Abstract
The allergist is frequently called on to evaluate patients after episodes of anaphylaxis to determine the cause and implement preventive measures that will reduce the patient’s risk from future episodes. The etiology of anaphylaxis can be the result of numerous causes that may go undiagnosed if a thorough evaluation is not performed. We present a 71-year-old man with no history of food allergy or atopy who presented to the emergency room and then our allergy clinic for evaluation after suffering anaphylaxis after a meal of grits and shrimp. The underlying diagnosis, which was subsequently determined, requires a high index of suspicion and should be included in the differential diagnosis of any patient presenting with unexplained anaphylaxis.
CASE PRESENTATION
Chief Complaint
Food-induced anaphylaxis.
History of Present Illness
A 71-year-old man with a history of hypertension, gastroesophageal reflux, and dyslipidemia presented to our clinic after an allergic reaction. His allergic reaction occurred within 1 hour of his evening meal, which consisted of shrimp with grits and bacon. The reaction started with his eyes becoming itchy and watery, followed by nasal congestion and clear rhinorrhea. His tongue and lip then became swollen and he had excessive drooling. He developed an itchy rash on his arms and full body flushing. Next, he experienced shortness of breath, which prompted him to seek emergency medical care. He denied nausea, diarrhea, abdominal cramps, light headedness, or syncope. After evaluation in the emergency room, he was treated with methylprednisolone, diphenhydramine, and nebulized epinephrine. He was admitted for observation but had no further symptoms and was discharged home with an epinephrine autoinjector device and a prednisone taper and told to avoid seafood.
He presented to our clinic the next day. He had no similar prior episodes. He denied any history of atopic disease. He had eaten shrimp from the same bag and not had similar reactions. His wife consumed the same meal that he did and did not have similar symptoms. His review of systems was unremarkable. His medications had not changed recently. He had no known drug allergies and specifically no history of reactions to non-steroidal anti-inflammatory drugs (NSAIDs).
Physical Examination
His vital signs were normal. He was a well-appearing man, in no distress, with clear conjunctivae. Nasal, oropharyngeal, and respiratory exam were normal. Skin examination was normal with no signs of eczema, urticaria, or other rash.
Initial Laboratory and Diagnostic Findings
His complete blood count with differential and basic metabolic panel were unremarkable. His total IgE drawn 2 days after his episode was 747 IU/mL and specific IgE to the components of the meal including corn, shrimp, milk, pork, and tomato were all <0.35 kU/L. Skin testing was not performed because of the proximity to the anaphylactic episode and recent use of antihistamines.
QUESTIONS
What Is the Differential Diagnosis of the Patient’s Food-Induced Anaphylaxis?
In 2006, a panel of experts in allergy and immunology developed a definition of anaphylaxis as that of clinical scenarios that include a combination of the acute onset of reactions that involve the skin, mucosal tissue, or both and respiratory or vascular compromise.1 Anaphylactic reactions can occur as a result of IgE-mediated and non–IgE-mediated pathways.2 The patient in our case developed symptoms within 2 hours of a meal, which prompted a thorough evaluation for a food allergen as the cause of his reaction. His serum did not contain specific IgE to any of the components of the meal and therefore we considered alternative diagnoses. Other ingested food-associated antigens and toxins mimic anaphylaxis. For example, cross-reactivity of aeroallergens and profilin contained within fruits and vegetables can cause oral allergy syndrome.3–5 Latex allergy is also associated with cross-reactivity to fruits, which has recently been linked to 1,3β-glucanase.6 Many other immunologic and nonimmunologic mimics of food allergy have been described (Table 1).
Table 1.
Differential diagnosis of immunologic and nonimmunologic mimics of food allergy
| Masquerading Food | Actual Trigger | Diagnosis |
|---|---|---|
| Fruits and vegetables | Birch, mugwort, ragweed, and profilin5 |
Oral allergy syndrome* |
| Corn, wheat, and flour |
Der p 1 | Oral mite anaphylaxis* |
| Avocado, banana, kiwi, and others | Latex (hevein,1,3β-glucanase)6,7 | Latex-fruit syndrome* |
| Wheat, celery, shellfish, cabbage, peaches, grapes, apples, and others |
Exercise8 | Food dependent, exercise- induced anaphylaxis* |
| Mammalian meat | Galactose-α-1,3-galactose | Delayed meat anaphylaxis* |
| Dairy, eggs, and meats | Enterotoxin |
Staphylococcus aureus food poisoning |
| Dairy | Lactose | Lactase deficiency |
| Pork | Cat albumin | Pork-cat syndrome9* |
| Soy sauce, tomatoes, meat, parmesan cheese, and mushrooms |
Monosodium glutamate10 | Preservative intolerance |
| Cheese, smoked meats, alcohol, and chocolate |
Tyramine | Pheochromocytoma crisis and carcinoid syndrome11,12 |
| Salad bars, dehydrated fruits, wine, and others |
Metabisulfites and other sulfites13 | Preservative intolerance |
| Fish and cheese | Scombroid14 and ciguatera toxin | Scombroid and ciguatera poisoning |
IgE-mediated reaction.
What Additional Investigations Would Be Helpful in This Patient?
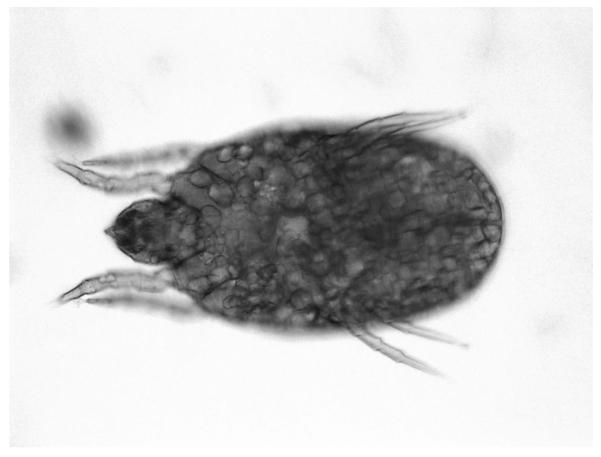
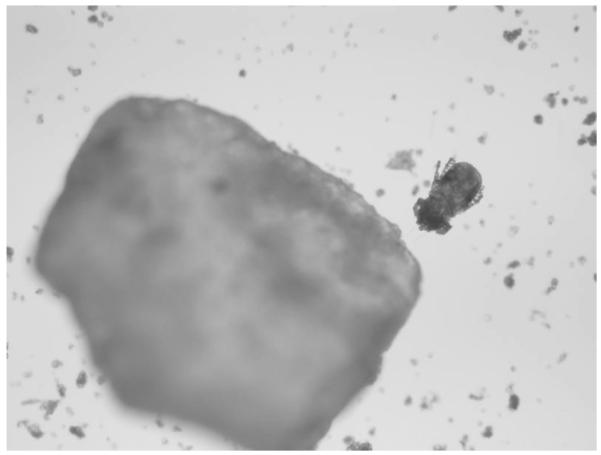
Given that his reaction occurred immediately before our first visit, we had the patient return after 6 weeks for skin-prick testing. His total IgE was repeated and was 416 IU/mL. Skin testing was performed with the grits and frozen shrimp (freshly thawed) used in the original meal by making a protein extract in normal saline. Positive control with histamine resulted in a 10-mm wheal with flare and there was no reaction to saline. The protein extract made from his grits produced an 11-mm wheal with flare. Skin testing after heating the grit extract in a microwave oven for 3 minutes on high power continued to induce a 10-mm wheal with flare. The shrimp mixture produced no reaction. A new bag of grits was obtained and the skin testing protocol was repeated with negative results. Gross inspection of the grits revealed no abnormalities. Light microscopic examination of the grits, however, revealed numerous mites consistent with the appearance of Dermatophagoides farinae15 (Figs. 1 and 2). Subsequently, specific IgE to various mite species was obtained and D. farinae was shown to be 92.30 kU/L. In addition, the patient showed sensitivity to Dermatophagoides pteronyssinus (33.50 kU/L), Tyrophagus putrescentiae (3.65 kU/L), Acarus siro (4.12 kU/L), and Lepidoglyphus destructor (5.07 kU/L).
Figure 1.
Mite obtained from grits at 100× magnification.
Figure 2.
Mite adjacent to grit at 40× magnification.
Clinical Course
On further questioning, the patient and his wife reported that the grits they had used in the meal the evening of the reaction had been in their pantry for over a year in a cloth sack. They had obtained the grits while on vacation in South Carolina and this was the first time they used them for a meal. The results of his evaluation indicated that his reaction likely occurred because of ingestion of large quantities of mites to which he was sensitized. We advised him that he could safely add back shrimp, tomato, milk, and corn to his diet. We asked him to discard the grits mixture. He was instructed on a food allergy action plan and on avoidance measures for the mites. These included appropriate bedding covers and to store his grains and cereals in airtight containers in the refrigerator because this hinders mite reproduction and to continue to carry an epinephrine injector because newly purchased grain products can occasionally contain mites.16 He has experienced no further reactions in the last 4 months following our recommendations for avoidance.
DISCUSSION
The lifetime prevalence of anaphylaxis is estimated at 0.05–2%.17 The most common causes of anaphylaxis are foods, followed by medications and then venom. Other rare causes of anaphylaxis are exposure to semen, latex, or exercise, although many cases of anaphylaxis are deemed idiopathic when no identifiable cause is established.18 According to prior case series, ~32–37% of cases of anaphylaxis are left undetermined and are labeled as idiopathic.19,20
Oral mite anaphylaxis (OMA) involves the ingestion of wheat, flour, or grains infested with mites, either dust or storage, and their associated allergens by a patient with underlying sensitization and clinically presents with the signs and symptoms of a systemic IgE-mediated hypersensitivity reaction. OMA been commonly referred to as “pancake syndrome” given its frequency after ingestion of contaminated pancakes.21 OMA was first reported by Erben et al. in 1993 after an episode of anaphylaxis that occurred in a mite-allergic patient after ingestion of D. farinae–contaminated beignets from New Orleans.22 The word farinae is derived from the Latin word farina, for “flour,” or a meal made from cereal grains, reflecting the recognized tendency of these insects to infest flour stores. The incidence of OMA is unknown. OMA has been mainly reported in areas of increased humidity such as the tropical/sub-tropical climates, although it has been seen outside the tropics, e.g., in Philadelphia, Detroit, and Massachusetts.23,24 Prior cases have typically occurred in patients known to have allergic rhinitis and/or asthma, who are sensitized to dust or storage mites, and episodes occurred when they ingested food such as wheat, flour, or other grains infested with the mites.
Our patient was unusual in that he had no history of allergic rhinitis or sensitivity to dust mites as an inhalant allergen. His total IgE was markedly elevated and >10% of this was specific for D. farinae. This striking elevation of total IgE, with much of the total comprised of specific IgE targeting a single antigen, is similar to the recently reported syndrome of adult-onset food anaphylaxis to mammalian meats associated with IgE to galactose-α-1,3-galactose.25 OMA can occur regardless of whether or not the grain is cooked because the relevant allergens appear to be thermoresistant both clinically and by skin testing, as shown previously by Sánchez-Borges et al.16 Although not present in our case, there has been an association between OMA and NSAID hypersensitivity, which is thought to reflect the presence of components in dust-mite extracts capable of inhibiting cyclooxygenase pathways.21,26 There has also been a presentation of the OMA associated with exercise.27
Criteria for the diagnosis of OMA include (1) compatible symptoms occurring after the intake of foods prepared with wheat flour; (2) previous history of rhinitis, asthma, atopic dermatitis, and/or food allergy; (3) in vivo or in vitro demonstration of IgE-mediated sensitization to mite allergens; (4) positive immediate-type skin test with extract of the suspected flour; (5) negative skin tests to wheat and to uncontaminated flour extract; (6) clinical tolerance to foods made with uncontaminated wheat flour; (7) microscopic identification of mites in the suspected flour; (8) presence of mite allergens in the flour; and (9) hypersensitivity to NSAIDs.21
Final Diagnosis
Oral mite anaphylaxis.
CONCLUSION
Many cases of food-related anaphylaxis presenting to the emergency room or to an allergy clinic may be erroneously regarded as idiopathic when testing to seemingly relevant food ingredients are negative because they may contain hidden allergens.28,29 This case reinforces that food-induced anaphylaxis can be caused by many IgE-mediated and non—IgE-mediated mechanisms. Among the IgE-mediated reactions, OMA should be a consideration in patients presenting with food-induced allergic reactions.
Footnotes
The authors have no conflicts to declare pertaining to this article
REFERENCES
- 1.Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: Summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman P, Nicklas R, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 Update. J Allergy Clin Immunol. 2010;126:477–480. doi: 10.1016/j.jaci.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Morrow J, Margolies G, Rowland J, et al. Evidence that histamine is the causative toxin of scombroid-fish poisoning. N Engl J Med. 1991;324:716–720. doi: 10.1056/NEJM199103143241102. [DOI] [PubMed] [Google Scholar]
- 4.Ortolani C, Ispano M, Pastorello E, et al. The oral allergy syndrome. Ann Allergy Asthma Immunol. 1988;61:47–52. [PubMed] [Google Scholar]
- 5.van Ree R, Fernández-Rivas M, Cuevas M, et al. Pollen-related allergy to peach and apple: An important role for profilin. J Allergy Clin Immunol. 1995;95:726–734. doi: 10.1016/s0091-6749(95)70178-8. [DOI] [PubMed] [Google Scholar]
- 6.Barre A, Culerrier R, Granier C, et al. Mapping of IgE-binding epitopes on the major latex allergen Hev b 2 and the cross-reacting 1,3 β glucanase fruit allergens as a molecular basis for the latex-fruit syndrome. Mol Immunol. 2009;43:1595–1604. doi: 10.1016/j.molimm.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Nel A, Gujuluva C. Latex antigens: Identification and use in clinical and experimental studies, including crossreactivity with food and pollen allergens. Ann Allergy Asthma Immunol. 1998;81:388–396. doi: 10.1016/S1081-1206(10)63135-3. [DOI] [PubMed] [Google Scholar]
- 8.Baek CH, Bae YJ, Cho YS, et al. Food-dependent exercise-induced anaphylaxis in the celery–mugwort–birch–spice syndrome. Allergy. 2010;65:791–804. doi: 10.1111/j.1398-9995.2009.02233.x. [DOI] [PubMed] [Google Scholar]
- 9.Savi E, Rossi A. Cat–pork syndrome: A case report with a three year follow-up. Eur Ann Allergy Clin Immunol. 2006;38:366–368. [PubMed] [Google Scholar]
- 10.Williams AN, Woessner KM. Monosodium glutamate “allergy”: Menace or myth? Clin Exp Allergy. 2009;39:640–646. doi: 10.1111/j.1365-2222.2009.03221.x. [DOI] [PubMed] [Google Scholar]
- 11.Manger WM. An overview of pheochromocytoma. Ann NY Acad Sci. 2006;1073:1–20. doi: 10.1196/annals.1353.001. [DOI] [PubMed] [Google Scholar]
- 12.Srirajaskanthan R, Shanmugabavan D, Ramage JK. Carcinoid syndrome. BMJ. 2010;341:603–606. doi: 10.1136/bmj.c3941. [DOI] [PubMed] [Google Scholar]
- 13.Randhawa S, Bahna S. Hypersensitivity reactions to food additives. Curr Opin Allergy Clin Immunol. 2009;9:278–283. doi: 10.1097/ACI.0b013e32832b2632. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman JA, Sicherer SH. The diagnosis of food allergy. Am J Rhinol Allergy. 2010;24:439–443. doi: 10.2500/ajra.2010.24.3515. [DOI] [PubMed] [Google Scholar]
- 15.Hughes AM. The Mites of Stored Food and Houses. Vol. 206. Her Majesty’s Stationary Office; London, England: 1976. [Google Scholar]
- 16.Sánchez-Borges M, Capriles-Hulett A, Fernández-Caldas E, et al. Mite-contaminated foods as a cause of anaphylaxis. J Allergy Clin Immunol. 1997;99:738–743. doi: 10.1016/s0091-6749(97)80005-x. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman P, Camargo C, Bohlke K, et al. Epidemiology of anaphylaxis: Findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97:596–602. doi: 10.1016/S1081-1206(10)61086-1. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman P, Kemp S, Oppenheimer J, et al. The diagnosis and management of anaphylaxis: An updated practice parameter. J Allergy Clin Immunol. 2005;115:S483–S523. doi: 10.1016/j.jaci.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Kemp S, Lockey R, Wolf B, et al. Anaphylaxis: A review of 266 cases. Arch Intern Med. 1995;155:1749–1754. doi: 10.1001/archinte.155.16.1749. [DOI] [PubMed] [Google Scholar]
- 20.Yocum M, Butterfield J, Klein J, et al. Epidemiology of anaphylaxis in Olmsted County: A population-based study. J Allergy Clin Immunol. 1999;104:452–456. doi: 10.1016/s0091-6749(99)70392-1. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez-Borges M, Suárez-Chacon R, Capriles-Hulett A, et al. Pancake syndrome (oral mite anaphylaxis) J World Allergy Org. 2009;2:91–96. doi: 10.1186/1939-4551-2-5-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erben AM, Rodriguez JL, McCullough J, et al. Anaphylaxis after ingestion of beignets contaminated with Dermatophagoides farinae. J Allergy Clin Immunol. 1993;92:846–849. doi: 10.1016/0091-6749(93)90062-k. [DOI] [PubMed] [Google Scholar]
- 23.Hannaway PJ, Miller JD. The pancake syndrome (oral mite anaphylaxis) by ingestion and inhalation in a 52-year old woman in the northeastern United States. Ann Allergy Asthma Immunol. 2008;100:397–398. doi: 10.1016/S1081-1206(10)60607-2. [DOI] [PubMed] [Google Scholar]
- 24.Guerra Bernd LA, Arruda LK, Barros Antunes HB. Oral anaphylaxis to mites. Allergy. 2001;56:83–84. doi: 10.1034/j.1398-9995.2001.00922.x. [DOI] [PubMed] [Google Scholar]
- 25.Commins S, Satinover S, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-α-1,3-galactose. J Allergy Clin Immunol. 2009;123:426–433. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanco C, Quiralte J, Quiralte R, et al. Anaphylaxis after ingestion of wheat flour contaminated with mites. J Allergy Clin Immunol. 1997;99:308–312. doi: 10.1016/s0091-6749(97)70047-2. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez-Borges M, Iraola V, Fernández-Caldas E, et al. Dust mite ingestion-associated, exercise-induced anaphylaxis. J Allergy Clin Immunol. 2007;120:714–716. doi: 10.1016/j.jaci.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Puglisi G, Frieri M. Update on hidden food allergens and food labeling. Allergy Asthma Proc. 2007;28:634–639. doi: 10.2500/aap.2007.6.3066. [DOI] [PubMed] [Google Scholar]
- 29.Luccioli S, Malka-Rais J, Nsouli TM, Bellanti JA. Clinical reactivity to ingestion challenge with mixed mold extract may be enhanced in subjects sensitized to molds. Allergy Asthma Proc. 2009;30:433–442. doi: 10.2500/aap.2009.30.3254. [DOI] [PubMed] [Google Scholar]