Abstract
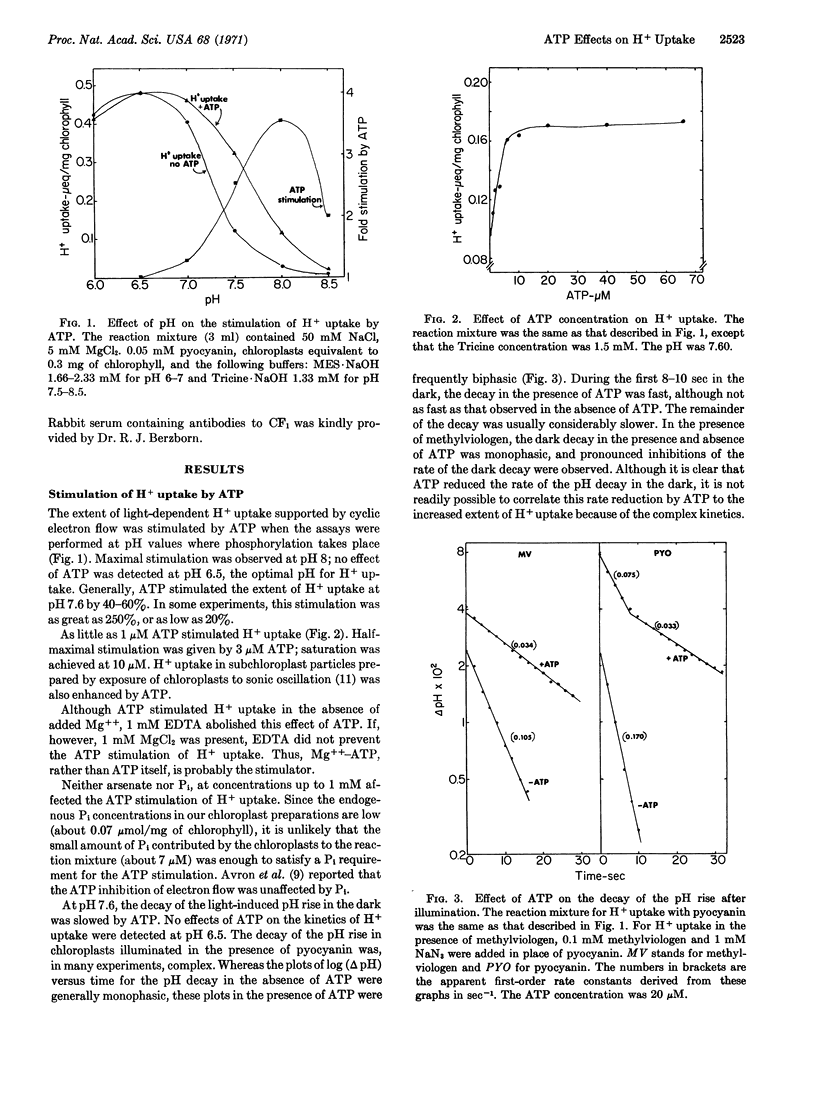
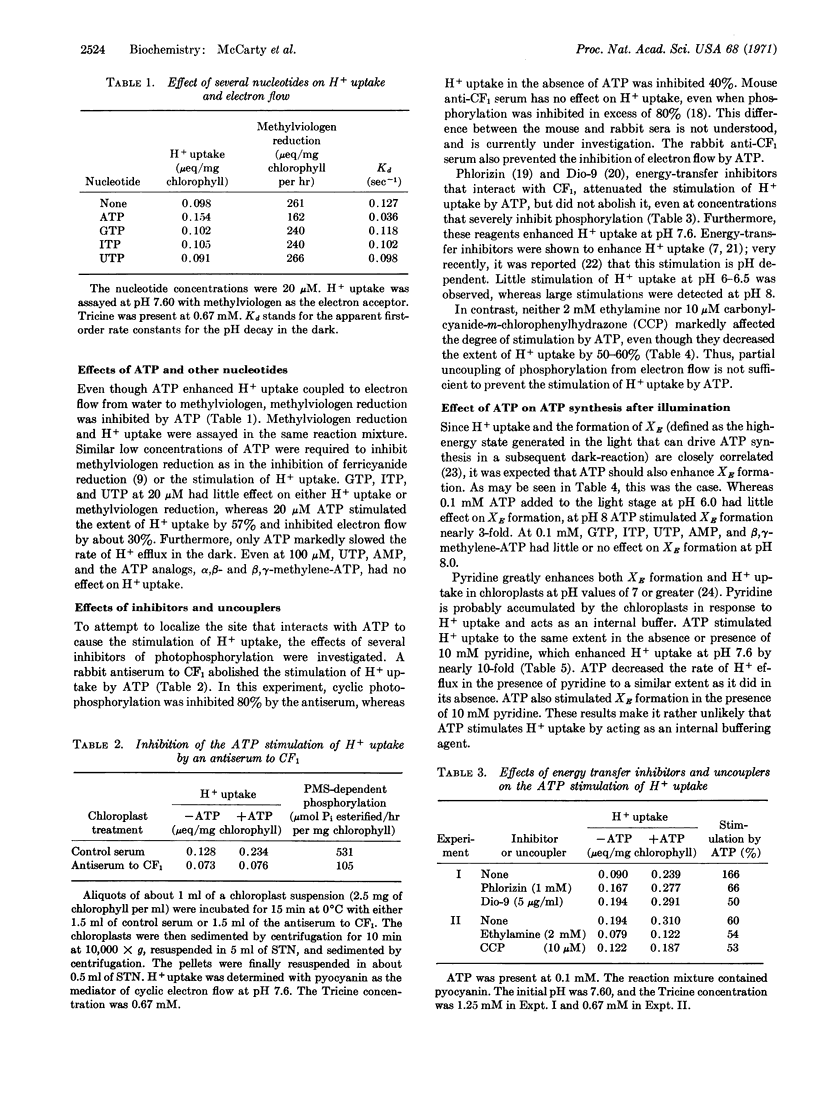
1-10 μM ATP stimulated H+ uptake and slowed the release of H+ in the dark in chloroplasts illuminated at pH values at which photophosphorylation can occur, but not at pH 6.5. This ATP stimulation of H+ uptake was abolished by an antiserum to the chloroplast coupling factor and was reduced by the energy transfer inhibitors phlorizin and Dio-9. ATP synthesis after illumination was also enhanced by ATP. Electron flow from water to methyl viologen was inhibited by the same low concentrations of ATP.
ADP also increased the extent of H+ uptake in chloroplasts, even in the presence of arsenate and MgCl2. In the presence of hexokinase and glucose, as well as arsenate and Mg++, ADP inhibited H+ uptake. The failure of previous investigators to observe a direct inhibition of H+ uptake by phosphorylation was probably caused by a masking of the inhibition by the stimulation of H+ uptake by ATP. Furthermore, the stimulation of H+ uptake by ATP provides an explanation for its inhibition of electron flow.
Keywords: spinach, coupling factor 1, photophosphorylation, electron transport, membrane permeability
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., KROGMANN D. W., JAGENDORF A. T. The relation of photosynthetic phosphorylation to the Hill reaction. Biochim Biophys Acta. 1958 Oct;30(1):144–153. doi: 10.1016/0006-3002(58)90251-8. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeli C. Proton translocation induced by ATPase activity in chloroplasts. FEBS Lett. 1970 Apr 16;7(3):297–300. doi: 10.1016/0014-5793(70)80187-9. [DOI] [PubMed] [Google Scholar]
- Crofts A. R. Uptake of ammonium ion by chloroplasts, and its relation to photophosphorylation. Biochem Biophys Res Commun. 1966 Sep 8;24(5):725–731. doi: 10.1016/0006-291x(66)90385-8. [DOI] [PubMed] [Google Scholar]
- Dilley R. A., Shavit N. On the relationship of H+ transport to photophosphorylation in spinach chloroplasts. Biochim Biophys Acta. 1968 Jul 16;162(1):86–96. doi: 10.1016/0005-2728(68)90217-x. [DOI] [PubMed] [Google Scholar]
- Dilley R. A. The effect of various energy-conversion states of chloroplasts on proton and electron transport. Arch Biochem Biophys. 1970 Mar;137(1):270–283. doi: 10.1016/0003-9861(70)90434-0. [DOI] [PubMed] [Google Scholar]
- ERNSTER L., LINDBERG O. Determination of organic phosphorus compounds by phosphate analysis. Methods Biochem Anal. 1956;3:1–22. doi: 10.1002/9780470110195.ch1. [DOI] [PubMed] [Google Scholar]
- HOCH G., MARTIN I. Photo-potentiation of adenosine triphosphate hydrolysis. Biochem Biophys Res Commun. 1963 Jul 26;12:223–228. doi: 10.1016/0006-291x(63)90194-3. [DOI] [PubMed] [Google Scholar]
- Hind G., Jagendorf A. T. SEPARATION OF LIGHT AND DARK STAGES IN PHOTOPHOSPHORYLATION. Proc Natl Acad Sci U S A. 1963 May;49(5):715–722. doi: 10.1073/pnas.49.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Winget G. D., Good N. E. Phlorizin, a specific inhibitor of photophosphorylation and phosphorylation-coupled electron transport in chloroplasts. Biochem Biophys Res Commun. 1966 Jan 24;22(2):223–226. doi: 10.1016/0006-291x(66)90436-0. [DOI] [PubMed] [Google Scholar]
- Jagendorf A. T., Uribe E. ATP formation caused by acid-base transition of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Jan;55(1):170–177. doi: 10.1073/pnas.55.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagendorf A. T., Uribe E. Photophosphorylation and the chemi-osmotic hypothesis. Brookhaven Symp Biol. 1966;19:215–245. [PubMed] [Google Scholar]
- Karlish S. J., Avron M. Energy transfer inhibition and ion movements in isolated chloroplasts. Eur J Biochem. 1971 May 11;20(1):51–57. doi: 10.1111/j.1432-1033.1971.tb01361.x. [DOI] [PubMed] [Google Scholar]
- McCarty R. E., Guillory R. J., Racker E. Dio-9, an inhibitor of coupled electron transport and phosphorylation in chloroplasts. J Biol Chem. 1965 Dec;240(12):4822–4823. [PubMed] [Google Scholar]
- McCarty R. E., Racker E. Effect of a coupling factor and its antiserum on photophosphorylation and hydrogen ion transport. Brookhaven Symp Biol. 1966;19:202–214. [PubMed] [Google Scholar]
- McCarty R. E. Relation of photophosphorylation to hydrogen ion transport. Biochem Biophys Res Commun. 1968 Jul 11;32(1):37–43. doi: 10.1016/0006-291x(68)90422-1. [DOI] [PubMed] [Google Scholar]
- McCarty R. E. The uncoupling of photophosphorylation by valinomycin and ammon-ium chloride. J Biol Chem. 1969 Aug 25;244(16):4292–4298. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- NEUMANN J., JAGENDORF A. T. LIGHT-INDUCED PH CHANGES RELATED PHOSPHORYLATION BY CHLOROPLASTS. Arch Biochem Biophys. 1964 Jul;107:109–119. doi: 10.1016/0003-9861(64)90276-0. [DOI] [PubMed] [Google Scholar]
- PETRACK B., CRASTON A., SHEPPY F., FARRON F. STUDIES ON THE HYDROLYSIS OF ADENOSINE TRIPHOSPHATE BY SPINACH CHLOROPLASTS. J Biol Chem. 1965 Feb;240:906–914. [PubMed] [Google Scholar]
- Polya G. M., Jagendorf A. T. Light-induced change in the buffer capacity of spinach chloroplast suspensions. Biochem Biophys Res Commun. 1969 Aug 15;36(4):696–703. doi: 10.1016/0006-291x(69)90362-3. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Light induced proton gradient links electron transport and phosphorylation. Nature. 1968 Aug 31;219(5157):915–919. doi: 10.1038/219915a0. [DOI] [PubMed] [Google Scholar]
- Shavit N., Herscovici A. On the interaction of ATP with the phosphorylation system in chloroplasts. FEBS Lett. 1970 Nov 18;11(2):125–128. doi: 10.1016/0014-5793(70)80508-7. [DOI] [PubMed] [Google Scholar]


