Abstract
Using archival data, we conducted a secondary analysis to examine race-differences in the relation of serum vitamins A, C, E and β-carotene to insulin resistance (IR), fasting insulin and glucose, high sensitivity C-reactive protein (hsCRP), and leukocyte count in 176 non-smoking, healthy, white and African American (AA) adults aged 18-65 years (48% women, 33% AA). We hypothesized that micronutrient concentrations would be associated with early risk markers of cardiometabolic diseases in a race-dependent manner. Fasting blood samples were analyzed for micronutrients, insulin, glucose, hsCRP, and leukocyte count. Insulin resistance was estimated using the homeostatic model assessment (HOMA). After adjusting for age, body mass index, gender, educational level, use of vitamin supplements, alcohol intake, leisure time physical activity, menopausal status, and total cholesterol, we observed that β-carotene was significantly associated with insulin resistance and fasting insulin in a race-dependent manner. Among AA, lower β-carotene levels were associated with higher estimates of insulin resistance and fasting insulin; whereas, these same associations were not significant for whites. Race also significantly moderated the relation of vitamin C to leukocyte count, with lower vitamin C being associated with higher leukocyte count only in AA but not whites. For all subjects, lower β-carotene was associated with higher hsCRP. In AA, but not whites, lower levels of β-carotene and vitamin C were significantly associated with early risk markers implicated in cardiometabolic conditions and cancer. Whether or not lower levels of micronutrients contribute uniquely to racial health disparities is a worthwhile aim for future research.
Keywords: Human, micronutrients, risk biomarkers, African Americans, whites
1. INTRODUCTION
Despite improvements in the overall health of the population of the United States, racial health disparities continue to pose a major challenge [1]. When comparing African Americans (AA) to whites, it is overwhelmingly evident that AA have worse health outcomes and higher mortality rates from type 2 diabetes and cardiovascular disease (CVD) [2-4]. Similarly, AA have a lower rate of survival for a variety of cancers [5, 6]. At this time, factors contributing to race-related differences are not well understood [7]. However, it has been postulated that racial health disparities may reflect race differences in the prevalence of obesity [8, 9], hypertension [10-12], hyperinsulinemia [13, 14], insulin resistance (IR) [15, 16], and inflammation [15, 17], which are factors implicated in heart disease, type 2 diabetes, and obesity-related cancers [18-22]. While the best risk models are successful in predicting disease-related outcomes, they fall short of fully explaining the underlying causes of racial health disparities [23].
It has been reported that cardiometabolic conditions and various forms of cancers are closely linked to dietary intake and blood levels of micronutrients [24-27]. To date, the most consistent findings have been reported for carotenoids where both dietary intake and serum concentrations have been inversely related to occurrence of type 2 diabetes [28, 29] and CVD mortality [30]. As suggested by a recent meta-analysis, lower levels of carotenoids are also associated with an increased risk of breast cancer [31], with recent evidence suggesting that this association is stronger when carotenoids are assessed in blood [32]. Low serum levels of β – carotene have also been associated with an increased risk of colon and colorectal cancers [33] and non-Hodgkin lymphoma [34]. In contrast, higher levels of β-carotene appear to be associated with an increased risk of prostate cancer [35]. For vitamins E and C, however, findings have been inconsistent, with some studies reporting inverse, albeit modest, associations with CVD [30, 36-38], type 2 diabetes [39, 40], and various forms of cancer [41, 42]. In light of these prior findings, the greater prevalence of cardiometabolic conditions, and some forms of cancers in AA, we examined whether the relation of serum micronutrient concentrations to early risk biomarkers differed in AA and whites. For the most part, prior studies either adjusted for the effects of race or did not include sufficient numbers of AA participants to conduct analysis [29, 43-45]. Interestingly, one study observed that the relation of serum β-carotene concentration to C-reactive protein (CRP) was moderated by race, although no additional details were provided [46]. More recently, one study suggested that the relation of a serum indicator of vitamin D mediates race-differences in the prevalence of insulin resistance in AA and whites [47].
Surprisingly, race differences in early risk factors of cardiometabolic conditions and some forms of cancer, such as hyperinsulinemia and insulin resistance, have not been associated with race differences in dietary intake [24], even when intake of fruits and vegetables has been reported to be higher for AA compared to whites [24, 48]. Those findings may explain the results of one meta-analysis that suggested that blood concentrations of micronutrients, relative to dietary assessment of intake, were more strongly associated with breast cancer risk [32]. Given the lack of evidence for the relation of early risk markers to dietary intake and the relative strength of the relation of blood concentrations to disease, we determined whether blood concentrations of micronutrients are associated with early markers of disease risk and whether these associations are race-dependent. More specifically, we examined the cross-sectional relationship of β-carotene, vitamin A, vitamin C, and vitamin E to metabolic and inflammatory biomarkers in a sample of apparently healthy, non-smoking community volunteers who self-identified as either white or AA. The data were derived from a study that examined the relation of psychosocial factors to early risk biomarkers of CVD and type 2 diabetes [49-52]. The aim of this secondary analysis, however, was to examine the relation of micronutrients to early risk biomarkers and whether these associations differed by race. We hypothesized that the relationship between micronutrient concentrations and early risk markers of cardiometabolic conditions would be race-dependent. Analyses focused on determining whether or not race moderated the relation of vitamin A, C, E and β-carotene to fasting insulin and glucose, estimation for insulin resistance, high sensitivity (hs) CRP and white blood cell count. Given the high prevalence of nutrition related cardiometabolic conditions among AA, we speculated that micronutrients would be more strongly associated with early risk markers in AA than in whites.
2. METHODS and MATERIALS
2.1 Participants and recruitment
Subjects in these analyses were 176, apparently healthy, adults (age: 18-65 years) that were recruited between 1999 and 2004 and self-identified as being white or AA. The 176 subjects in these analyses represent a subsample of 210 adults who enrolled in the initial study [53]. The remaining 34 subjects self-identified as being of another race or ethnicity. The procedures described in this article are the same as those used in the original study [53]. Individuals were initially screened for health criteria using a self-report health questionnaire and in-person interview. Inclusion criteria included the following: negative history and no current diagnosis of psychiatric conditions; no current or previous use of anti-depressant medications; and no chronic medical conditions, such as asthma, allergies, arthritis, diabetes, cancer, and cardiovascular diseases. Subjects who had a history of smoking were excluded. We excluded women if they reported use of oral contraceptives or hormone replacement therapy within the previous 6 months. This study was approved by the Institutional Review Board of Duke University and informed consent was obtained prior to the collection of data.
2.2 Protocol
Following an overnight fast, subjects reported to the laboratory. Subjects were instructed to not use prescription or over-the-counter medications, including low-dose aspirin, during the two-weeks prior to the study visit. On the day of the study visit, staff verified via interview that participants were free of acute infections, had not incurred any injuries, and had not undergone any medical/dental procedures two weeks prior, which are conditions known to increase hsCRP and other markers of inflammation. To minimize menstrual cycle effects, pre-menopausal women were studied during the follicular phase (days 4-9 of the menstrual cycle).
2.3 Biomarkers
Fasting blood samples were analyzed for vitamins A, C, E, β-carotene, lipids, and hsCRP. Blood samples used to assess micronutrients were drawn in chilled serum separator tubes containing Na-heparin. Careful attention was placed on protecting samples from light. Samples were immediately centrifuged and serum was transferred to an amber plastic transport tube. Due to issues with stability, analyses were performed on the day of sample collection. Samples were analyzed isocratically by reverse-phase high performance liquid chromatography (HPLC) with photodiode array detection. As previously noted, these data were collected between 1999 and 2004, a period of time during which a number of large intervention studies were examining the potential cardioprotective effects of vitamins A, C, E, and β-carotene [38, 54-57]. Thus, the selection of the micronutrient panel was based on those studies. For analysis of vitamin, 9 subjects had missing vitamin C values (4 whites, 5 AA), thus the vitamin C results are based on only 167 subjects (113 whites and 54 AA). For remaining micronutrients, analysis is based on 172 subjects (117 whites and 55 AA).
High sensitivity CRP was measured using an ultrasensitive, enzyme-linked, immunometric latex-enhanced assay (Diagnostic Products Corporation, Los Angeles, CA) using purified protein and polyclonal anti-CRP antibodies from Diagnostic Products Corporation (Los Angeles, CA). This system has a low detection threshold of < 0.10 mg/L with coefficients of variation ranging from 6.6% to 9.3%. Measurements of hsCRP were done on fasting venous blood samples collected between the hours of 8:30 AM and 9:30 AM while subjects were seated in a reclined position. Measures of fasting insulin, glucose and lipids (total, triglycerides, low density lipoprotein (LDL)) levels were conducted by the Duke University Clinical Laboratories. Estimated insulin resistance was calculated using the Homeostatic Model Assessment (HOMA-IR) [58] with the following equation: HOMA-IR =[fasting glucose (mg/dl) X fasting insulin (unit/mL)]/405. HOMA-IR values have been positively associated with an increased risk of type 2 diabetes [59], CVD [60] and cancer [61-63].
2.4 Demographics
On the day of the study visit, subjects’ heights and weights were measured and used to calculate BMI. Race was determined by self-report. Data were collected for vitamin supplement use, alcohol consumption, and leisure time physical activity. Vitamin supplement use was evaluated for the 6 months prior to the study visit. Alcohol consumption was classified using a modification of the scheme used by Albert et al. [64]: never/former (< 1 drink in past 12-months), infrequent (1-3 drinks/month), occasional (1-7 drinks/week), and regular (1-4 drinks/day). For the purpose of analysis, we combined the subjects who responded “occasional” and “regular.” Leisure time physical activity was assessed by a yes/no response to the following question: “Do you exercise on a regular basis (2 or more hours/week)?”. Educational attainment was defined as the highest level achieved. To confirm menopausal status and menstrual cycle phase, we assessed estradiol, progesterone, and follicular stimulating hormone (FSH).
2.5 Statistical Analyses
Statistical analyses were conducted using SAS 9.3 software (SAS Institute Inc., Cary, NC, USA). Tests of the hypothesis, that race moderates the relation of micronutrients to HOMA-IR, glucose, insulin, and hsCRP, were conducted using the SAS procedure for general linear models (PROC GLM). Guided by previous research, covariates were selected a priori and included in all models. Covariates included age, gender, body mass index (BMI), race, educational level, alcohol use, physical activity, vitamin supplement usage, menopausal status, and total cholesterol. For models predicting HOMA-IR, insulin, and glucose, log-transformed hsCRP was included as a covariate. Logarithmic transformation was performed on all micronutrient concentrations, HOMA-IR, fasting insulin, glucose, and CRP. Graphic and tabular means represent adjusted means or predicted means derived from multiple linear regression models.
Regression models included all covariates, main effects for vitamins A, C, and E and β-carotene, as well as the 2-way interactions between race and micronutrient levels (race X vitamin A, race X vitamin C, race X vitamin E, and race X β-carotene). A significant interaction suggests that race moderates the relation of vitamin level to biomarker, thus, significant interactions were followed by race-specific analysis that included the same set of covariates.
3 RESULTS
3.1 Bivariate Analyses
Data for demographic, biometric and clinical characteristics are presented by race with accompanying p-values for tests of race differences in Table 1. No race differences were observed for gender distribution, age, educational attainment, fasting triglycerides, glucose, and leisure time physical activity (> 2 hr/week). AA exhibited significantly higher BMI, fasting total cholesterol, high density lipoprotein (HDL) cholesterol, resting systolic and diastolic blood pressure (BP), fasting insulin, and estimated IR. No significant ethnic difference in the use of vitamin supplements was observed (χ2(1) = 2.33, ns), with approximately one-third of the total sample reporting regular use in the 6 months prior to study visit, which is a percentage of subjects consistent with recently published population reports of adults living in United States [65].
Table 1.
Participant Characteristics
| Caucasian | African American |
Race Difference (p-value) |
|
|---|---|---|---|
| Gender (% female) | 42 | 57 | NS |
| Age, years | 29.6 | 29.2 | NS |
| BMI, kg/m2 | 24.4 | 28.5 | <.0001 |
| Total Cholesterol (mg/dL) | 167.4 | 179.2 | .047 |
| HDL Cholesterol (mg/dL) | 48.0 | 52.7 | .01 |
| Triglycerides (mg/dL) | 98.6 | 82.7 | NS |
| Fasting Glucose (mg/dL) | 85.8 | 85.6 | NS |
| Fasting Insulin (mcU/mL) | 6.53 | 10.04 | .0004 |
| Insulin Resistance (units) | 1.40 | 2.21 | .0005 |
| Systolic Blood Pressure (mmHg) | 106.6 | 116.1 | .0083 |
| Diastolic Blood Pressure (mmHg) | 61.1 | 67.0 | <.0001 |
| Education level (%) | NS | ||
| Less than High School | 0.9 | 0 | |
| High School Graduate | 6.9 | 5.1 | |
| Some College | 31.9 | 34.5 | |
| College Graduate | 19.8 | 34.5 | |
| Post-College | 40.5 | 25.9 | |
| Use of Vitamin Supplements (%)a | NS | ||
| Yes | 38.8 | 27.6 | |
| No | 61.2 | 72.4 | |
| Leisure Time Physical Activity (%) b | .03 | ||
| Yes | 86.2 | 72.9 | |
| No | 13.8 | 27.1 | |
| Micronutrient Levels c | |||
| vitamin A (μg/dL) | 53.2 | 44.3 | <.0001 |
| vitamin C (mg/dL) | 0.91 | 0.99 | NS |
| vitamin E (mg/L) | 11.1 | 10.5 | NS |
| β-carotene (μg/dL) | 17.1 | 14.0 | NS |
| C-reactive protein (mg/L) | 0.99 | 1.92 | <.0001 |
in past 6 months;
2 hours/week or more;
adjusted for multivitamin use
Continuous parametric results are given as Mean (SD); categorical results as percentage; and continuous non-parametric results as median (95% confidence interval); NS = not significant
Analysis of vitamin concentrations, adjusting for vitamin supplement use, revealed no significant race differences in vitamin C, vitamin E, and β-carotene. African Americans, however, had significantly lower mean adjusted concentration of vitamin A (p = .003) (see Table 1). It is important to note that none of our subjects met National Center for Health Statistics (NCHS) criteria for at-risk status for serum retinol deficiency (< 20 μg/dL), vitamin C (< 2.0 mg/l), and vitamin E (< 5μg/ml) [66]. For β-carotene, subject levels were above 0.3 mol/L a level considered acceptable for adults.
Univariate analysis controlling for use of vitamin supplements revealed that BMI was negatively correlated with concentrations of vitamin C (r = −0.23, p = .003) and β-carotene (r = −0.22, p = .004) but not with vitamin A or vitamin E. Race-specific analysis showed that for AA, BMI was significantly associated with vitamin C (r = −0.35, p = .007), β-carotene (r = −0.28, p =.037), and vitamin A (partial r = 0.31, p = .02) but not vitamin E. For whites, BMI was not associated with any of the micronutrients (all p-values > .05) although we did observe a marginally significant association between β-carotene and BMI (r = −0.17, p = .080).
We conducted multivariate analysis to determine if BMI was associated with micronutrient concentrations and whether this association was moderated by race. Regression analysis included age, gender, educational level, alcohol use, leisure time physical activity, vitamin supplements usage, and menopausal status as covariates. The BMI by race interaction did not significantly predict levels of any of the micronutrients. Adjusting for confounding factors revealed that BMI (= −0.04, p = .01) was significantly associated with β-carotene levels, and this association was independent of race. Consistent with results of univariate analysis, multivariate analysis revealed significant race differences (F = 6.67, p = .01) for vitamin A, with AA having lower concentrations. While results of univariate analyses suggested stronger associations between BMI and micronutrient concentrations in AA than whites, adjusting for potential confounding factors attenuated most of the associations between BMI and micronutrients. Consistent with results of univariate analysis, multivariate analysis revealed a significant race-related difference in vitamin A that was independent of BMI.
3.2 Interaction between serum micronutrients and ethnicity predicting insulin resistance, fasting insulin, and glucose
3.2.1 Insulin Resistance
Log-transformed HOMA-IR values were analyzed using multivariate general linear models adjusted for age, gender, BMI, vitamin supplement usage, alcohol consumption, leisure time physical activity, educational level, total cholesterol, menopausal status, and hsCRP. Inspection of the two-way interactions revealed that only the race X β-carotene interaction was significant (p = .016). The interactions between race and vitamin A, vitamin C, and vitamin E and the main effects of vitamin A, vitamin C, and vitamin E did not predict HOMA-IR.
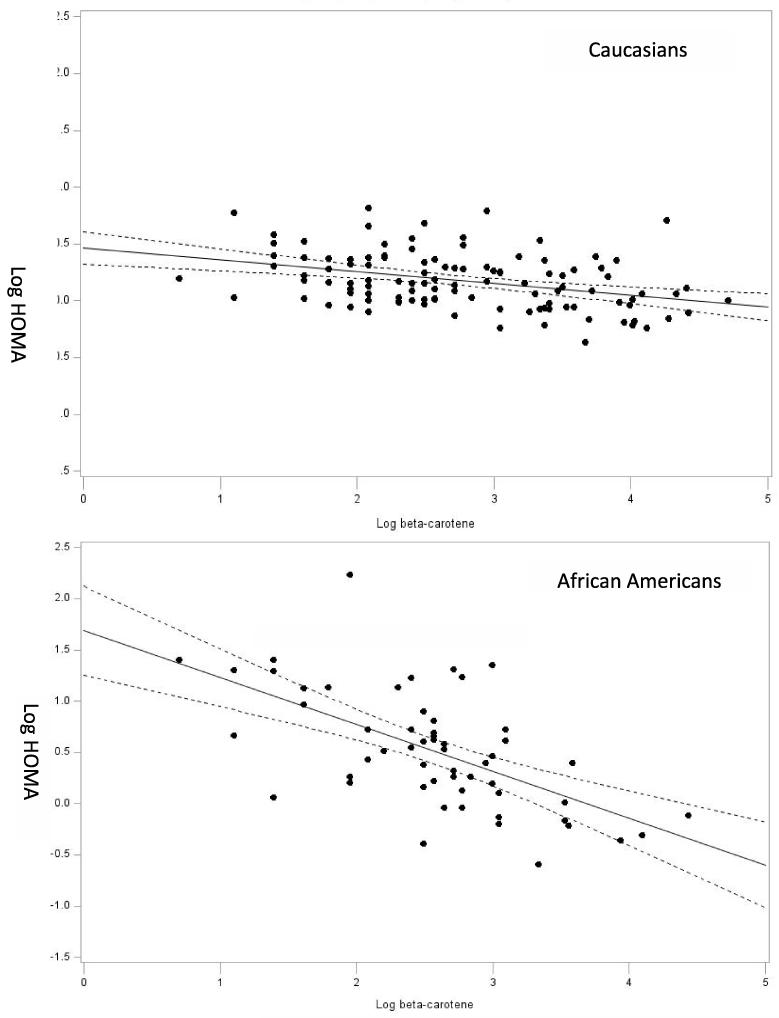
We conducted race-specific analysis to determine the relation of log-HOMA-IR to β-carotene in whites and AA. The regression model included all covariates and micronutrients to examine the unique association of β-carotene to log-HOMA-IR. As shown in Figure 1, greater HOMA-IR was associated with lower β-carotene (β = −0.253, p = .0398). For whites, HOMA-IR was not associated with β-carotene (β = −0.015, p = .84).
Figure 1.
Illustrated are scatterplots with regression line (95% confidence interval) for the relation of HOMA-estimated insulin resistance and β-carotene concentration for African Americans and whites. Regression lines are adjusted for all covariates in the model.
3.2.2 Insulin and Glucose
The race X β-carotene interaction significantly predicted insulin level (p = .021). Neither the main effects of vitamins A, C, and E nor their interactions with race predicted fasting insulin (all p > .05). Decomposition of the race X β-carotene interaction revealed that for AA, higher fasting insulin was significantly associated with lower β-carotene (β = −0.250, p = .028). In contrast, for whites, β-carotene (β = −0.034, p = .64) was not associated with fasting insulin.
For glucose, none of the interactions were significant and only the main effect of vitamin A approached significance (= .034, p = .06).
3.3 The relation of insulin resistance and fasting insulin to micronutrient concentrations as a function of adiposity
Given the significant race-difference in BMI and the observation that β-carotene concentrations were significantly associated with BMI in multivariate analysis, we tested the interactions of race X β-carotene and BMI X β-carotene for their effect on HOMA-IR, insulin levels, and glucose levels. First, we performed regression analysis that included the β-carotene X BMI interaction (as a continuous variable), with race as a covariate. Results showed that the BMI X β-carotene interaction significantly predicted HOMA-IR (= −0.034, p = .034) and fasting insulin level (= −0.58, p < .0001) with race included as a covariate.
Having established the significance of the BMI by β-carotene interaction, we then conducted analysis to evaluate whether the β-carotene by race interaction was due, in part, to the effect of carotene and BMI. If these analyses revealed that the β-carotene X race interaction was no longer significant, the results would suggest that our initial observation of a race by β-carotene interaction was likely due to race being a proxy for BMI. Conversely, if the β-carotene by BMI interaction was no longer significant, then it would suggest that BMI served as a proxy for race. Results indicated that when both BMI X β-carotene and race X β-carotene interactions were included in the model, the effects of both interactions lost significance for HOMA-IR (p’s > .05). Analysis of fasting insulin, however, revealed that both the β-carotene X race and the β-carotene X BMI interactions remained significant (p’s < .05), thus suggesting that the effects of these interactions were independent in predicting insulin levels.
3.4 The relation of inflammatory biomarkers to micronutrient concentrations in whites and African Americans
Using the same analytic approach, we examined the moderating effect of race on the relationship between micronutrients, leukocyte count, and hsCRP. None of the 2-way interactions were significant at the 0.05 level. We then examined the main effects of micronutrients on hsCRP. Higher hsCRP was significantly associated with lower β-carotene (β = −0.30, p = .007). There was a non-significant trend for a positive association between hsCRP and vitamin E (= 0.64, p = .064). No other micronutrient was related to hsCRP levels.
For leukocyte count, the race X vitamin C interaction (p < .0001) and race X vitamin A interaction (p = .021) were significant. Post-hoc analyses revealed that for AA, a higher leukocyte count was significantly associated with lower vitamin C (β = −1.73, p = .0001), where in whites, leukocyte count was not associated with vitamin C (β = 0.075, p = .76). This was not the pattern observed for vitamin A where for whites, but not AA, higher leukocyte count was associated with higher vitamin A concentrations (= 1.18, p = .024). For the total sample, higher leukocyte count tended to be associated with lower β-carotene concentration (β = −0.396, p = .072), although the association only approached significance.
4. DISCUSSION
The current findings are consistent with our hypothesis that the relation of micronutrient concentrations to early risk biomarkers of cardiometabolic conditions is moderated by race. In a sample of healthy, non-smoking adult men and women, we found statistically significant race-related differences in the relationship of fasting levels of serum micronutrients to early markers of disease risk. For AA, lower β-carotene concentrations were associated with greater IR and elevated fasting insulin. For whites, however, we found no such relationships. These observations were noted in the presence of no significant race differences in β-carotene concentrations and were independent of the effects of other measured micronutrients and potential confounders such as BMI, age, alcohol use, leisure time physical activity, educational level, fasting total cholesterol, menopausal status, and hsCRP. In addition, race also moderated the relation of vitamin C and vitamin A to leukocyte count, a measure of inflammation. While whites and AA did not differ in mean level of vitamin C, lower vitamin C concentrations were associated with higher leukocyte count in AA but not whites. Conversely, higher levels of vitamin A were associated with higher leukocyte count in whites but not in AA. Lastly, lower β-carotene concentrations were associated with higher levels of hsCRP in both races, a relation that has been previously reported [67, 68]. Combined, our observations suggest that race is a key moderator of the relation of β-carotene and vitamin C to early risk markers and that race differences may explain, in part, the previously reported lack of an association between micronutrients and early biomarkers [69]. More importantly, the observation that lower levels of β-carotene were significantly associated with HOMA-IR and greater inflammation suggests that for AA, β-carotene may be an important early marker of risk given the synergistic effects of inflammation and insulin resistance on progression of both coronary artery disease [70] and cancer [18]. Our findings complement recent evidence suggesting that serum 23 [OH] D levels mediate the increased prevalence of IR among AA [47].
Given the novelty of our findings, it is important that we stress that the study sample included only nonsmokers with no evidence of vitamin deficiencies in any of our participants. Similar to large intervention studies, such as the β-carotene and Retinal Efficacy Trial (CARET) [71], we assessed smoking history via self-report and interview and not with an objective measure such as cotinine levels. Thus, these findings may not be generalized to smokers, a speculation consistent with recent findings suggesting that β-carotene is associated with the metabolic syndrome only in nonsmokers [72, 73]. Given that insulin resistance is a principle component of the metabolic syndrome, it is likely that race differences observed in the current study likely would not be applicable to smokers.
At this time, the exact physiological and biochemical mechanisms for the relation of β-carotene to an IR/secretion profile in AA but not whites are unknown. One possibility is adiposity. In univariate analysis, micronutrient concentrations were associated with BMI, an association that has been previously reported [74-76]. However, with the exception of the interaction between β-carotene to BMI, the remaining associations were attenuated once confounding factors were included in the analysis. Thus, it was important to determine if race-differences in the relation of β-carotene to insulin and IR remained significant when the β-carotene by BMI interaction was included in the model. Results of the concomitant testing of the interactions revealed that for HOMA-IR, the independent effect of each interaction was attenuated. For insulin, however, both interactions remained significant. Combined, these findings suggest that the observed race-related differences in the relation of β-carotene to metabolic factors are complex and may involve BMI in predicting insulin resistance but not fasting insulin.
We also observed significant associations between lower vitamin C and higher leukocyte count in AAs and not whites, as well as an association between lower β-carotene and higher CRP that was independent of race. It has been suggested that antioxidant micronutrients provide protection against oxidative damage via inactivation of reactive oxygen species (ROS), reduction of lipid peroxidation, and reduction of cholesterol uptake implicated in formation of atherosclerotic lesions. Each of these actions are thought to inhibit the onset and progression of atherosclerosis and type 2 diabetes [77-79], as well as certain types of cancer [41, 80]. The benefits of vitamin C may also be due, in part, to its effects on increasing immune cell proliferation and activity, as well as protecting cells from oxidative DNA damage. Thus, among AA, lower serum vitamin C may contribute to inflammation via increased production of ROS.
It is interesting to note that serum levels of micronutrients have been associated with self-reports of dietary intake in a race dependent manner. Arab et al. [81] showed that dietary intake of carotenoids was correlated with serum levels in whites, but not AA, suggesting the possibility of metabolic or pharmacokinetic differences in micronutrient processing between the two races. Such observations argue for the importance of measuring serum concentrations of β-carotene, since dietary intake appears to not be a reliable measure of circulating levels in AA. Interestingly, previous studies have shown that relative to whites, AA have a greater intake of fruits and vegetables [24, 48], an observation that is inconsistent with the increased burden of IR among AA [24].
At this time, we can only speculate on the putative mechanisms underlying our findings, and specifically, those with regards to β-carotene. One possibility is the enzyme β-carotene monooxygenase (BCMO)-1 which converts β-carotene to vitamin A [82, 83]. In this study, whites and AA exhibited equivalent levels of β-carotene. Yet AA had significantly lower levels of vitamin A, relative to whites, alluding to potential differences in the conversion of β-carotene to vitamin A. Interestingly, evidence from one study has suggested that BCMO-1 exhibits variation in activity among ethnic groups [84]. Moreover, recent evidence stemming from a mouse model with a disruption on the BCMO-1 gene showed that, compared to wild-type mice, the BCMO-1 −/− knockout mouse exhibited lower levels of vitamin A but no difference in β-carotene levels, even when both groups were fed a diet supplemented with β-carotene [85]. In that same study, when animals were fed a high fat diet, the BCMO-1 −/− mice, relative to C57BL/6 control mice, gained significantly more weight and showed greater increases in serum cholesterol ester levels [85]. Interestingly, in our study, AA subjects had significantly higher mean BMI and fasting cholesterol with lower vitamin A levels, which were significantly associated with higher BMI, an association not found in whites. Thus, it may be that the race-related difference in BCMO-1 gene expression is an important mechanism underlying our findings of race-related differences in the relation of β-carotene to early metabolic risk markers. Gene-diet interactions have been previously reported, and while this is an emerging research area, it points to the possibility that the effect of diet is dependent upon genetic phenotypes [86].
The strength of our study rests upon a number of factors, one of which is stringent methodological controls. This is most notable in including only participants who were healthy, non-smoking, non-medicated, and limiting female subjects to those not using exogenous hormones (either oral contraceptives or hormone replacement therapy). We implemented these methodological constraints so as to reduce the sources of error variance that are frequently controlled for in statistical models employed in studies with considerably larger samples. Our aim was to allow for statistical models to include tests of effects commensurate with sample size while recognizing the possibility that results may have limited generalizability. Smoking status was determined by self-report and interview and not serum cotinine level, an objective measure of smoking status. Nevertheless, we are relatively confident that all participants were non-smokers. To control for the effects of female sex hormones on inflammatory biomarkers, blood samples were collected during the follicular phase (days 5-9) when sex hormones are at their nadir. Implementing these methodological constraints and statistically adjusting for other potential confounders such as socioeconomic and lifestyle factors adds to the strength of the study. Although sample size was small compared to large population studies, our sample size was sufficient to replicate many findings previously reported by larger studies.
Nevertheless, our study has several limitations. Most notable are the cross-sectional design and the lack of a dietary intake tool. The design of our study did not allow for tests of causality among the variables of interest. Thus, we could not determine if low concentrations of β-carotene and vitamin C precede the development of IR, hyperinsulinemia, and inflammation as a result of increased oxidative stress or vice-versa. We also did not collect dietary histories to assess energy intake. It is widely accepted that serum concentrations of micronutrients reflect dietary intake and supplementation. As noted in the preceding section, recent findings by Arab et al. suggest that the strength of the relation of dietary carotenoid intake to serum β-carotene concentration is significantly lower in AA relative to whites [87]. In that study, Arab et al. attributed their findings to differences in dietary reporting or genetic differences between races, possibilities that can be tested in future studies. Interestingly, results of another study suggest that race-related dietary differences do not account for the higher prevalence of IR and hyperinsulinemia in AA, particularly since AA reported greater intake of fruits and vegetables [24].
To our knowledge, the current findings are the first to show that the relationship between β-carotene and vitamin C levels and early risk markers of chronic diseases are moderated by race. Lower levels of β-carotene and vitamin C were associated with elevated risk markers in AA but not in whites, despite the fact that we observed no race differences in serum levels of these micronutrients. Our findings complement recent evidence suggesting that lower serum vitamin D may account, in part, for the greater prevalence of IR and hyperinsulinemia in AA [47]. Thus, we hypothesize that micronutrients may contribute in multiple ways to racial disparities in incidence of CVD and type 2 diabetes. The current paper suggests that even when there are no differences in serum levels (implying no difference in dietary intake or serum uptake), there still may be differences in how micronutrients confer protection from or vulnerability to disease.
Acknowledgment
This work was supported by a grant from the National Institutes of Health (HL67459) to ECS. We thank Anne Dennos for her editorial assistance in the preparation of this manuscript.
Abbreviations
- AA
African American
- HOMA
homeostatic model assessment
- IR
insulin resistance
- CVD
cardiovascular disease
- hsCRP
high sensitivity C-reactive protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: ECS and NLSS have no conflicts of interest related to the submitted manuscript.
References
- 1.Bosma H, van de Mheen HD, Borsboom GJ, Mackenbach JP. Neighborhood socioeconomic status and all-cause mortality. Am J Epidemiol. 2001;153:363–71. doi: 10.1093/aje/153.4.363. [DOI] [PubMed] [Google Scholar]
- 2.Jones DW, Chambless LE, Folsom AR, et al. Risk factors for coronary heart disease in african americans: The atherosclerosis risk in communities study, 1987-1997. Arch Intern Med. 2002;162:2565–71. doi: 10.1001/archinte.162.22.2565. [DOI] [PubMed] [Google Scholar]
- 3.Gillum RF, Mussolino ME, Madans JH. Coronary Heart Disease Incidence and Survival in African-American Women and Men: The NHANES I Epidemiologic Follow-up Study. Ann Intern Med. 1997;127:111–18. doi: 10.7326/0003-4819-127-2-199707150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Brancati FL, Kao W, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in african american and white adults: The atherosclerosis risk in communities study. JAMA. 2000;283:2253–59. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 5.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer Disparities by Race/Ethnicity and Socioeconomic Status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 6.Wells TS, Bukowinski AT, Smith TC, Smith B, Dennis LK, Chu LK, Gray GC, Ryan MA. Racial differences in prostate cancer risk remain among US servicemen with equal access to care. Prostate. 2010;70:727–34. doi: 10.1002/pros.21105. [DOI] [PubMed] [Google Scholar]
- 7.Woolf SH, Braverman P. Where health disparities begin: the role of social and economic determinants - and why current policies may make matters worse. Health Aff (Millwood) 2011;30:1830–36. doi: 10.1377/hlthaff.2011.0685. [DOI] [PubMed] [Google Scholar]
- 8.Beydoun MA, Gary TL, Caballero BH, Lawrence RS, Cheskin LJ, Wang Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. The American Journal of Clinical Nutrition. 2008;87:1914–25. doi: 10.1093/ajcn/87.6.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17:143–52. [PubMed] [Google Scholar]
- 10.Woodside JV, McCall D, McGartland C, Young S. Micronutrients: dietary intake v supplement use. Proc Nutrition Society. 2005;64:543–53. doi: 10.1079/pns2005464. [DOI] [PubMed] [Google Scholar]
- 11.Khattar RS, Swales JD, Senior R, Lahiri A. Racial variation in cardiovascular morbidity and mortality in essential hypertension. Heart. 2000;83:267–71. doi: 10.1136/heart.83.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flack JM, Ferdinand KC, Nasser SA. Epidemiology of Hypertension and Cardiovascular Disease in African Americans. The Journal of Clinical Hypertension. 2003;5:5–11. doi: 10.1111/j.1524-6175.2003.02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falkner B, Sherif K, Sumner A, Kushner H. Hyperinsulinism and sex hormones in young adult African Americans. Metabolism: Clinical & Experimental. 1999;48:107–12. doi: 10.1016/s0026-0495(99)90018-5. [DOI] [PubMed] [Google Scholar]
- 14.He J, Klag MJ, Caballero B, Appel LJ, Charleston J, Whelton PK. Plasma insulin levels and incidence of hypertension in African Americans and whites. Arch Intern Med. 1999;159:498–503. doi: 10.1001/archinte.159.5.498. [DOI] [PubMed] [Google Scholar]
- 15.Binder EB, Holsboer F. Low Cortisol and Risk and Resilience to Stress-Related Psychiatric Disorders. Biol Psychiatry. 2012;71:282–83. doi: 10.1016/j.biopsych.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Goodman E, Must A, Daniels SR, Dolan LM, Goodman E, Must A, Daniels SR, Dolan LM. Hostility and adiposity mediate disparities in insulin resistance among adolescents and young adults. J Pediatr. 2010;157:572–7. doi: 10.1016/j.jpeds.2010.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jara L, Medina G, Saavedra M, Vera-Lastra O, Navarro C. Prolactin and autoimmunity. Clin Rev Allergy Immunol. 2009;40:50–59. doi: 10.1007/s12016-009-8185-3. [DOI] [PubMed] [Google Scholar]
- 18.Arcidiacono B, Iiritano S, Nocera A, Possidente K, Nevolo MT, Ventura V, Foti D, Chiefari E, Brunetti A. Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res. 2012;2012:789174. doi: 10.1155/2012/789174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makino T, Noguchi Y, Yoshikawa T, Doi C, Nomura K. Circulating interleukin 6 concentrations and insulin resistance in patients with cancer. Br J Surg. 1998;85:1658–62. doi: 10.1046/j.1365-2168.1998.00938.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsugane S, Inoue M. Insulin resistance and cancer: Epidemiological evidence. Cancer Science. 2010;101:1073–79. doi: 10.1111/j.1349-7006.2010.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodwin P, Ennis M, Bahl M, Fantus IG, Pritchard K, Trudeau M, Koo J, Hood N. High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res Treat. 2009;114:517–25. doi: 10.1007/s10549-008-0019-0. [DOI] [PubMed] [Google Scholar]
- 22.Albanes D, Weinstein SJ, Wright ME, Männistö S, Limburg PJ, Snyder K, Virtamo J. Serum Insulin, Glucose, Indices of Insulin Resistance, and Risk of Prostate Cancer. J Natl Cancer Inst. 2009;101:1272–79. doi: 10.1093/jnci/djp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harle P, Straub R, Wiest R, Mayer A, Scholmerich J, Atzeni F, Carrabba M, Cutolo M, Sarzi-Puttini P. Increase of sympathetic outflow measured by neuropeptide Y and decrease of the hypothalamic-pituitary-adrenal axis tone in patients with systemic lupus erythematosus and rheumatoid arthritis: another example of uncoupling of response systems. Ann Rheum Dis. 2006;65:51–56. doi: 10.1136/ard.2005.038059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindquist CH, Gower BA, Goran MI. Role of dietary factors in ethnic differences in early risk of cardiovascular disease and type 2 diabetes. The American Journal of Clinical Nutrition. 2000;71:725–32. doi: 10.1093/ajcn/71.3.725. [DOI] [PubMed] [Google Scholar]
- 25.Kromhout D. Diet and cardiovascular diseases. J Nutr Health Aging. 2001;5:144–9. [PubMed] [Google Scholar]
- 26.Fung TT, Schulze M, Manson JE, Willett WC, Hu FB. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med. 2004;164:2235–40. doi: 10.1001/archinte.164.20.2235. [DOI] [PubMed] [Google Scholar]
- 27.Knekt P, Ritz J, Pereira MA, O’Reilly EJ, Augustsson K, Fraser GE, Goldbourt U, Heitmann BL, Hallmans G, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Rimm EB, Ascherio A. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. The American Journal of Clinical Nutrition. 2004;80:1508–20. doi: 10.1093/ajcn/80.6.1508. [DOI] [PubMed] [Google Scholar]
- 28.Robins SJ, Lyass A, Zachariah JP, Massaro JM, Vasan RS. Insulin Resistance and the Relationship of a Dyslipidemia to Coronary Heart Disease. Arterioscler Thromb Vasc Biol. 2011;31:1208–14. doi: 10.1161/ATVBAHA.110.219055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford ES, Will JC, Bowman BA, Narayan KMV. Diabetes Mellitus and Serum Carotenoids: Findings from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 1999;149:168–76. doi: 10.1093/oxfordjournals.aje.a009783. [DOI] [PubMed] [Google Scholar]
- 30.Strickland PL, Deakin JFW, Percival C, Dixon J, Gater RA, Goldberg DP. Bio-social origins of depression in the community: Interactions between social adversity, cortisol and serotonin neurotransmission. The British Journal of Psychiatry. 2002;180:168–73. doi: 10.1192/bjp.180.2.168. [DOI] [PubMed] [Google Scholar]
- 31.Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, Dorgan JF, Franke AA, Gao Y-t, Goodman MT, Hallmans G, Helzlsouer KJ, Hoffman-Bolton J, Hultén K, Sesso HD, Sowell AL, Tamimi RM, Toniolo P, Wilkens LR, Winkvist A, Zeleniuch-Jacquotte A, Zheng W, Hankinson SE. Circulating Carotenoids and Risk of Breast Cancer: Pooled Analysis of Eight Prospective Studies. J Natl Cancer Inst. 2012;104:1905–16. doi: 10.1093/jnci/djs461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aune D, Chan DS, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, Norat T. Dietary compared with blood concentrations of carotenoids and breast cancer risk: a systematic review and meta-analysis of prospective studies. The American Journal of Clinical Nutrition. 2012;96:356–73. doi: 10.3945/ajcn.112.034165. [DOI] [PubMed] [Google Scholar]
- 33.Kabat GC, Kim MY, Sarto GE, Shikany JM, Rohan TE. Repeated measurements of serum carotenoid, retinol and tocopherol levels in relation to colorectal cancer risk in the Women’s Health Initiative. Eur J Clin Nutr. 2012;66:549–54. doi: 10.1038/ejcn.2011.207. [DOI] [PubMed] [Google Scholar]
- 34.Ollberding NJ, Maskarinec G, Conroy SM, Morimoto Y, Franke AA, Cooney RV, Wilkens LR, Le Marchand L, Goodman MT, Hernandez BY, Henderson BE, Kolonel LN. Prediagnostic circulating carotenoid levels and the risk of non-Hodgkin lymphoma: the Multiethnic Cohort. Blood. 2012;119:5817–23. doi: 10.1182/blood-2012-02-413609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karppi J, Kurl S, Laukkanen JA, Kauhanen J. Serum β-Carotene in Relation to Risk of Prostate Cancer: The Kuopio Ischaemic Heart Disease Risk Factor Study. Nutr Cancer. 2012;64:361–67. doi: 10.1080/01635581.2012.658949. [DOI] [PubMed] [Google Scholar]
- 36.Rimm EB, Stampfer MJ, Ascherio A, Giovanucci E, Colditz GA, Willet WC. Vitamin E consumption and the risk of coronary heart disease in men. New England Journal Medicine. 1993;328:1450–56. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 37.Oswald LM, Zandi P, Nestadt G, Potash JB, Kalaydjian AE, Wand GS. Relationship between Cortisol Responses to Stress and Personality. Neuropsychopharmacology. 2006;31:1583–91. doi: 10.1038/sj.npp.1301012. [DOI] [PubMed] [Google Scholar]
- 38.Hak AE, Stampfer MJ, Campos H, Sesso HD, Gaziano JM, Willett W, Ma J. Plasma carotenoids and tocopherols and risk of myocardial infarction in a low-risk population of US male physicians. Circulation. 2003;108:802–7. doi: 10.1161/01.CIR.0000084546.82738.89. [see comment] [DOI] [PubMed] [Google Scholar]
- 39.Beaudreau SA, Spira AP, Stewart A, Kezirian EJ, Lui L-Y, Ensrud K, Redline S, Ancoli-Israel S, Stone KL. Validation of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older black and white women. Sleep Medicine. 2012;13:36–42. doi: 10.1016/j.sleep.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knight JM, Avery EF, Janssen I, Powell LH. Cortisol and Depressive Symptoms in a Population-Based Cohort of Midlife Women. Psychosom Med. 2010;72:855–61. doi: 10.1097/PSY.0b013e3181f4ab87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banim PJR, Luben R, McTaggart A, Welch A, Wareham N, Khaw K-T, Hart AR. Dietary antioxidants and the aetiology of pancreatic cancer: a cohort study using data from food diaries and biomarkers. Gut. 2012 doi: 10.1136/gutjnl-2011-301908. [DOI] [PubMed] [Google Scholar]
- 42.Park Y, Spiegelman D, Hunter D, Albanes D, Bergkvist L, Buring J, Freudenheim J, Giovannucci E, Goldbohm RA, Harnack L, Kato I, Krogh V, Leitzmann M, Limburg P, Marshall J, McCullough M, Miller A, Rohan T, Schatzkin A, Shore R, Sieri S, Stampfer M, Virtamo J, Weijenberg M, Willett W, Wolk A, Zhang S, Smith-Warner S. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes & Control. 2010;21:1745–57. doi: 10.1007/s10552-010-9549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beydoun MA, Canas JA, Beydoun HA, Chen X, Shroff MR, Zonderman AB. Serum Antioxidant Concentrations and Metabolic Syndrome Are Associated among U.S. Adolescents in Recent National Surveys. The Journal of Nutrition. 2012;142:1693–704. doi: 10.3945/jn.112.160416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beydoun MA, Shroff MR, Chen X, Beydoun HA, Wang Y, Zonderman AB. Serum Antioxidant Status Is Associated with Metabolic Syndrome among U.S. Adults in Recent National Surveys. The Journal of Nutrition. 2011;141:903–13. doi: 10.3945/jn.110.136580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Gaziano JM, Norkus EP, Buring JE, Sesso HD. Associations of plasma carotenoids with risk factors and biomarkers related to cardiovascular disease in middle-aged and older women. The American Journal of Clinical Nutrition. 2008;88:747–54. doi: 10.1093/ajcn/88.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ford ES, Liu S, Mannino DM, Giles WH, SMith SJ. C-reactive protein concentration and concentrations of blood vitamins, carotenoids, and selenium among United State adults. Euro J Clinical Nutrition. 2003;57:1157–63. doi: 10.1038/sj.ejcn.1601667. [DOI] [PubMed] [Google Scholar]
- 47.Williams SK, Fiscella K, Winters P, Martins D, Ogedegbe G. Association of racial disparities in the prevalence of insulin resistance with racial disparities in vitamin D levels: National Health and Nutrition Examination Survey (2001-2006) Nutrition Research. 2013;33:266–71. doi: 10.1016/j.nutres.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muñoz KA, Krebs-Smith SM, Ballard-Barbash R, Cleveland LE. Food Intakes of US Children and Adolescents Compared With Recommendations. Pediatrics. 1997;100:323–29. doi: 10.1542/peds.100.3.323. [DOI] [PubMed] [Google Scholar]
- 49.Greeson J, Lewis JG, Achanza K, Zimmerman E, Young KH, Suarez EC. Stress-induced changes in the expression of monocytic β2-integrins: The impact of arousal of negative affect and adrenergic responses to the Anger Recall Interview. Brain, Behavior, Immunity. 2008 doi: 10.1016/j.bbi.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suarez EC, Boyle SH, Lewis JG, Hall RP, Young KH. Increases in stimulated secretion of proinflammatory cytokines by blood monocytes following arousal of negative affect: The role of insulin resistance as moderator. Brain, Behavior, and Immunity. 2006;20:331–38. doi: 10.1016/j.bbi.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced cytokine and chemokine expressions by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29:1119–28. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Suarez EC. C-reactive protein is associated with psychologic risk factors of cardiovascular disease in apparently healthy adults. Psychosom Med. 2004;66:684–91. doi: 10.1097/01.psy.0000138281.73634.67. [DOI] [PubMed] [Google Scholar]
- 53.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: Evidence for gender disparity. Brain, Behavior, and Immunity. 2008;22:960–68. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pryor WA. Vitamin E and heart disease: basic science to clinical intervention trials. Free Radic Biol Med. 2000;28:141–64. doi: 10.1016/s0891-5849(99)00224-5. [DOI] [PubMed] [Google Scholar]
- 55.Singhal S, Gupta R, Goyle A. Comparison of antioxidant efficacy of vitamin E, vitamin C, vitamin A and fruits in coronary heart disease: a controlled trial. J Assoc Physicians India. 2001;49:327–31. [PubMed] [Google Scholar]
- 56.Osganian SK, Stampfer MJ, Rimm E, Spiegelman D, Hu FB, Manson JE, Willett WC. Vitamin C and risk of coronary heart disease in women. J Am Coll Cardio. 2003;42:246–52. doi: 10.1016/s0735-1097(03)00575-8. [see comment] [DOI] [PubMed] [Google Scholar]
- 57.Mayer-Davis EJ, Costacou T, King I, Zaccaro DJ, Bell RA. Plasma and dietary vitamin E in relation to incidence of type 2 diabetes: The Insulin Resistance and Atherosclerosis Study (IRAS) Diabetes Care. 2002;25:2172–77. doi: 10.2337/diacare.25.12.2172. [DOI] [PubMed] [Google Scholar]
- 58.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–19. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 59.Haffner SM. Epidemiology of Type 2 Diabetes: Risk Factors. Diabetes Care. 1998;21:C3–C6. doi: 10.2337/diacare.21.3.c3. [DOI] [PubMed] [Google Scholar]
- 60.Haffner SM. Epidemiology of insulin resistence and its relation to coronary artery disease. Am J Cardiol. 1999;84:11J–14J. doi: 10.1016/s0002-9149(99)00351-3. [DOI] [PubMed] [Google Scholar]
- 61.Sasaki Y, Takeda H, Sato T, Orii T, Nishise S, Nagino K, Iwano D, Yaoita T, Yoshizawa K, Saito H, Tanaka Y, Kawata S. Serum Interleukin-6, Insulin, and HOMA-IR in Male Individuals with Colorectal Adenoma. Clin Cancer Res. 2012;18:392–99. doi: 10.1158/1078-0432.CCR-11-0896. [DOI] [PubMed] [Google Scholar]
- 62.Capasso I, Esposito E, Pentimalli F, Montella M, Crispo A, Maurea N, D’Aiuto M, Fucito A, Grimaldi M, Cavalcanti E, Esposito G, Brillante G, Lodato S, Pedicini T, D’Aiuto G, Ciliberto G, Giordano A. Homeostasis model assessment to detect insulin resistance and identify patients at high risk of breast cancer development: National Cancer Institute of Naples experience. J Exp Clin Cancer Res. 2013;32:14. doi: 10.1186/1756-9966-32-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yun SJ, Min B-D, Kang H-W, Shin K-S, Kim T-H, Kim W-T, Lee SC, Kim W-J. Elevated Insulin and Insulin Resistance Are Associated with the Advanced Pathological Stage of Prostate Cancer in Korean Population. J Korean Med Sci. 2012;27:1079–84. doi: 10.3346/jkms.2012.27.9.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107:443–47. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- 65.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261–6. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bialostosky K, Wright JD, Kennedy-Stephenson J, McDowell M, Johnson CL. Dietary intake of macronutrients, micronutrients, and other dietary constituents: United States 1988-94. Vital and health statistics Series 11, Data from the national health survey. 2002:1–158. [PubMed] [Google Scholar]
- 67.Kritchevsky SB, Bush AJ, Pahor M, Gross MD. Serum carotenoids and markers of inflammation in nonsmokers. Am J Epidemiol. 2000;152:1065–71. doi: 10.1093/aje/152.11.1065. [DOI] [PubMed] [Google Scholar]
- 68.Hu P, Reuben DB, Crimmins EM, Harris TB, Huang MH, Seeman TE. The effects of serum beta-carotene concentration and burden of inflammation on all-cause mortality risk in high-functioning older persons: MacArthur studies of successful aging. Journals of Gerontology Series A Biological Sciences & Medical Sciences. 2004;59:849–54. doi: 10.1093/gerona/59.8.m849. [DOI] [PubMed] [Google Scholar]
- 69.de Oliveira Otto MCC, Alonso A, Lee D-H, Delclos GL, Jenny NS, Jiang R, Lima JA, Symanski E, Jacobs DR, Nettleton JA. Dietary Micronutrient Intakes Are Associated with Markers of Inflammation but Not with Markers of Subclinical Atherosclerosis. The Journal of Nutrition. 2011;141:1508–15. doi: 10.3945/jn.111.138115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arcury TA, Preisser JS, Gesler WM, Powers JM. Access to Transportation and Health Care Utilization in a Rural Region. The Journal of Rural Health. 2005;21:31–38. doi: 10.1111/j.1748-0361.2005.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 71.Raison Cl, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suzuki K, Ito Y, Inoue T, Hamajima N. Inverse association of serum carotenoids with prevalence of metabolic syndrome among Japanese. Clin Nutr. 2011;30:369–75. doi: 10.1016/j.clnu.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Matsumoto H, Ando F, Shimokata H, Yano M. Associations of serum carotenoid concentrations with the metabolic syndrome: interaction with smoking. Br J Nutr. 2008;100:1297–306. doi: 10.1017/S0007114508978302. [DOI] [PubMed] [Google Scholar]
- 74.Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Shavit Y, Ovadia H, Weidenfeld J, Morag A, Newman ME, PollmÄCher T. Illness, Cytokines, and Depression. Ann N Y Acad Sci. 2000;917:478–87. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 75.Chai W, Conroy SM, Maskarinec G, Franke AA, Pagano IS, Cooney RV. Associations between obesity and serum lipid-soluble micronutrients among premenopausal women. Nutrition Research. 2010;30:227–32. doi: 10.1016/j.nutres.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson K, Gullone E. The relationship between personality and affect over the lifespan. Personality and Individual Differences. 1999;27:1141–56. [Google Scholar]
- 77.Fletcher RH, Fairfield KM. Vitamins for chronic disease prevention in adults: Clinical applications. JAMA. 2002;287:3127–29. doi: 10.1001/jama.287.23.3127. [DOI] [PubMed] [Google Scholar]
- 78.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–23. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 79.Opara EC. Oxidative stress, micronutrients, diabetes mellitus and its complications. The Journal of the Royal Society for the Promotion of Health. 2002;122:28–34. doi: 10.1177/146642400212200112. [DOI] [PubMed] [Google Scholar]
- 80.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. Journal of Carcinogenesis. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Straub R, Buttgereit F, Cutolo M. Alterations of the hypothalamic-pituitary-adrenal axis in systemic immune diseases: a role for misguided energy regulation. Clin Rheumatol. 2011;29:S23–S31. [PubMed] [Google Scholar]
- 82.Lobo GP, Amengual J, Li HNM, Golczak M, Bonet ML, Palczewski K, von Lintig J. β,β-Carotene Decreases Peroxisome Proliferator Receptor γ Activity and Reduces Lipid Storage Capacity of Adipocytes in a β,β-Carotene Oxygenase 1-dependent Manner. J Biol Chem. 2010;285:27891–99. doi: 10.1074/jbc.M110.132571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferrucci L, Perry JRB, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, Fried LP, Albanes D, Corsi A-M, Cherubini A, Guralnik J, Bandinelli S, Singleton A, Virtamo J, Walston J, Semba RD, Frayling TM. Common Variation in the β-Carotene 15,15′-Monooxygenase 1 Gene Affects Circulating Levels of Carotenoids: A Genome-wide Association Study. The American Journal of Human Genetics. 2009;84:123–33. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lietz G, Oxley A, Leung W, Hesketh J. Single nucleotide polymorphisms upstream from the beta-carotene 15,15′-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr. 2012;142:161S–5S. doi: 10.3945/jn.111.140756. [DOI] [PubMed] [Google Scholar]
- 85.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A. CMO1 Deficiency Abolishes Vitamin A Production from β-Carotene and Alters Lipid Metabolism in Mice. J Biol Chem. 2007;282:33553–61. doi: 10.1074/jbc.M706763200. [DOI] [PubMed] [Google Scholar]
- 86.Paul NA, Stanton SJ, Greeson JM, Smoski MJ, Wang L. Psychological and neural mechanisms of trait mindfulness in reducing depression vulnerability. Social Cognitive and Affective Neuroscience. 2013;8:56–64. doi: 10.1093/scan/nss070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arab L, Cambou MC, Craft N, Wesseling-Perry K, Jardack P, Ang A. Racial differences in correlations between reported dietary intakes of carotenoids and their concentration biomarkers. The American Journal of Clinical Nutrition. 2011 doi: 10.3945/ajcn.110.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]