Abstract
Objectives
To describe the CD4 cell count at the start of combination antiretroviral therapy (cART) in low-income (LIC), lower middle-income (LMIC), upper middle-income (UMIC) and high-income (HIC) countries.
Methods
Patients aged ≥16 years starting cART in a clinic participating in a multi-cohort collaboration spanning six continents (International epidemiological Databases to Evaluate AIDS and ART Cohort Collaboration) were eligible. Multi-level linear regression models were adjusted for age, gender and calendar year; missing CD4 counts were imputed.
Findings
379,865 patients from nine LIC, four LMIC, four UMIC and six HIC were included. In LIC the median CD4 cell count at cART initiation increased by 83% from 80 to 145 cells/μl between 2002 and 2009. Corresponding increases in LMIC, UMIC and HIC were from 87 to 155 cells/μl (76% increase), 88 to 135 cells/μl (53%) and 209 to 274 cells/μl (31%). In 2009, compared to LIC, median counts were 13 cells/μl (95% CI -56 to +30) lower in LMIC, 22 cells/μl (-62 to +18) lower in UMIC and 112 /μl (+75 to +149) higher in HIC. They were 23 cells/μl (95% CI +18 to +28) higher in women than men. Median counts were 88 cells/μl (95% CI +35 to +141) higher in countries with an estimated national cART coverage >80%, compared to countries with <40% coverage.
Conclusions
Median CD4 cell counts at start of cART increased 2000-2009 but remained below 200 cells/μl in LIC and MIC and below 300 cells/μl in HIC. Earlier start of cART will require substantial efforts and resources globally.
Introduction
The prognosis of HIV-positive patients has dramatically improved with the advent, in 1996, of combination antiretroviral therapy (cART) [1, 2]. Suppressed viral replication allows reconstitution of the immune system: peripheral CD4 cell counts increase rapidly first from redistribution from lymphoid tissues, and then gradual by de novo synthesis [3, 4]. Since 2002, the Global Fund for Tuberculosis, AIDS and Malaria (GFTAM), US President’s Emergency Plan for AIDS Relief (PEPFAR) and other funders have sharply increased global cART availability. The World Health Organization (WHO) estimated, by 2010, that 6.6 million of the 15 million who needed cART in low and middle income countries had access [5].
When to initiate cART to maximize the benefit of therapy has been debated for years [6]. Benefits of early initiation, at high CD4 cell counts, must be balanced against drug toxicities and the potential for drug resistance. Conversely, starting therapy late, as measured clinically or by CD4 count, is associated with poorer prognosis and increased mortality [7]. A sub-study of the Strategies for Management of Antiretroviral Therapy (SMART) trial showed that delaying cART until the count fell below 250 cells/μl more than tripled the rate of AIDS or death compared to starting above 350 cells/μl [8]. Analyses that combined data from cohort studies also indicated that starting cART above 350 CD4 cells/μl is beneficial, and some, but not all, showed benefit with a threshold of 500 cells/μl [9-11]. The START (NCT00821171) and TEMPRANO (NCT00495651) trials will provide further data on the efficacy of early versus late initiation of cART.
However, many patients enter care at late. An analysis of treatment programs in 12 countries in sub-Saharan Africa, South America and Asia showed that while CD4 cell counts at initiation increased from 2001 to 2005/2006, most started well below recommended thresholds [12]. Similarly, a United States of America (USA) and Canada cohort showed that median CD4 cell count at first presentation for HIV care was 317 cells/μl in 2007: more than half of patients initiated therapy below 350 cells/μl [13]. A recent Latin American study reported that the percentage of patients initiating cART late ranged from 56% in Argentina to 91% in Honduras [14].
Early initiation of cART is recognized as having a broader role in HIV prevention [15]. Already established as a means to prevent mother-to-child transmission [5], the HIV Prevention Trials Network (HPTN) 052 trial found cART reduced heterosexual HIV transmission by 96% between discordant couples [16]. Combined with other proven prevention tools, immediate or early cART might contribute to achieving the goal of an AIDS-free generation [17].
We examined trends and determinants of the CD4 cell count at cART initiation in patients starting therapy between 2002 and 2010 in low, middle and high income countries by combining data from two HIV cohort consortia, which together span six continents.
Methods
Data sources
The International epidemiological Databases to Evaluate AIDS (IeDEA) is a global consortium structured through regional centres to pool clinical and epidemiological data on HIV-positive individuals, particularly patients on cART. The seven regions included in IeDEA are North America, Caribbean/Central and South America, Asia/Pacific, East Africa, West Africa, Central Africa and Southern Africa. Regional cohorts of IeDEA have been described in detail elsewhere [18-21]. The European cohorts of the ART Cohort Collaboration, a network of cohort studies of patients on cART in high-income countries, were also included [7]. Pooling of data and their use in collaborative analyses were approved by local ethics committees and institutional review boards. For the present study, regional centers sent de-identified data to the University of Bern, Switzerland for cleaning and analysis.
Inclusion criteria and definitions
Patients aged ≥16 years at cART initiation were eligible. cART was defined as a regimen of at least three antiretroviral drugs, typically from two drug classes. Baseline CD4 cell count was defined as the count nearest to the date of cART start with a window of 6 months before to 1 month after starting. CD4 cell counts above 5,000 cells/μl (i.e. >3 times above the upper reference range in Caucasians [22]) were considered invalid and set to missing. Countries were grouped according to the World Bank classification of Gross National Income per Capita 2010 as low-income (LIC, US$1,005 or less per year); lower middle-income (LMIC, US$1,006 to 3,975 per year); upper middle-income (UMIC, US$3,976 to 12,275 per year) and high-income (HIC, US$12,276 or more per year) [23]. Data on national cART coverage for 2009 (based on the WHO 2006 treatment guidelines [24]) were obtained from the 2010 progress report on the Global HIV/AIDS response published by WHO [25] for LIC and MIC.
Statistical analysis
Descriptive analyses were stratified by country, gender and World Bank country income group. CD4 cell counts at cART start and other baseline characteristics were summarized as medians with interquartile ranges (IQR) or numbers with percentages. To address the problem of generalizing data from a small sample to an entire country, further analyses were restricted to countries that contributed at least 500 patients with CD4 cell counts from two or more sites and had observations after 2007.
Missing CD4 counts were multiply imputed using predictive mean matching and chained equations stratified by gender, age and country income level. Technical details on the multiple imputation are provided in the supplementary materials. All analyses were performed both for the imputed data and for the complete case dataset. To assess trends and examine individual-level and country-level predictors of median baseline CD4 cell counts, we aggregated the data by sex, age, year and country. We then fitted three types of weighted linear regression models. First, a simple linear regression estimated gender-specific annual changes in median baseline CD4 cell counts by country (model 1). In sensitivity analyses, we weighted individual country data to create a dataset that was representative, within each income group, of the number of patients on cART in each country in 2009, as estimated by WHO [5]. The derivation of the weighting factors is shown in supplementary Table S1.
Second, a weighted mixed-effects linear regression was used to estimate the median CD4 cell counts at start of cART in 2009 and to examine the influence of age, sex, country income level and, in an analysis restricted to LIC and MIC, of national cART coverage (model 2). Age, calendar year, gender and country income level were entered as fixed effects and country as random effect. The third model included calendar year, gender and country income level and was used to model median CD4 cell count trends between 2002 and 2010 (model 3). Finally, the proportions of patients starting cART with CD4 cell counts below 50, 100 and 200 cells/μl were analyzed using generalized linear mixed effects models. Age, calendar year, gender and country income level were entered as fixed effects and intercept and slope as random effects, by country. Data were analysed using Stata 12.0 (Stata Corporation, College Station, Texas, USA) and R 2.12 (The R Development Core Team, Vienna, Austria).
Results
Descriptive analyses
Data from 492,915 patients ≥16 years of age who started cART in 48 countries were submitted to the data center (Figure 1). Among 437,230 ART-naïve patients with known age, gender and start date, 309,564 (63%) had a CD4 cell count at start of cART. Compared to the 127,666 patients without a CD4 cell count, patients with counts at cART initiation were younger, more likely to be male, and more likely to be from a high income country. Importantly, those with CD4 cell counts were less likely to have advanced disease (WHO stage III/IV) than patients without CD4 cell counts (Table S2). Only 4.6% (20,217 patients) had an earlier CD4 cell count, 6 months or more before starting ART.
Figure 1.
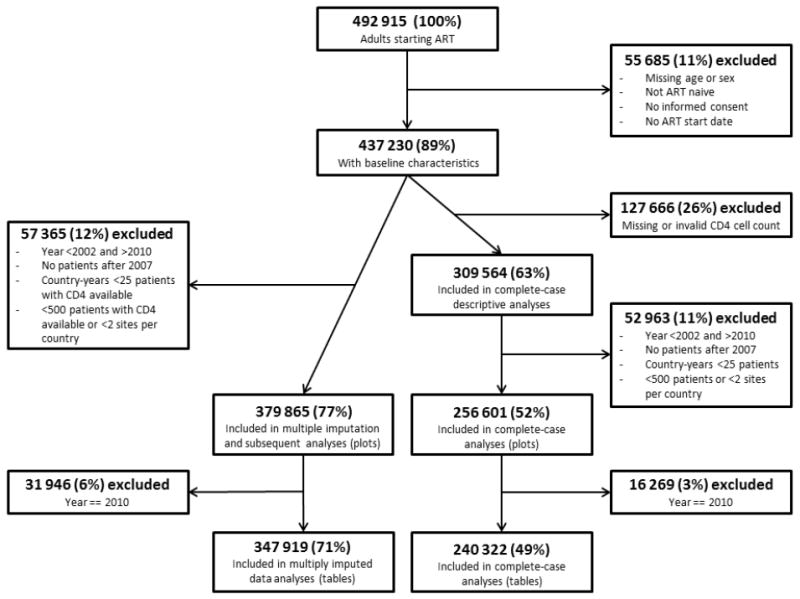
Flow chart of patients included and excluded from analyses.
The number of included patients from each country varied from 60 from Japan to 147,029 from Zambia (Table 1). There were 12 countries with up to 500 patients, 23 with 501 to 5,000 patients, four with 5,001 to 10,000 patients, seven with 10,001 to 50,000 and two with more than 50,000 patients (Figure S1). The percentage of female patients ranged from 4% in Taiwan to 73% in Burundi, and median age ranged from 30.9 years in Indonesia to 41.5 years in Nigeria (Table 1). The median year of cART initiation ranged from 2000 in Australia and Italy to 2009 in the Philippines. The median CD4 cell count at cART-initiation for the entire study period ranged from 56 cells/μl in Indonesia to 290 cells/μl in Australia.
Table 1.
Characteristics of patients starting cART by country and World Bank income groups.
| Country | No. of patients
|
Median age in years (IQR)
|
Median calendar year of starting cART (IQR)
|
Median CD4 cell count at start of cART in cells/μl (IQR)
|
||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | Women | Men | |
|
Low income
| ||||||||
| Benin | 413 | 298 | 33.8 | 40.1 | 2005 | 2005 | 119 | 90 |
|
| ||||||||
| Burkina Faso | 822 | 348 | 35.7 | 41.3 | 2007 | 2006 | 182 | 137.5 |
|
| ||||||||
| Burundi | 332 | 139 | 36.4 | 43.0 | 2009 | 2009 | 209.5 | 157 |
|
| ||||||||
| Cambodia | 107 | 110 | 33.8 | 36.6 | 2005 | 2005 | 103 | 70.5 |
|
| ||||||||
| DR Congo | 1828 | 800 | 38.5 | 43.3 | 2008 | 2008 | 145 | 139.5 |
|
| ||||||||
| Gambia | 140 | 77 | 37.4 | 45.0 | 2006 | 2006 | 160 | 130 |
|
| ||||||||
| Haiti | 780 | 668 | 38.0 | 40.0 | 2004 | 2004 | 115.5 | 92.5 |
|
| ||||||||
| Kenya | 19454 | 10571 | 35.2 | 39.6 | 2007 | 2006 | 135 | 109 |
|
| ||||||||
| Malawi | 4337 | 3365 | 33.3 | 37.2 | 2008 | 2008 | 150 | 122 |
|
| ||||||||
| Mali | 1020 | 650 | 32.7 | 40.2 | 2006 | 2006 | 134 | 101 |
|
| ||||||||
| Mozambique | 284 | 177 | 32.1 | 37.5 | 2008 | 2008 | 242 | 204 |
|
| ||||||||
| Rwanda | 1500 | 521 | 33.8 | 38.0 | 2006 | 2007 | 218 | 183 |
|
| ||||||||
| Tanzania | 2168 | 1050 | 36.5 | 41.4 | 2007 | 2007 | 107 | 106 |
|
| ||||||||
| Uganda | 4229 | 2552 | 34.2 | 38.3 | 2007 | 2007 | 130 | 114 |
|
| ||||||||
| Zimbabwe | 3112 | 1388 | 37.1 | 39.7 | 2009 | 2009 | 133 | 96 |
|
| ||||||||
| Overall | 40526 | 22714 | 35.1 (29 - 41) | 39.4 (33 - 46) | 2007 (2006 - 2008) | 2007 (2006 - 2008) | 139 (65 - 213) | 113 (40 - 186) |
|
| ||||||||
|
Lower middle income
| ||||||||
| Cameroon | 1821 | 829 | 33.9 | 39.3 | 2009 | 2009 | 158 | 139 |
|
| ||||||||
| Côte d’Ivoire | 8531 | 4857 | 34.5 | 41.1 | 2006 | 2005 | 156 | 125 |
|
| ||||||||
| Honduras | 101 | 159 | 35.0 | 38.0 | 2005 | 2005 | 120 | 98 |
|
| ||||||||
| India | 91 | 364 | 31.9 | 34.1 | 2004 | 2003 | 177 | 129.5 |
|
| ||||||||
| Indonesia | 92 | 227 | 29.1 | 31.4 | 2008 | 2008 | 89.5 | 45 |
|
| ||||||||
| Nigeria | 4728 | 2678 | 41.2 | 41.3 | 2006 | 2006 | 162 | 127 |
|
| ||||||||
| Philippines | 27 | 234 | 39.3 | 30.5 | 2008 | 2009 | 186 | 180 |
|
| ||||||||
| Senegal | 183 | 126 | 35.4 | 42.0 | 2004 | 2004.5 | 122 | 97.5 |
|
| ||||||||
| Zambia | 50096 | 32054 | 33.5 | 37.3 | 2008 | 2008 | 150 | 129 |
|
| ||||||||
| Overall | 65670 | 41528 | 34.5 (28 - 41) | 38.4 (33 - 44) | 2007 (2006 - 2008) | 2007 (2006 - 2008) | 152 (76 - 228) | 128 (55 - 201) |
|
| ||||||||
|
Upper middle income
| ||||||||
| Argentina | 208 | 479 | 34.0 | 36.0 | 2004 | 2004 | 181 | 148 |
|
| ||||||||
| Botswana | 549 | 367 | 34.3 | 38.4 | 2003 | 2003 | 140 | 116 |
|
| ||||||||
| Brazil | 371 | 740 | 37.0 | 36.1 | 2007 | 2007 | 208 | 186 |
|
| ||||||||
| Chile | 56 | 383 | 34.1 | 36.6 | 2003 | 2003 | 138 | 112 |
|
| ||||||||
| China | 124 | 409 | 38.9 | 39.6 | 2005 | 2007 | 169.5 | 91 |
|
| ||||||||
| Malaysia | 111 | 380 | 34.1 | 36.2 | 2007 | 2008 | 159 | 113 |
|
| ||||||||
| Mexico | 49 | 349 | 35.0 | 35.0 | 2004 | 2004 | 131 | 84 |
|
| ||||||||
| Peru | 208 | 495 | 32.0 | 35.0 | 2005 | 2005 | 85.5 | 74 |
|
| ||||||||
| South Africa | 32920 | 21673 | 33.6 | 39.0 | 2007 | 2007 | 124 | 111 |
|
| ||||||||
| Thailand | 731 | 907 | 35.7 | 36.3 | 2007 | 2008 | 95 | 89 |
|
| ||||||||
| Overall | 35327 | 26182 | 33.7 (28 - 39) | 38.5 (32 - 45) | 2007 (2006 - 2008) | 2006 (2004 - 2008) | 124 (60 - 188) | 111 (42 - 180) |
|
| ||||||||
|
High income
| ||||||||
| Australia | 74 | 1261 | 33.7 | 39.1 | 2001 | 1999 | 280 | 290 |
|
| ||||||||
| Canada | 850 | 3651 | 36.0 | 41.0 | 2002 | 2002 | 210 | 220 |
|
| ||||||||
| France | 10023 | 20739 | 34.3 | 38.8 | 2003 | 2001 | 237 | 233 |
|
| ||||||||
| Germany | 269 | 955 | 33.9 | 38.5 | 2004 | 2002 | 177 | 173 |
|
| ||||||||
| Italy | 915 | 2312 | 35.4 | 37.6 | 2000 | 2000 | 265 | 244 |
|
| ||||||||
| Japan | 4 | 56 | 46.1 | 38.1 | 2006 | 2007 | 236 | 253.5 |
|
| ||||||||
| Netherlands | 1617 | 5125 | 32.4 | 39.4 | 2003 | 2002 | 210 | 189 |
|
| ||||||||
| Singapore | 19 | 83 | 33.9 | 41.4 | 2004 | 2003 | 134 | 57 |
|
| ||||||||
| South Korea | 10 | 182 | 41.2 | 38.0 | 2003.5 | 2006 | 123 | 222.5 |
|
| ||||||||
| Spain | 2596 | 8406 | 34.0 | 36.8 | 2002 | 2003 | 224 | 201 |
|
| ||||||||
| Switzerland | 1414 | 3124 | 33.9 | 38.4 | 2001 | 2001 | 201 | 205 |
|
| ||||||||
| Taiwan | 8 | 186 | 32.2 | 32.9 | 2001.5 | 2003 | 132 | 225 |
|
| ||||||||
| UK | 369 | 1016 | 34.3 | 36.9 | 2003 | 2002 | 158 | 206 |
|
| ||||||||
| USA | 3362 | 8991 | 39.0 | 40.0 | 2003 | 2003 | 241.5 | 215 |
|
| ||||||||
| Overall | 21530 | 56087 | 34.9 (28 - 41) | 38.9 (33 - 45) | 2002 (2000 - 2004) | 2002 (1999 - 2005) | 230 (110 - 350) | 217 (89 - 345) |
Results from descriptive analyses based on 309,564 patients.
Regression models
A total of 379,865 patients from 23 countries were included in the regression models, including 86,390 patients from nine LIC (23%), 176,858 from four LMIC (47%), 82,152 from four UMIC (22%) and 34,465 from six HIC (9%). The Democratic Republic of the Congo, Kenya, Malawi and Mali were overrepresented when compared to the WHO estimates of the number of patients on cART in the LIC included in our study. In contrast, Tanzania and Zimbabwe were underrepresented. The group of LMIC countries was dominated by Zambia, and that of UMIC countries by South Africa. Among HIC, France contributed almost half of all patients whereas the United States and Italy were underrepresented (Table S1).
The annual increase in median CD4 cell counts at start of cART initiation from 2002 to 2009, estimated through linear regression (model 1), varied within and across World Bank country groups and tended to be greater among women than men (Table 2). Among LIC, the annual change in median CD4 cell counts ranged from -14 cells/μl (95% CI -37 to +9 cells/μl) among women in the Democratic Republic of the Congo to +32 cells/μl (95% CI +22 to +42 cells/μl) among Rwandan women. For LMIC the corresponding range was from -2 cells/μl (95% CI -14 to +9 cells/μl) among Nigerian men to +13 cells/μl (95% CI +8 to +19 cells/μl) among women in Côte d’Ivoire. In UMIC the range was from -1 cells/μl (-17 to +15 cells/μl) in Botswana men to +15 cells/μl (95% CI 0 to +29 cells/μl) among Brazilian men. Finally, in HIC the range was from -3 cells/μl (95% CI -12 to +7 cells/μl) in Australian men to +16 cells/μl (95% CI +12 to +20 cells/μl) in Canadian men. Crude (unweighted) and weighted pooled estimates of annual increases in CD4 cell count at ART-initiation by country group were generally similar (Table 2). Also, results were similar in the complete case analysis (supplementary Tables S2 and S3).
Table 2.
Annual change between 2002 and 2009 in median CD4 cell count at the start of cART in low income, lower middle income, upper middle income and high income countries, by gender.
| Country | Women
|
Men
|
|||
|---|---|---|---|---|---|
| CD4 cells/μl | (95% CI) | CD4 cells/μl | (95% CI) | ||
|
Low income
| |||||
| Benin | +8 | (-15 to +31) | +4 | (-11 to +19) | |
|
| |||||
| DR Congo | -14 | (-37 to +9) | +6 | (-11 to +22) | |
|
| |||||
| Kenya | +15 | (+13 to +18) | +11 | (+8 to +14) | |
|
| |||||
| Malawi | +9 | (+2 to +15) | +12 | (+4 to +21) | |
|
| |||||
| Mali | +12 | (-2 to +27) | +4 | (-1 to +10) | |
|
| |||||
| Rwanda | +32 | (+22 to +42) | +31 | (+12 to +51) | |
|
| |||||
| Tanzania | +7 | (-1 to +14) | +2 | (-4 to +8) | |
|
| |||||
| Uganda | +23 | (+11 to +35) | +16 | (+1 to +31) | |
|
| |||||
| Zimbabwe | +8 | (+1 to +15) | +5 | (-1 to +11) | |
|
| |||||
| Pooled | crude | +12 | (+6 to +17) | +11 | (+7 to +15) |
| weighted* | +11 | (+4 to +17) | +9 | (+4 to +14) | |
|
Lower middle income
| |||||
| Cameroon | +10 | (-4 to +24) | +13 | (-4 to +31) | |
|
| |||||
| Côte d’Ivoire | +13 | (+8 to +19) | +10 | (-0 to +20) | |
|
| |||||
| Nigeria | +4 | (-13 to +21) | -2 | (-14 to +9) | |
|
| |||||
| Zambia | +11 | (+9 to +13) | +8 | (+4 to +11) | |
|
| |||||
| Pooled | crude | +9 | (+6 to +12) | +7 | (+4 to +10) |
| weighted* | +6 | (+1 to +11) | +5 | (+1 to +9) | |
|
Upper middle income
| |||||
| Botswana | +9 | (-4 to +23) | -1 | (-17 to +15) | |
|
| |||||
| Brazil | +8 | (-8 to +24) | +15 | (+0 to +29) | |
|
| |||||
| South Africa | +9 | (+8 to +11) | +4 | (+1 to +7) | |
|
| |||||
| Thailand | +10 | (+2 to +17) | +6 | (-6 to +18) | |
|
| |||||
| Pooled | crude | +9 | (+6 to +11) | +4 | (+1 to +7) |
| weighted* | +7 | (+1 to +13) | +6 | (-1 to +13) | |
|
High income
| |||||
| Australia | +10 | (-18 to +38) | -3 | (-12 to +7) | |
|
| |||||
| Canada | +7 | (-1 to +15) | +16 | (+12 to +20) | |
|
| |||||
| France | +11 | (+8 to +13) | +9 | (+3 to +15) | |
|
| |||||
| Italy | +4 | (-3 to +11) | +6 | (-3 to +14) | |
|
| |||||
| Spain | +3 | (-1 to +7) | +6 | (+2 to +10) | |
|
| |||||
| USA | +9 | (+2 to +16) | +13 | (+6 to +20) | |
|
| |||||
| Pooled | crude | +9 | (+6 to +11) | +9 | (+6 to +12) |
| weighted* | +7 | (+4 to +11) | +9 | (+6 to +13) | |
Results from linear regression (model 1) based on 347,919 patients, with missing values imputed using multiple imputation.
Weighted by the number of patients on cART in the respective country, as estimated by WHO [5].
Table 3 shows estimated median CD4 cell counts at start of cART in 2009 by age, gender and country income level, as estimated from the mixed effects linear regression (model 2). Median CD4 cell counts were higher in women compared to men (difference +23 cells/μl, 95% CI +18 to +28 cells/μl) and lower for patients aged from 30 to <40 (difference -16 cells/μl, 95% CI -21 to -10) and 40 to <50 years (difference -17 cells/μl, 95% CI -24 to -10) compared to those below 30 years of age. Median counts were similar in LIC, LMIC and UMIC, but were higher in HIC (difference compared to low income countries +112 cells/μl, 95% CI +75 to +149 cells/μl). In the analysis restricted to LIC and MIC, those with cART coverage of 80% or greater (Botswana, Brazil, Cambodia, Rwanda and Zambia) had substantially higher CD4 cell counts at cART initiation (difference +88 cells/μl; 95% CI +35 to +141 cells/μl) than countries with a coverage below 40% (Côte d’Ivoire, Democratic Republic of the Congo, Indonesia, Nigeria and Malaysia). Results were again similar in the complete case analysis (Table S4).
Table 3.
Individual-level and country-level predictors of the median CD4 cell count at the start of cART in 2009.
| Variable | Median CD4 cell count (cells/μl) |
|---|---|
| Sex | |
| Male | 164 (intercept, 140 to 189) |
| Female | 23 (18 to 28) |
| Income group | |
| Low | 164 (intercept, 140 to 189) |
| Lower middle | -13 (-56 to 30) |
| Upper middle | -22 (-62 to 18) |
| High | 112 (75 to 149) |
| Age group (years) | |
| < 30 | 164 (intercept, 140 to 189) |
| 30 to <40 | -16 (-21 to -10) |
| 40 to <50 | -17 (-24 to -10) |
| ≥50 | -3 (-12 to 6) |
| National cART coverage (%)* | |
| < 40 | 144 (intercept, 103 to 185) |
| 40 to < 60 | 10 (-38 to 57) |
| 60 to < 80 | -6 (-58 to 46) |
| ≥80 | 88 (35 to 141) |
Results from mixed effects linear regression (model 2) based on 55,007 patients starting cART in 2009, missing values imputed using multiple imputation. Intercepts and coefficients (95% confidence intervals) are shown. All models include calendar year, age, gender and income group. The intercept of 165 cells/μl corresponds to men in low income countries.
Separate analysis based on 52,482 patients starting cART in 2009 in low income and middle income countries (model 2). The intercept of 146 cells/μl corresponds to men in low income countries. Estimates of national cART coverage in 2009, based on WHO 2006 guidelines were as follows [25]: Benin 72%, Democratic Republic of the Congo 26%, Kenya 65%, Malawi 63%, Mali 65%, Rwanda >95%, Tanzania 44%, Uganda 53%, Zimbabwe 49%, Cameroon 41%, Côte d’Ivoire 39%, Nigeria 31%, Zambia 85%, Botswana >95%, Brazil 80%, South Africa 56%, Thailand 76%.
Figure S2 shows trajectories of median CD4 cell counts at cART initiation and the percentage of patients starting cART below 200 cells/μl and below 100 cells/μl, by country income group and gender, estimated by model 3. In LIC median CD4 cell count at start of cART in women increased by 91% from 2002-2009, from 82 cells/μl to 157 cells/μl, and by 62% from 79 cells/μl to 127 in men. Corresponding increases for LMIC were from 83 cells/μl to 166 cells/μl in women (a 102% increase) and from 97 cells/μl to 138 cells/μl in men (a 42% increase). The increases in UMIC were from 86 cells/μl to 141 cells/μl in women (a 63% increase) and from 91 cells/μl to 125 cells/μl in men (a 36% increase). Finally, in HIC median CD4 cell counts at cART initiation increased by 26% from 211 cells/μl to 266 cells/μl in women and by 34% from 208 cells/μl to 280 cells/μl in men.
In LIC the percentage of patients starting cART below 200 cells/μl declined from 85% in 2002 to 81% in 2009 in women and from 88% to 70% in men. Corresponding figures for LMIC were from 78% to 59% in women and from 79% to 67% in men and for UMIC from 86% to 65% in women and from 78% to 71% in men. In HIC the decline was from 46% to 31% in women and from 49% to 35% in men. For threshold below 100 cells/μl the declines were from 50% to 28% in women and from 57% to 36% in men in LIC, from 47% to 27% in women and from 51% to 36% in men in LMIC, from 54% to 31% in women and from 49% to 40% in men in UMIC and from 22% to 15% in women and from 27% to 17% in men in HIC. Trends were similar in the complete case analysis (Figure S2).
Discussion
This global analysis of CD4 cell counts at cART initiation between 2002 and 2010 was conducted in two HIV cohort collaborations, which together span six continents [7, 18-21]. We found that median CD4 cell counts at cART initiation were substantially higher in HIC, with only small differences between LIC and MIC. Median CD4 cell counts at start of cART increased over the study period in most countries. These increases were greater in LIC and MIC than in HIC, and greater in women than in men. Among LIC and MIC, median CD4 cell counts at start of cART were substantially higher in the few countries with national cART coverage of 80% or above.
In LIC and MIC the median CD4 cell count remained well below 200 cells/μl between 2002 and 2009, despite the 2001WHO recommendation to start when the CD4 gets near or falls below 200 cells/μl or, in persons with advanced clinical disease (WHO stage IV), irrespective of the CD4 cell count [26]. Following a 2006 recommendation to consider treatment at CD4 cell counts below 350 cells/μl for patients in WHO stage III, WHO indicated in 2009 that cART should be initiated at 350 cells/μl irrespective of clinical symptoms [27]. National guidelines in resource-limited settings generally echoed WHO guidelines [28], while HIC have more rapidly increased the CD4 cell count threshold for initiation. Of note, North American guidelines recently converged in their recommendation that cART should be offered to all HIV-positive individuals, irrespective of the CD4 cell count [29, 30].
A substantial rise HIV testing in many countries, supported by GFTAM, PEPFAR, national or state/provincial level governments and other donors may have contributed to increasing CD4 cell counts at the start of ART. A monitoring and evaluation analysis of PEPFAR-supported HIV care clinics in eight sub-Saharan African countries found that CD4 cell counts at cART initiation increased with HIV testing coverage in the region [31]. In sub-Saharan Africa, PEPFAR supported over 140 million testing and counseling sessions between 2004 to 2011, with the number of sessions increasing from 1.9 million in 2004 to over 40 million in 2011 [32]. Two Demographic and Health Surveys (DHS) from seven PEPFAR countries conducted between 2003 and 2010 showed a dramatic increase in population level coverage of HIV testing and counseling [32]. In Kenya, for example, the percentage of men reporting testing in the last 12 months increased from 7.5% to 22.7%. The corresponding increase was even greater in women, from 6.7% to 29.3%. Similarly, in Lesotho, testing increased from 6.3% to 42.0% in women and from 4.8% to 24.0% in men. Provider-initiated testing and counseling may not, however, be sufficient to prevent late HIV diagnosis. Uganda adopted provider-initiated HIV testing in the health care setting in 2005, but in a recent randomized controlled trial half of HIV-positive patients screened had CD4 cell counts below or equal to 250 cells/μl [33].
The steeper increase of HIV testing coverage among women compared to men may be explained by scale-up of programs to prevent mother to child transmission (PMTCT). More frequent testing leads to earlier diagnosis and then earlier initiation of cART in eligible women. The scale-up of PMTCT could thus also account for the greater increases of CD4 cell counts at the start of cART in women than men observed in the present study. A review of national program data for 2004 to 2005 showed that the scale-up of PMTCT had gained momentum in many countries, and that provider-initiated (opt-out) HIV testing had become nearly universal in some regions (for example in East and Southern Africa) but not in others (for example in West Africa) [34]. A systematic review and meta-analysis of 44 studies of pregnant women who attended PMTCT programs in sub-Saharan Africa showed that uptake at antenatal care services was 94% for opt-out testing, compared to 58% for opt-in testing [35]. Coverage with any type of antiretroviral prophylaxis was 70%, and 62% of pregnant women eligible for cART received treatment [35]. Similarly, a meta-analysis of six studies that reported on numbers of adults followed between HIV diagnosis and start of cART showed that of every 100 patients with a positive HIV test, 72 had a CD4 cell count measured, 40 were eligible for cART and 25 started cART. Of note, men were more likely to be lost to program and less likely to start cART than women [36].
Our study has several limitations. We could only examine information up to 2010, because more recent data were not yet available from many sites participating in the IeDEA and ART cohort collaborations. CD4 cell counts at start of cART were missing in approximately one quarter of patients, who were more likely to be from LIC and MIC and in a more advanced stage of disease than patients with measured CD4 cell counts. It is thus likely that our estimates of median CD4 cell counts at cART initiation are biased upwards for these countries. Data from some countries were limited to a small number of patients from a single clinic. We decided to include these in descriptive analyses, but because the data are probably not representative of all patients on cART in those countries, we excluded them from analyses of time trends and predictors of CD4 cell count at start of cART. In sensitivity analyses we weighted individual country data, with the aim of creating a dataset that was representative, within each income group, of the number of patients on cART in each country, as estimated by WHO [5]. Some of the data included in these sensitivity analyses may, however, not be representative of all patients on cART in the country. In particular, the clinics from LIC and MIC participating in IeDEA are mainly urban and capture data in electronic databases, indicating a higher level of resources. They may more closely reflect best practice in urban settings than in the country as a whole. Nevertheless, our study is a unique source of information on trends and determinants of the CD4 cell count in adult patients starting cART across the globe.
In conclusion, our results illustrate the enormous challenges that lie ahead. Despite the massive scale up of cART in LIC, from 300,000 patients on cART in 2002 to 6.6 million by the end of 2010, the increases in median CD4 cell count at the start of cART during this time period have been modest. In 2009, most patients in LIC and MIC countries and many patients in HIC started cART with CD4 cell counts below 200 cells/μl, which means that they were already at high risk of complications and had spent years at high risk of HIV transmission. Substantial efforts and resources are needed to achieve earlier implementation of cART globally. Finally, continued monitoring of the CD4 cell count at which HIV-positive patients start cART in LIC, middle and high income countries is needed to evaluate the results of these efforts.
Supplementary Material
Figure 2.
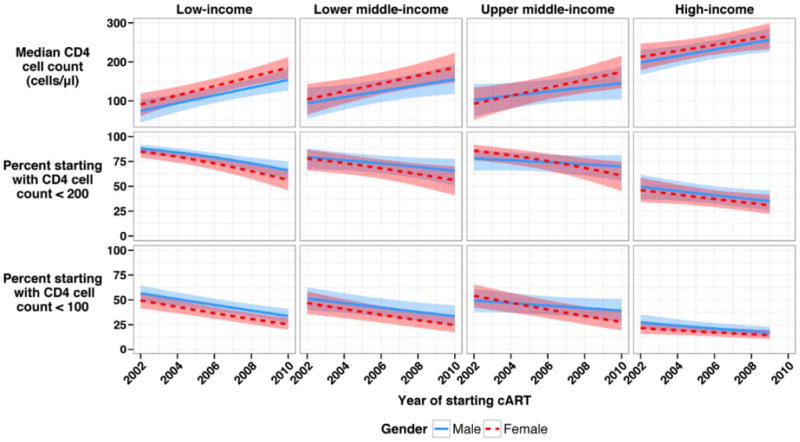
Trends of median CD4 cell counts at the start of cART (upper panel) and in proportions of men and women starting cART below 200 cells/μl (middle panel) or below 100 cells/μl (lower panel) in low, middle and high income countries, 2002 to 2010.
The shaded areas represent the 95% confidence intervals for each year. Results from mixed effects linear regression (model 3) based on 379,865 patients, missing values imputed using multiple imputation.
Acknowledgments
We are grateful to all patients, care givers and data managers involved in the participating cohorts and treatment programs.
The African regions of the International Epidemiological Databases to Evaluate AIDS (IeDEA) are supported by the National Cancer Institute (NCI), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Allergy And Infectious Diseases (NIAID) as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) (Grants 5U01AI069919-04, 5U01-AI069924-05, 1U01 AI069927, U01AI069911-01). The Caribbean, Central and South America network (CCASAnet) of IeDEA is supported by NIAID grant 5U01-AI69923-06. The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA is supported by grants U01-AI069918, U10-AA13566, U01-AI31834, U01-AI34989, U01-AI34993, U01-AI34994, U01-AI35004, U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, U01-AI35043, U01-AI37613, U01-AI37984, U01-AI38855, U01-AI38858, U01-AI42590, U01-AI68634, U01-AI68636, U01-HD32632, U10-EY08057, U10-EY08052, U10-EY08067, UL1-RR024131, UL1-RR024131, M01-RR-00052, M01-RR00071, M01-RR00079, M01-RR00083, M01-RR00722, M01-RR025747, P30-AI27757, P30-AI27767, P30-AI27763, P30-AI50410, P30-AI54999, R01-DA04334, R01-DA12568, R01-DA11602, R01-AA16893, R24-AI067039, Z01-CP010176, AHQ290-01-0012, N02-CP55504, AI-69432, AI-69434, K01-AI071725, K23-AI610320, K23-EY013707, K24-DA00432, K01-AI093197, U10-AA13566, R01-AG029154 and K23 AG024896 from the National Institutes of Health; contract CDC200-2006-18797 from the CDC; grants TGF-96118, HCP-97105, CBR-86906, CBR-94036, KRS-86251, and 169621 from the Canadian Institutes of Health Research; the Canadian HIV Trials Network, project 24; and the Government of British Columbia. The TREAT Asia HIV Observational Database, TREAT Asia Studies to Evaluate Resistance, and the Australian HIV Observational Database are initiatives of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the Dutch Ministry of Foreign Affairs through a partnership with Stichting Aids Fonds, and the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute, as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907). Queen Elizabeth Hospital and the Integrated Treatment Centre received additional support from the Hong Kong Council for AIDS Trust Fund. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales. The ART Cohort Collaboration is supported by UK Medical Research Council grants MR/J002380/1 and G0700820.
The content of this publication is solely the responsibility of the authors and does not represent the official views of any of the institutions and funders mentioned.
Appendix
Writing committee
Dorita Avila1, Keri N Althoff2, Catrina Mugglin1, Kara Wools-Kaloustian3, Manuel Koller1, François Dabis4, Denis Nash5, Thomas Gsponer1Somnuek Sungkanuparph6, Catherine McGowan7, Margaret May8, David Cooper9, Cleophas Chimbetete10, Marcelo Wolff11, Ann Collier12, Hamish McManus9, Mary-Ann Davies13, Dominique Costagliola14, Brenda Crabtree-Ramirez15, Romanee Chaiwarith16, Angela Cescon17, Morna Cornell13, Lameck Diero18, Praphan Phanuphak19, Adrien Sawadogo20, Jochen Ehmer21, Serge P. Eholie22, Patrick CK Li23, Matthew P Fox24,25, Neel E Ghandi26, Elsa González27, Christopher KC Lee28, Christopher J Hoffmann29,30, Andrew Kambugum31, Olivia Keiser1, Rossana Ditangco32, Hans Prozesky33, Fiona Lampe34, Nagalingeswaran Kumarasamy35, Mari Kitahata12, Emmanuel Lugina36, Rita Lyamuya37, Saphonn Vonthanak38, Valeria Fink39, Antonella d’Arminio Monforte40, Paula M Luz41, Yi-Ming A Chen42, Albert Minga43, Jordi Casabona44,45, Albert Mwango46, Jun Y Choi47, Marie-Louise Newell48, Elizabeth A. Bukusi49, Kapella Ngonyani50, Tuti P Merati51, Juliana Otieno52, Mwebesa B Bosco53, Sam Phiri54, Oon T. Ng55, Kathryn Anastos56, Jürgen Rockstroh57, Ignacio Santos58, Shinichi Oka59, Geoffrey Somi60, Christoph Stephan61, Ramon Teira62, Deo Wabwire63, Gilles Wandeler64, Andrew Boulle13, Peter Reiss65, Robin Wood66, Benjamin H Chi67, Carolyn Williams68, Jonathan A Sterne9, Matthias Egger1,13
1) Institute of Social and Preventive Medicine (ISPM), University of Bern, Switzerland; 2) Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, USA; 3) Indiana University School of Medicine, Indianapolis, IN, USA; 4) Institut de Santé Publique, Epidémiologie et Développement (ISPED), Université Bordeaux Segalen, Bordeaux, France; 5) CUNY School of Public Health, New York, USA; 6) Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 7) Vanderbilt University School of Medicine, Nashville, TN, USA; 8) School of Social and Community Medicine, University of Bristol, UK; 9) The Kirby Institute, The University of New South Wales, Sydney, Australia; 10) Newlands Clinic, Harare, Zimbabwe; 11) Fundación Arriarán, Universidad de Chile, Santiago, Chile; 12) University of Washington School of Medicine Center, Seattle, USA; 13) Centre for Infectious Disease Epidemiology and Research, School of Public Health and Family Medicine, University of Cape Town, South Africa; 14) 1-UPMC Univ Paris 06, UMR_S 943, F-75013, Paris, France; 15) Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán, México D.F., México; 16) Research Institute for Health Sciences, Chiang Mai, Thailand; 17) British Columbia Centre for Excellence in HIV/AIDS, Vancouver, Canada; 18) Academic Model Providing Access to Health Care (AMPATH), School of Medicine, Faculty of Health Sciences, Moi University, Eldoret, Kenya; 19) HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand; 20) CHU Sourô Sanou, Bobo-Dioulasso, Burkina Faso; 21) SolidarMed African HIV prevention and treatment program, Lucerne, Switzerland; 22) Service de Maladies Infectieuses et Tropicales (SMIT), CHU de Treichville, Abidjan, Côte d’Ivoire; 23) Queen Elizabeth Hospital, Hong Kong SAR, China; 24) Center for Global Health and Development and Department of Epidemiology, Boston University and Boston University School of Public Health, Boston, USA; 25) Health Economics and Epidemiology Research Office, University of the Witwatersrand, Johannesburg, South Africa; 26) Departments of Epidemiology, Global Health & Infectious Diseases, Emory University Rollins School of Public Health, Atlanta, GA USA; 27) Instituto de Medicina Tropical Alexander von Humboldt, Universidad Peruana Cayetano Heredia, Lima, Peru; 28) Hospital Sungai Buloh, Sungai Buloh, Malaysia; 29) Aurum Institute, Johannesburg, South Africa; 30) John Hopkins University School of Medicine, Baltimore, USA; 31) Infectious Diseases Institute (IDI), Kampala, Uganda; 32)Research Institute for Tropical Medicine, Manila, Philippines; 33) Division of Infectious Diseases, University of Stellenbosch and Tygerberg Hospital, Cape Town, South Africa; 34) Research Department of Infection and Population Health, University College London, UK; 35) YRG Centre for AIDS Research and Education, Chennai, India; 36) Ocean Road Cancer Institute (ORCI), Dar-es-Salaam, Tanzania; 37) Morogoro Regional Hospital, Morogoro, Tanzania; 38) National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia; 39) Fundación Huésped, Buenos Aires, Argentina; 40) Clinic of Infectious Diseases & Tropical Medicine, San Paolo Hospital, University of Milan, Italy; 41) Fundação Oswaldo Cruz, Rio de Janeiro, Brazil; 42) Taipei Veterans General Hospital and AIDS Prevention and Research Centre, National Yang-Ming University, Taipei, Taiwan; 43) Centre Médical de Suivi de Donneurs de Sang/CNTS/PRIMO-CI, Abidjan, Cote d’Ivoire; 44) Centre d’Estudis Epidemiològics sobre ITS/VIH/SIDA de Catalunya, Badalona, Spain; 45) CIBER Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain; 46) Zambian Ministry of Health, Lusaka, Zambia; 47) Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea; 48) Africa Centre for Health and Population Studies, University of KwaZulu-Natal, Mtubatuba, South Africa; 49) Family AIDS Care and Education Services (FACES), Kisumu, Kenya; 50) Tumbi Regional Hospital, Kibaha, Tanzania; 51) Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia; 52) Nyanza Provincial Hospital, MTCT-plus, Kisumu, Kenya; 53) Mbarara University of Science and Technology (MUST), ISS Clinic, Mbarara, Uganda; 54) Lighthouse Trust, Lilongwe, Malawi; 55) Tan Tock Seng Hospital, Singapore; 56) Departments of Medicine and Epidemiology and Population Health, Albert Einstein College of Medicine, New York, USA; 57) Department of Internal Medicine I, University Hospital Bonn, 53105 Bonn, Germany; 58) Hospital La Princesa, Madrid, Spain; 59) National Center for Global Health and Medicine, Tokyo, Japan; 60) National AIDS Control Program, Dar es Salaam, Tanzania; 61) HIVCENTER am Klinikum der Johann Wolfgang Goethe-Universität, Frankfurt, Germany; 62) Hospital Sierrallana, Torrelavega, Spain; 63) Mulago University JHU Collaboration (MUJHU MTCT-plus), Kampala, Uganda; 64) Department of Infectious Diseases, Bern University Hospital and University of Bern, Switzerland; 65) Academisch Medisch Centrum bij de Universiteit van Amsterdam, Amsterdam, The Netherlands; 66) Desmond Tutu HIV Centre, University of Cape Town, South Africa; 67) Center for Infectious Disease Research in Zambia, Lusaka, Zambia; 68) Epidemiology Branch, Division of AIDS, National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA.
Footnotes
CONFLICTS: The authors declare that they have no conflicts of interest.
References
- 1.Egger M, Hirschel B, Francioli P, Sudre P, Wirz M, Flepp M, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. BMJ. 1997;315:1194–1199. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. New England Journal of Medicine. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Nash D, Katyal M, Brinkhof MW, Keiser O, May M, Hughes R, et al. Long-term immunologic response to antiretroviral therapy in low-income countries: a collaborative analysis of prospective studies. AIDS. 2008;22:2291–2302. doi: 10.1097/QAD.0b013e3283121ca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 5.World Health O, UNAIDS, UNICEF. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. WHO; Geneva: 2011. [Google Scholar]
- 6.Phillips AN, Gazzard BG, Clumeck N, Losso MH, Lundgren JD. When should antiretroviral therapy for HIV be started? BMJ. 2007;334:76–78. doi: 10.1136/bmj.39064.406389.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 8.Emery S, Neuhaus JA, Phillips AN, Babiker A, Cohen CJ, Gatell JM, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 9.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collaboration H-C, Cain LE, Logan R, Robins JM, Sterne JA, Sabin C, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154:509–515. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keiser O, Anastos K, Schechter M, Balestre E, Myer L, Boulle A, et al. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13:870–879. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Althoff KN, Gange SJ, Klein MB, Brooks JT, Hogg RS, Bosch RJ, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010;50:1512–1520. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crabtree-Ramirez B, Caro-Vega Y, Shepherd BE, Wehbe F, Cesar C, Cortes C, et al. Cross-sectional analysis of late HAART initiation in Latin America and the Caribbean: late testers and late presenters. PLoS One. 2011;6:e20272. doi: 10.1371/journal.pone.0020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Group HIVMCTaPEW. HIV treatment as prevention: models, data, and questions--towards evidence-based decision-making. PLoS Med. 2012;9:e1001259. doi: 10.1371/journal.pmed.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fauci AS, Folkers GK. Toward an AIDS-free generation. JAMA : the journal of the American Medical Association. 2012;308:343–344. doi: 10.1001/jama.2012.8142. [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, et al. Cohort Profile: The international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. International Journal of Epidemiology. 2012;41:1256–64. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gange SJ, Kitahata MM, Saag MS, Bangsberg DR, Bosch RJ, Brooks JT, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007;36:294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGowan CC, Cahn P, Gotuzzo E, Padgett D, Pape JW, Wolff M, et al. Cohort Profile: Caribbean, Central and South America Network for HIV research (CCASAnet) collaboration within the International Epidemiologic Databases to Evaluate AIDS (IeDEA) programme. Int J Epidemiol. 2007;36:969–976. doi: 10.1093/ije/dym073. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Kumarasamy N, Ditangco R, Kamarulzaman A, Lee CK, Li PC, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38:174–179. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bofill M, Janossy G, Lee CA, MacDonald-Burns D, Phillips AN, Sabin C, et al. Laboratory control values for CD4 and CD8 T lymphocytes. Implications for HIV-1 diagnosis. Clin Exp Immunol. 1992;88:243–252. doi: 10.1111/j.1365-2249.1992.tb03068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bank TW. [15 June 2013];How we classify countries. Available from http://data.worldbank.org/about/country-classifications.
- 24.World Health Organization. Antiretroviral therapy for HIV Infection in adults and adolescents in resource-limited settings: towards universal access. Recommendations for a public health approach. Geneva: World Health Organization; 2006. [Google Scholar]
- 25.World Health Organization. Progress Report 2010. WHO; Geneva: 2010. Towards universal access. Scaling up priority HIV/AIDS interventions in the health sector. [Google Scholar]
- 26.World Health O. Scaling up antiretroviral therapy in resource-limited settings. Guidelines for a public health approach. Geneva: World Health Organization; 2002. [PubMed] [Google Scholar]
- 27.World Health O. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Geneva: World Health Organization; 2010. 2010 revision. [PubMed] [Google Scholar]
- 28.Beck EJ, Vitoria M, Mandalia S, Crowley S, Gilks CF, Souteyrand Y. National adult antiretroviral therapy guidelines in resource-limited countries: concordance with 2003 WHO guidelines? AIDS. 2006;20:1497–1502. doi: 10.1097/01.aids.0000237365.18747.13. [DOI] [PubMed] [Google Scholar]
- 29.Panel on Antiretroviral Guidelines for Adult and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Washington DC: Department of Health & Human Services; 2012. [Google Scholar]
- 30.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA : the journal of the American Medical Association. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 31.Nash D, Wu Y, Elul B, Hoos D, El Sadr W, et al. International Center for AC. Program-level and contextual-level determinants of low-median CD4+ cell count in cohorts of persons initiating ART in eight sub-Saharan African countries. AIDS. 2011;25:1523–1533. doi: 10.1097/QAD.0b013e32834811b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marum E, Taegtmeyer M, Parekh B, Mugo N, Lembariti S, Phiri M, et al. “What took you so long?” The impact of PEPFAR on the expansion of HIV testing and counseling services in Africa. J Acquir Immune Defic Syndr. 2012;60(Suppl 3):S63–69. doi: 10.1097/QAI.0b013e31825f313b. [DOI] [PubMed] [Google Scholar]
- 33.Wanyenze RK, Kamya MR, Fatch R, Mayanja-Kizza H, Baveewo S, Sawires S, et al. Missed opportunities for HIV testing and late-stage diagnosis among HIV-infected patients in Uganda. PLoS One. 2011;6:e21794. doi: 10.1371/journal.pone.0021794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo C, Akwara P, Ngongo N, Doughty P, Gass R, Ekpini R, et al. Global progress in PMTCT and paediatric HIV care and treatment in low- and middle-income countries in 2004-2005. Reprod Health Matters. 2007;15:179–189. doi: 10.1016/S0968-8080(07)30327-3. [DOI] [PubMed] [Google Scholar]
- 35.Wettstein C, Mugglin C, Egger M, Blaser N, Salazar L, Estill J, et al. Missed Opportunities to Prevent Mother-to-Child-Transmission in sub-Saharan Africa: Systematic Review and Meta-Analysis. AIDS. 2012;26:2361–73. doi: 10.1097/QAD.0b013e328359ab0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mugglin C, Estill J, Wandeler G, Bender N, Egger M, Gsponer T, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health. 2012 Sep 20; doi: 10.1111/j.1365-3156.2012.03089.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


