR mouse brain at similar age (Figure 3A and 3D). In 32 weeks huApoE4 TR mice, Akt phosphorylation (T308) was reduced by 61%, whereas the reduction in huApoE4 at 72 weeks was 48%.
Figure 3. Akt expression and phosphorylation in huApoE TR mice.
(A) Immunoblotting of total Akt, phosphoryalted Akt (S473) and phosphorylated Akt (T308) in huApoE3 and huApoE4 TR mice at 32 and 72 weeks of age. β-actin was immunoblotted to ensure similar gel loading of the starting material in each sample. The blot is a representative of three independent experiments. Blot images were cropped for comparison. Densitometry analysis of (B) total Akt level relative to β-actin level, (C) phosphorylated Akt(S437) and (D) phosphorylated Akt(T308) level relative to total Akt level, in 32 and 72 weeks old E3 (white bar) and E4 (grey bar) mice was performed using the NIH ImageJ software. Each value represents the mean ± SEM for individual mouse brain sample (n = 3 at each time point for each mouse line). Lower Akt phosphorylation (S473) was detected in 72 weeks E4 TR mice as compared to E3 TR mice at similar age. However, reduced Akt phosphorylation at T308 can be observed in E4 TR mice at 32 and 72 weeks of age as compared to E3 TR mice of similar age. (*p < 0.04; ** p < 0.01, using Student's t-test).
Brain insulin and glucose in huApoE TF mice
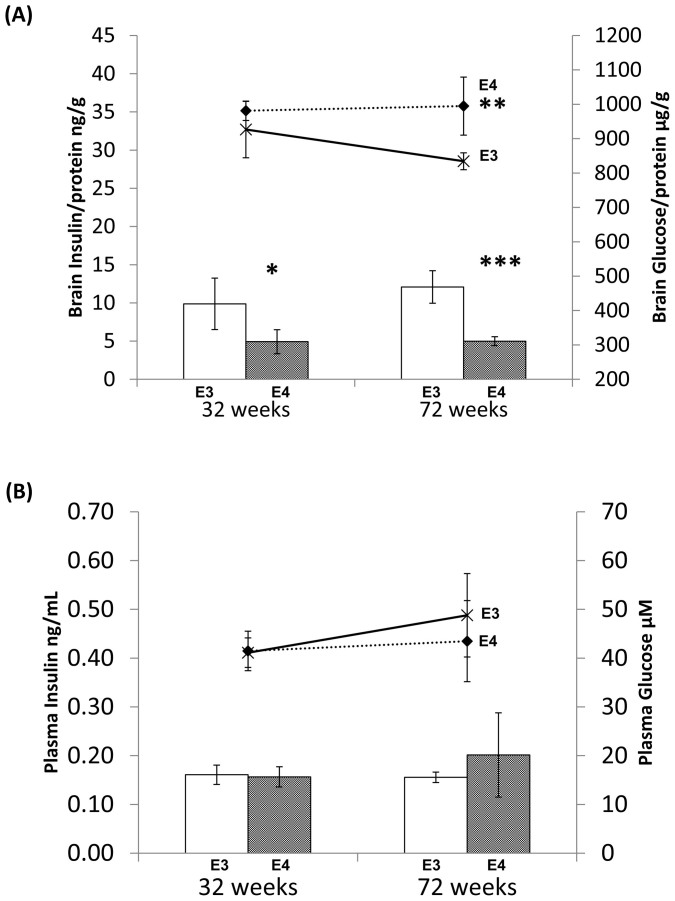
We then examined if changes in IRS expression and phosphorylation are linked to altered brain insulin. As shown in figure 4a, there were no significant different in brain insulin level between fasting huApoE3 and fasting huApoE4 TR mice at 32 weeks of age. At 72 weeks of age, brain insulin was 20% higher in the fasting huApoE4 TR mice as compared to the fasting huApoE3 TR mice (Figure 4a line).
Figure 4. Analysis of insulin and glucose contents in the brain and plasma of fasting huApoE TR mice.
(A) Lower brain glucose was detected in E4 TR (grey bar) mice at 32 and 72 weeks of age as compared to E3 TR mice (white bar) of similar age. But higher brain insulin was only detected in 72 weeks old E4 TR mice (dotted line) as compared to E3 TR mice (bold line) at similar age. (B) No significant change in plasma insulin and glucose contents between E3 TR and E4 TR mice at 32 and 72 weeks of age. Each value represents the mean ± SEM of duplicate assays for individual samples (n = 5). (*p < 0.04; ** p < 0.001; *** p < 0.004, using Student's t-test).
Since insulin signaling is associated to cerebral glucose metabolism34,35, it is possible that the mouse brain glucose content could be affected in our mouse lines. Unlike the late stage (72 weeks) change in brain insulin, brain glucose was affected as early as 32 weeks of age (Figure 3a bar). Brain glucose level was significant lower by 26% and 33% in the fasting huApoE4 TR mice as compared to the fasting huApoE3 TR mice at 32 and 72 weeks of age respectively. This observation is in line with recent clinical imaging study showing lower cerebral glucose metabolism in non-demented ApoE4 carriers as compared to non-demented non-ApoE4 carriers22.
Plasma insulin and glucose in huApoE TF mice
We next determine if the biochemical change in the mouse brain was related to the animal blood system. As shown in figure 4b, no change in plasma glucose and insulin was detected between the fasting huApoE3 TR and huApoE4 TR mice at both ages.
Brain and plasma cholesterol in huApoE TR mice
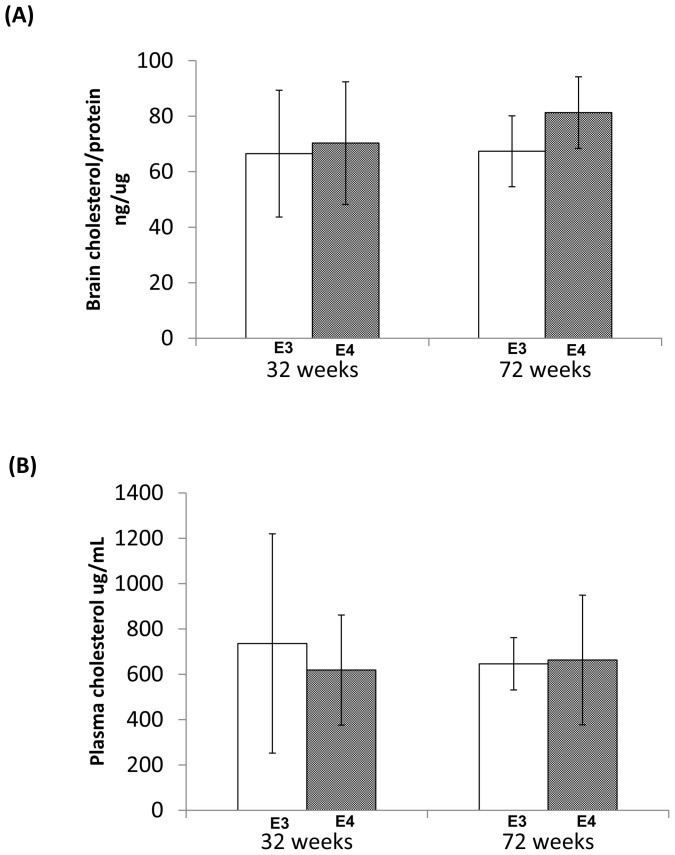
ApoE is a major player in cholesterol metabolism2. We decided to examine if changes in brain insulin and glucose also affect brain cholesterol. We did not detect any significant different in brain cholesterol between fasting huApoE4 and huApoE3 TR mice at 32 and 72 weeks of age (Figure 5a). Similarly, the plasma cholesterol content was not significant altered between fasting huApoE3 and huApoE4 mice at both ages (Figure 5b). This is expected since studies have shown that plasma cholesterol in these two TR mouse lines are only affected when they are kept on high-fat diet36,37,38.
Figure 5. Analysis of brain and plasma cholesterol contents in fasting huApoE TR mice.
No significant different in (A) brain and (B) plasma cholesterol contents between E3 TR (white bar) and E4 TR (grey bar) mice at 32 and 72 weeks of age was detected. Each value represents the mean ± SEM of duplicate assays for individual samples (n = 5).
ApoE expression in huApoE TR mouse brain
Reduced ApoE levels have been reported in the brain of huApoE4 TR mice39,40 and in non-demented APOE4 carriers40. However, it is unclear if this altered ApoE content persists during development.
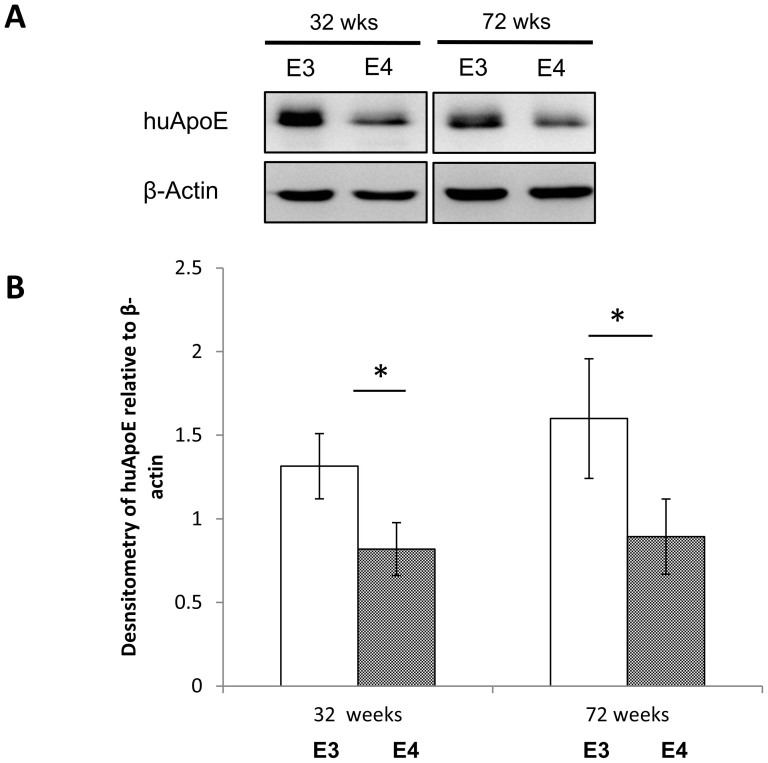
We therefore immunoblotted for ApoE in the brain of fasting huApoE3 and huApoE4 TR mice at 32 and 72 weeks of age. As show in figure 6, lower ApoE content was detected in the brain of young and old huApoE4 TR mice as compared to huApoE3 TR mice at similar age.
Figure 6. Lower human apolipoprotein E (huApoE) level in the brain of huApoE4 targeted replacement (TR) mice as compared to huApoE3 TR mice.
(A) Western blot and (B) densitometric analysis of huApoE levels in E3 (white bar) and E4 (grey bar) TR mice at 32 and 72 weeks of age. (A) The blot is a representative of three independent experiments. Blot images were cropped for comparison. β-actin was immunoblotted to ensure similar gel loading of the starting material in each sample. (B) Densitometric analysis was performed the NIH ImageJ software. Each value represents the mean ± SEM for individual mouse brain sample (n = 3 at each time point for each mouse line). Brain huApoE level was significant reduced in E4 TR mice as compared to E3 TR mice of similar age (*p < 0.05 using Student's t-test).
Discussion
Intranasal insulin can improve cognition29,30, but not in ApoE4 elderly subjects31. In this study, we are reporting an ApoE genotype-dependent effect on brain insulin signaling. Our results shows that ApoE4 expression is linked to lower brain insulin signaling and brain glucose content, and higher brain insulin.
Earlier studies have reported significant cognitive impairment in female ApoE4 mice as compared to male ApoE4 mice41,42,43,44. We therefore decided to examine insulin signaling in the brain of female ApoE3 and ApoE4 TR mice.
In this study, mice are fasted to trigger a catabolic state when examining peripheral insulin signaling45. This process will reduce variability in baseline insulin signaling activation during experiments.
We found that ApoE4 expression reduce the phosphorylation of IRS1 at S636/639 and at Y608. Altered phosphorylation of this serine residue (S636/639) on IRS1 has been shown to affect Akt phosphorylation46,47 and deregulate mTOR and MAPK activation32,33. Lower phosphorylation of p38 and p44/42 were detected in aged ApoE4 TR mice.
One possible mechanism linking IRS1 and Akt activation is ApoE expression. Studies have showed that ApoE treatment of primary neuron increases Akt phosphorylation48. Therefore, the lower brain ApoE level in huApoE4 TR mice as compared to huApoE3 TR mice39,40 could reduce Akt phosphorylation. This process may due to lower IRS1 phosphorylation at Y608 and S636/639.
The effect of ApoE4 on lowering Akt phosphorylation could also impair synaptic plasticity49 and reduce ApoER2 expression50. Aberrant Akt phosphorylation is reported to affect Reelin signaling51 leading to altered ApoER2 expression and synaptic function50. This synaptic dysfunction may contribute to the reported cognitive impairment in huApoE4 mice42.
Insulin is an important modulator of growth and metabolic function52. A reduction in insulin signalling activation can result in diseases such as diabetes and AD52,53,54. Most of our knowledge on insulin function is derived from observations in the peripheral organ systems55. However, recent clinical study has showed functional separation between brain insulin and peripheral insulin56. Our current study also show brain insulin and glucose is distinct from the blood glucose and insulin in our fasting mice.
However, as the lower brain glucose content was detected in 32 weeks old fasting hApoE4 TR mice, we were uncertain if the changes in the expression and activation of the brain insulin signaling proteins were the cause or consequence of the brain glucose alteration. Nevertheless, the late-stage (72 weeks old) elevation of brain insulin content in the fasting hApoE4 TR mice is likely due to impaired insulin signaling.
ApoE is extensive studied as a group of lipid carrier molecules that is vital in the cholesterol homeostasis of the body5, and has been proposed to have similar function in the brain57. However, in our fasting hApoE TR mice, changes in the brain insulin and glucose did not affect the brain cholesterol content. Further, blood cholesterol remains unchanged alongside blood insulin and glucose in the fasting hApoE TR mice. This is expected since our hApoE TR mice were not fed with high-fat diet37.
Reduced ApoE levels have been reported in the brain of huApoE4 TR mice39,40 and in non-demented APOE4 carriers40. Here, we have observed lower ApoE4 level in both young and aged huApoE TR mice. It is possible that ApoE level is lower in ApoE4 TR mice at birth.
There is increasing recognition that diabetes is an AD risk factor58,59,60,61,62. We and others have reported that impaired insulin signaling could enhance amyloid deposition and pathology54,58,59,60,61,62,63. PET imaging has detected lower cerebral glucose metabolism in overlapping brain regions in older asymptomatic ApoE4 carriers10,16,17 and in AD subjects18,19. It is possible that early changes in insulin signaling could be detected in these brain regions; including parietal, temporal, prefrontal and posterior cingulate.
Increasing connection between diabetes and AD has lead to growing interests to test the effect of anti-diabetic drugs on amyloid pathology in AD animal models and subjects64,65,66,67,68. Since ApoE can affect brain insulin signaling, the role of ApoE genotypes on the effect of anti-diabetic drugs on amyloid pathology in AD animal models and subjects should be examined.
In summary, our study indicates an interplay between ApoE and brain insulin signaling. This observation could underlie the ApoE genotype-dependent effect on cerebral glucose metabolism7,22 and intranasal insulin action on cognition31. ApoE4 is a strong genetic risk factor for AD9,10. But it is unclear if the insulin signaling dysfunction detected in AD52,69,70,71 is ApoE genotype-dependent. Further studies to examine the impact of ApoE on this pathogenic process will benefit the understanding and treatment of AD.
Methods
Animals
The experimental protocol (#009/10) involving the maintenance and euthanasia of laboratory mice was approved by the Institutional Animal Care and Use Committees (IACUC) at the National University of Singapore. The human apolipoprotein E3 and E4 targeted replacement mice were created as described37 and were purchased from Taconic. Briefly, the human APOE genomic fragments were used to replace the endogenous mouse ApoE gene via homologous recombination. The mice were kept on 2018 Teklad Global 18% Protein Rodent Diet (Harland Laboratories). Mice were fasted for ~12 hours prior to experiments. All experiments were performed on at least three (n ≥ 3) fasted female homozygous huApoE3 and huApoE4 mice at 32 and 72 weeks of age.
Preparation of brain homogenates
The mouse brain tissues were snapped frozen in liquid nitrogen when harvested and the wet weight of the tissues (in mg) was determined using an electronic balance. Twenty percent (w/v) brain homogenates were prepared with 1× cell lysis buffer (Cell Signaling Technology) with protease inhibitors cocktail (Roche Diagnostic).
The cell lysis buffer contains sodium orthovanadate, pyrophosphate and glycerophosphate, which can acts as phosphatase inhibitors. Lysates were then homogenized using a hand held motorized pestle (Sigma-Aldrich, St. Louis, USA) for 30 seconds on ice. Tissue lysates were subsequently centrifuged at 30,000 g for 30 minutes under 4C. The soluble portion of the lysates was collected for analysis.
Protein quantification of lysates
Tissue lysates were quantified using the Pierce™ MicroBCA assay kit (ThermoFisher Scientific, Waltham, USA) in a 96-well microplate format. Lysates were diluted in PBS and the working reagent was prepared and added in accordance to the manufacturer's instructions. Samples were then incubated at 37°C for 30 minutes before reading the absorbance values at 562 nm. Protein concentrations of samples were calculated based on a standard curve constructed from a range of BSA standards. The brain tissue lysates were aliquoted and stored at −80°C.
Immunoblot analysis
Fifty micrograms (μg) of soluble brain proteins from lysate samples were heated at 95°C for 5 minutes. Protein samples were then centrifuged at 14,000 g for 2 minutes on a bench top centrifuge before they were loaded on a 7.5–10% Tris-glycine polyacrylamide gel. The Precision Plus protein™ standard (Bio-Rad Laboratories, Hercules, California USA) was used as a molecular weight standard and run together with the samples on the same piece of gel.
The separated proteins were transferred onto a nitrocellulose membrane, probed with the respective antibodies and exposed to horseradish peroxidase (HRP)-conjugated secondary antibodies. The reactive protein bands were visualized by chemiluminescence on the Image Station 4000R (Carestream Health Inc) using the SuperSignal® West Dura Substrate (Pierce) system.
Immunoblotting of β-actin using a rabbit polyclonal antibody that binds to the C-terminal of β-actin (Sigma) was included in all western blot analysis to ensure comparable protein loading. The primary antibodies used include anti-huApoE (Santa Cruz Biotechn, Cat# 13521), anti-IRS1 (Cell Signaling Technology, Cat# 2382), anti-pIRS1(S636/639) (Cell Signaling Technology, Cat#2388), anti-pIRS1 (Y608) (Millipore, Cat# 09-432) anti-IRS2 (Cell Signaling Technology, Cat# 4502), anti-pIRS2 (S388) (Millipore, Cat# 07-1517), anti-pIRS2 (Y978) (Pierce, Cat# PA5-13025), anti-p38 (Cell Signaling Technology, Cat# 8690), anti-phospho-p38 (T180/Y182) (Cell Signaling Technology, Cat# 4511), anti-p44/42 (Cell Signaling Technology, Cat# 4695), anti-phospho-p44/42 (T202/Y204) (Cell Signaling Technology, Cat# 4370), anti-Akt (Cell Signaling Technology, Cat# 4691), anti-pAkt(S473) (Cell Signaling Technology, Cat# 4060), and anti-pAkt(T308) (Cell Signaling Technology, Cat# 2965).
Densitometry analysis was performed63 by measuring the optical densities of the targeted protein bands relative to the endogenous β-actin level from the same brain sample. For protein phosphorylation, the optical densities of the phosphorylated protein bands were measured relative to the targeted total protein level from the same brain sample. The analysis was performed using the NIH ImageJ software.
Glucose assay
Plasma and total brain glucose content was measuring using the amplex red glucose assay kit (Life Technologies) following the instructions provided by the manufacturer. Briefly plasma or brain lysate sample was mixed with equal volume of amplex red working reagent, and the reaction mixture was then incubated for 30 minutes at room temperature in the dark. The fluorescence values were read at an excitation wavelength of 545 nm and an emission wavelength of 590 nm. A series of glucose standards were prepared and run alongside the mouse plasma and brain samples.
Insulin assay
Plasma and total brain insulin content was measured using the sandwich ELISA mouse insulin assay system (Millipore) following the instructions provided by the manufacturer. Briefly, plasma or brain lysates were added to microtitre plate well pre-coated with anti-insulin antibody. After incubation and washing, a biotinylated anti-insulin antibody was added. This biotinylated antibody reacts against a distinctive epitope to that of the coated anti-insulin. The reaction was incubated for 15 minutes and the absorbance was read at 370 nm. The stop solution provided by the assay kit was added to the sample when the absorbance was read at 1.8. Immnoreactivity was immediately determined by measuring the absorbance at 450 nm and 590 nm.
Statistical analysis
Significant differences were analyzed using two-tailed Student's T-test. A p value of <0.05 is considered significant.
Author Contributions
Q.R.O., E.S.C. and M.L.L. performed the experiments. Q.R.O. and B.S.W. conceived and designed the experiments, and analyzed the data. Q.R.O., G.M.C. and B.S.W. wrote the paper.
Acknowledgments
This work was supported by grants to B.S.W. from the National Medical Research Council (NMRC/1148/2008) and the Biomedical Research Council (BMRC/05/1/21/19/401). Q.R.O. and E.S.C. were supported by graduate scholarships from Singapore Ministry of Education. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Rall S. C. Jr, Weisgraber K. H. & Mahley R. W. Human apolipoprotein E. The complete amino acid sequence. J Biol Chem 257, 4171–4178 (1982). [PubMed] [Google Scholar]
- Mahley R. W. & Rall S. C. Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet 1, 507–537 (2000). [DOI] [PubMed] [Google Scholar]
- Zannis V. I., McPherson J., Goldberger G., Karathanasis S. K. & Breslow J. L. Synthesis, intracellular processing, and signal peptide of human apolipoprotein E. J Biol Chem 259, 5495–5499 (1984). [PubMed] [Google Scholar]
- Zannis V. I., Kurnit D. M. & Breslow J. L. Hepatic apo-A-I and apo-E and intestinal apo-A-I are synthesized in precursor isoprotein forms by organ cultures of human fetal tissues. J Biol Chem 257, 536–544 (1982). [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240, 622–630 (1988). [DOI] [PubMed] [Google Scholar]
- Herz J. & Beffert U. Apolipoprotein E receptors: linking brain development and Alzheimer's disease. Nat Rev Neurosci 1, 51–58 (2000). [DOI] [PubMed] [Google Scholar]
- Jagust W. J. & Mormino E. C. Lifespan brain activity, beta-amyloid, and Alzheimer's disease. Trends in cognitive sciences 15, 520–526, 10.1016/j.tics.2011.09.004 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Weisgraber K. H., Mucke L. & Mahley R. W. Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer's disease. J Mol Neurosci 23, (2004). [DOI] [PubMed] [Google Scholar]
- Corder E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923 (1993). [DOI] [PubMed] [Google Scholar]
- Haan M. N., Shemanski L., Jagust W. J., Manolio T. A. & Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. Jama 282, 40–46 (1999). [DOI] [PubMed] [Google Scholar]
- Castellano J. M. et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med 3, 89ra57 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien-Ly N., Gillespie A. K., Walker D., Yoon S. Y. & Huang Y. Reducing human apolipoprotein E levels attenuates age-dependent Abeta accumulation in mutant human amyloid precursor protein transgenic mice. J Neurosci 32, 4803–4811 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. et al. Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-beta amyloidosis. J Neurosci 31, 18007–18012 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. C., Kanekiyo T., Xu H. & Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9, 106–118 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst A. J. et al. Cognition, glucose metabolism and amyloid burden in Alzheimer's disease. Neurobiol Aging 33, 215–225 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L. et al. Age and ApoE genotype interaction in Alzheimer's disease: an FDG-PET study. Psychiatry research 130, 141–151 (2004). [DOI] [PubMed] [Google Scholar]
- Samuraki M. et al. Glucose metabolism and gray-matter concentration in apolipoprotein E epsilon4 positive normal subjects. Neurobiol Aging 33, 2321–2323 (2012). [DOI] [PubMed] [Google Scholar]
- de Leon M. J. et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET). Proc Natl Acad Sci U S A 98, 10966–10971 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W. J. et al. Relationships between biomarkers in aging and dementia. Neurology 73, 1193–1199 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak E. et al. Neuropathology of Alzheimer's disease: what is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci 249 Suppl 3, 14–22 (1999). [DOI] [PubMed] [Google Scholar]
- Reiman E. M. et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med 334, 752–758 (1996). [DOI] [PubMed] [Google Scholar]
- Jagust W. J., Landau S. M. & Alzheimer's Disease Neuroimaging I. Apolipoprotein E, not fibrillar beta-amyloid, reduces cerebral glucose metabolism in normal aging. J Neurosci 32, 18227–18233 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Basak J. M. & Holtzman D. M. The role of apolipoprotein E in Alzheimer's disease. Neuron 63, 287–303 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong Q. R. & Wong B. S. The neuronal functions of human apolipoprotein E. OA Biochem In Press (2013). [Google Scholar]
- Wolf A. B., Caselli R. J., Reiman E. M. & Valla J. APOE and neuroenergetics: an emerging paradigm in Alzheimer's disease. Neurobiol Aging 34, 100710–100717 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. K. et al. APOE genotype and cognitive decline in a middle-aged cohort. Neurology 64, 268–276 (2005). [DOI] [PubMed] [Google Scholar]
- Cosentino S. et al. APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology 70, 1842–1849 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. et al. The apolipoprotein E gene and its age-specific effects on cognitive function. Neurobiology of aging 31, 1831–1833 (2010). [DOI] [PubMed] [Google Scholar]
- Ketterer C. et al. Insulin sensitivity of the human brain. Diabetes Res Clin Pract 93 Suppl 1, S47–S51 (2011). [DOI] [PubMed] [Google Scholar]
- Ott V., Benedict C., Schultes B., Born J. & Hallschmid M. Intranasal administration of insulin to the brain impacts cognitive function and peripheral metabolism. Diabetes, obesity & metabolism (2011). [DOI] [PubMed] [Google Scholar]
- Reger M. A. et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging 27, 451–458 (2006). [DOI] [PubMed] [Google Scholar]
- Hiratani K. et al. Roles of mTOR and JNK in serine phosphorylation, translocation, and degradation of IRS-1. Biochem Biophys Res Commun 335, 836–842 (2005). [DOI] [PubMed] [Google Scholar]
- Kang S., Chemaly E. R., Hajjar R. J. & Lebeche D. Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. J Biol Chem 286, 18465–18473 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson B. E., Seeley R. J. & Sandoval D. A. Wired on sugar: the role of the CNS in the regulation of glucose homeostasis. Nat Rev Neurosci 14, 24–37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubes G. Insulin insults may spur Alzheimer's disease. Science 301, 40–41 (2003). [DOI] [PubMed] [Google Scholar]
- Pendse A. A., Arbones-Mainar J. M., Johnson L. A., Altenburg M. K. & Maeda N. Apolipoprotein E knock-out and knock-in mice: atherosclerosis, metabolic syndrome, and beyond. J Lipid Res 50 Suppl, S178–182 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P. M. et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem 272, 17972–17980 (1997). [DOI] [PubMed] [Google Scholar]
- Johnson L. A. et al. Apolipoprotein E4 Exaggerates Diabetic Dyslipidemia and Atherosclerosis in Mice Lacking the LDL Receptor. Diabetes 60, 2285–2294, 10.2337/db11-0466 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell D. R. et al. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci 28, 11445–11453 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P. M. et al. Reduced levels of human apoE4 protein in an animal model of cognitive impairment. Neurobiol Aging 32, 791–801 (2011). [DOI] [PubMed] [Google Scholar]
- Raber J. et al. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci U S A 95, 10914–10919 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J. et al. Apolipoprotein E and cognitive performance. Nature 404, 352–354 (2000). [DOI] [PubMed] [Google Scholar]
- Leung L. et al. Apolipoprotein E4 causes age- and sex-dependent impairments of hilar GABAergic interneurons and learning and memory deficits in mice. PLoS One 7, e53569 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootendorst J. et al. Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behav Brain Res 159, 1–14 (2005). [DOI] [PubMed] [Google Scholar]
- Ayala J. E. et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 3, 525–534 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxhaj G., Dissanayake K. & Mackintosh C. Effect of IRS4 Levels on PI 3-Kinase Signalling. PLoS One 8, e73327 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copps K. D. & White M. F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55, 2565–2582 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. et al. Apolipoprotein E reduces food intake via PI3K/Akt signaling pathway in the hypothalamus. Physiol Behav 105, 124–128 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. et al. Human apoE4-targeted replacement mice display synaptic deficits in the absence of neuropathology. Neurobiol Dis 18, 390–398 (2005). [DOI] [PubMed] [Google Scholar]
- Chen Y., Durakoglugil M. S., Xian X. & Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci U S A 107, 12011–12016 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U. et al. Functional dissection of Reelin signaling by site-directed disruption of Disabled-1 adaptor binding to apolipoprotein E receptor 2: distinct roles in development and synaptic plasticity. J Neurosci 26, 2041–2052 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S. M. & Wands J. R. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer's disease. J Alzheimers Dis 7, 45–61 (2005). [DOI] [PubMed] [Google Scholar]
- Cohen E. & Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci 9, 759–767 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua L. M. et al. Impaired neuronal insulin signaling precedes Abeta(42) accumulation in APPsw/PS1deltaE9 mice. J Alzheimers Dis 29, 783–791 (2012). [DOI] [PubMed] [Google Scholar]
- Taubes G. Insulin resistance. Prosperity's plague. Science 325, 256–260 (2009). [DOI] [PubMed] [Google Scholar]
- Hirvonen J. et al. Effects of insulin on brain glucose metabolism in impaired glucose tolerance. Diabetes 60, 443–447 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B. Cholesterol and the biology of Alzheimer's disease. Neuron 41, 7–10 (2004). [DOI] [PubMed] [Google Scholar]
- Ballard C. et al. Alzheimer's disease. Lancet 377, 1019–1031 (2011). [DOI] [PubMed] [Google Scholar]
- Baker L. D. et al. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 68, 51–57 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan M. W., Reynolds R. M., Marioni R. E. & Price J. F. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol 7, 108–114 (2011). [DOI] [PubMed] [Google Scholar]
- Matsuzaki T. et al. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology 75, 764–770 (2010). [DOI] [PubMed] [Google Scholar]
- Irie F. et al. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: the Cardiovascular Health Study Cognition Study. Arch Neurol 65, 89–93 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong Q. R., Lim M. L., Chua C. C., Cheung N. S. & Wong B. S. Impaired insulin signaling in an animal model of Niemann-Pick Type C disease. Biochemical and Biophysical Research Communications 424, 482–487 (2012). [DOI] [PubMed] [Google Scholar]
- Bomfim T. R. et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease- associated Abeta oligomers. J Clin Invest 122, 1339–1353 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano L. et al. Rosiglitazone reverses memory decline and hippocampal glucocorticoid receptor down-regulation in an Alzheimer's disease mouse model. Biochem Biophys Res Commun 379, 406–410 (2009). [DOI] [PubMed] [Google Scholar]
- Nicolakakis N. et al. Complete rescue of cerebrovascular function in aged Alzheimer's disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci 28, 9287–9296 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean P. L., Parthsarathy V., Faivre E. & Holscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci 31, 6587–6594 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T. et al. Glucagon-Like Peptide-1 Cleavage Product GLP-1(9-36) Amide Rescues Synaptic Plasticity and Memory Deficits in Alzheimer's Disease Model Mice. J Neurosci 32, 13701–13708 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyt A. A. et al. The effect of insulin and glucose on the plasma concentration of Alzheimer's amyloid precursor protein. Neuroscience 95, 727–734 (2000). [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu F., Grundke-Iqbal I., Iqbal K. & Gong C. X. Deficient brain insulin signalling pathway in Alzheimer's disease and diabetes. J Pathol 225, 54–62 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K. et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122, 1316–1338 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]