Abstract
Background
Pulmonary hypertension (PH) is a key contributor to cardiovascular morbidity and early mortality; however, reports are lacking on the epidemiology of PH in at-risk patient populations.
Methods and Results
The echocardiography registries from two major Veterans Affairs hospitals were accessed to identify patients with at least moderate PH, defined here as a pulmonary artery systolic pressure (PASP) ≥60 mmHg detected echocardiographically. From a total of 10,471 individual patient transthoracic echocardiograms, we identified moderate or severe PH in 340 patients (332 men; mean 77 y, mean PASP 69.4 ± 10.5 mmHg), of which PH was listed as a diagnosis in the medical record for only 59 (17.3%). At a mean of 832 days (0-4817 d) following echocardiography diagnosing PH, 150 (44.1%) patients were deceased. Pulmonary hypertension was present without substantial left heart remodeling: the mean left ventricular (LV) ejection fraction was 0.50 ± 0.16, LV end-diastolic dimension was 4.9 ± 1.0 cm, and left atrial dimension was 4.4 ± 0.7 cm. Cardiac catheterization (n=122, 36%) demonstrated a mean pulmonary artery pressure of 40.5 ± 11.4 mmHg, pulmonary capillary wedge pressure of 22.6 ± 8.9 mmHg, and pulmonary vascular resistance of 4.6 ± 2.9 Wood units. Diagnostic strategies for PH were variable and often incomplete; for example, only 16% of appropriate patients were assessed with a nuclear ventilation/perfusion (V/Q) scan for thromboembolic causes of PH.
Conclusions
In an at-risk patient population, PH is under-diagnosed and associated with substantial mortality. Enhanced awareness is necessary among practitioners regarding contemporary PH diagnostic strategies.
Keywords: pulmonary hypertension, diagnostic method, population
Pulmonary hypertension (PH) is the principal intermediate pathophenotype responsible for right-sided congestive heart failure.1 When present, even subclinical pathological changes to cardiovascular function mediated by untreated PH are associated with increased morbidity and decreased longevity.2 Observations from epidemiological reports have indicated that compared with other community-based populations, the U.S. military Veteran patient population has an increased prevalence of PH-associated primary lung and cardiovascular disease;3 however, the epidemiology of PH for this population is not known. In the current study, we leveraged the unique strengths of the VA clinical database, which is a universal and centralized electronic medical record, to test our hypothesis that PH is a prevalent form of cardiovascular disease in a cohort of Veteran patients. We further aimed to investigate the clinical profile and diagnostic strategies used for PH in this patient population.
Methods
The study was approved by the VA investigational review board for patient safety and privacy, and complies with the Declaration of Helsinki. Databases hosting echocardiogram results were analyzed for all studies performed at the VA Boston Health Care System (VABHS, Boston, MA) and the Providence VA Medical Center (PVAMC, Providence, RI) from July 1, 2008 through July 1, 2011. These institutions function as referral centers for active or retired U.S. military Veterans residing in New Hampshire, Maine, Massachusetts, Vermont, and Rhode Island.
Echocardiographic Definition of Pulmonary Hypertension
At both VABHS and PVAMC, the Crystal Reports® software program was used to catalogue transthoracic echocardiography data. Focused studies to evaluate specific cardiovascular diseases (e.g., cardiac tamponade), exercise or dobutamine stress echocardiograms, and transesophageal echocardiograms were excluded from the analysis. Pulmonary hypertension was defined as pulmonary artery systolic pressure (PASP) ≥40 mmHg,4 which was calculated by the sum of the transtricuspid gradient plus estimated right atrial pressure.5
Electronic Medical Record
The centralized Veterans Health Information System and Technology Architecture (VistA) electronic medical record was accessed to acquire patients' medical information and clinical data. Active medications at the time of analysis were recorded. In the event patients were deceased at the time of analysis, then the most complete medication list prior to death was used for analysis.
Diagnostic Testing
Patients underwent a two-dimensional transthoracic echocardiogram as clinically indicated using standard ultrasound equipment by three major vendors (GE Healthcare, Milwaukee, WI, USA; Phillps, Bothel, WA, USA; and Siemens, Erlanger, Germany). A cardiologist experienced in echocardiography interpreted all studies using the Phillips Xcelera Cardiac Reporting™ system. Left atrial and left ventricular (LV) dimensions were measured in the parasternal long axis view. The LV ejection fraction was assessed using the biplane Simpson's method and diastolic functional classification was performed as outlined in the American Society of Echocardiography document for LV functional analysis.5,6 All right heart catheterizations were performed in the supine position according to standard, previously published methods for acquiring invasive cardiopulmonary hemodynamic measurements.7-9 The cardiopulmonary hemodynamic criteria used to consider patients for a diagnosis of World Health Organization Group 1 PH (i.e., pulmonary arterial hypertension [PAH]) or chronic thromboembolic PH (CTEPH) was: mean pulmonary artery pressure mPAP ≥25 mmHg, pulmonary vascular resistance (PVR) ≥3.0 Wood units, and pulmonary capillary wedge pressure (PCWP) ≤16 mmHg. Ultimately, the determination of WHO Group classification was adjudicated by two cardiologists and a pulmonologist, each with substantial clinical experience in the clinical management of patients with pulmonary vascular disease.
Statistical Analysis
All statistical analyses were performed using Origin 9.0 (Northampton, MA, USA). Categorical variables are reported as frequencies with percentages. Unless otherwise indicated, continuous data are expressed as the mean ± SD. Normality was tested by using the Shapiro-Wilk test. When samples were normally distributed, an unpaired Student t test was used to compare 2 independent groups. When data were not normally distributed, comparisons between 2 groups were made with use of the Mann-Whitney test. Statistical significance was defined as p<0.05.
Results
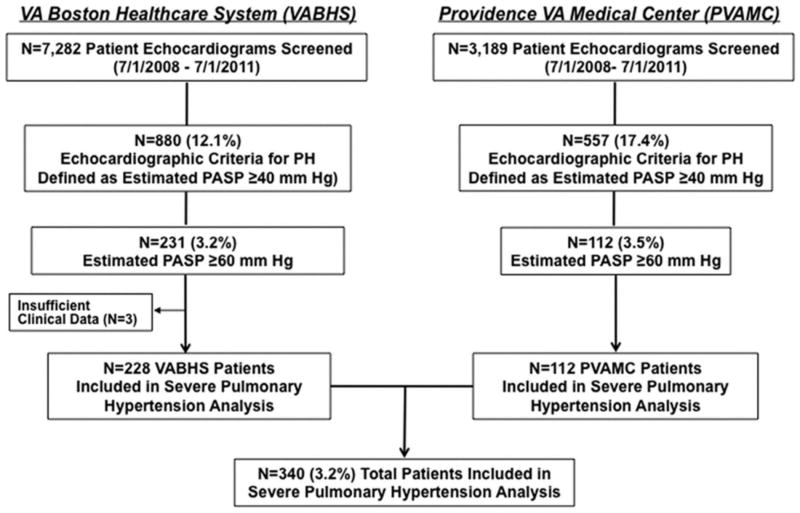
From a total of 10,471 individual patient echocardiograms screened, 1,437 (13.7%) patients met the predefined PH search criteria. We next elected to focus further analyses on patients with an estimated PASP ≥60 mmHg, a subpopulation of the cohort most likely to meet conventional clinical criteria for severe PH, and thereby express a representative profile of clinically meaningful PH in Veterans (Figure 1). We identified 343 patients with echocardiographically assessed PASP ≥60 mmHg; of these patients, three were excluded from further analysis due to the unavailability of clinical information. The remaining patient cohort (N=340) was largely male (97.6%, mean age 78 yr, range 52-100); at a mean of 832 days (0-4,817 d) following echocardiography demonstrating evidence of moderate or severe PH, 150 (44.1%) patients were deceased (Table 1). The mean number of comorbidities per patient listed in the problem list was 13.8 ± 6.1, with cardiovascular and pulmonary diseases accounting for 5.3 ± 2.7 and 1.3 ± 1.3 comorbidities per patient, respectively. Despite this, PH was reported as a diagnosis in the problem list for only 59 (17.3%) patients.
Figure 1. Methods for identifying the study population.
Results from transthoracic echocardiograms performed for any clinical indication at two Veterans Affairs (VA) medical centers were reviewed for the presence of pulmonary hypertension (PH), defined here as an estimated PASP ≥40 mmHg (calculated by the sum of the trantricuspid valve gradient plus estimated right atrial pressure). Patients likely to meet conventional criteria for severe PH were then selected for further analysis based on the presence of an estimated PASP ≥60 mmHg. PVAMC, Providence Veterans Affairs Medical Center; VABHS, Veterans Affairs Boston Healthcare System.
Table 1. Clinical and diagnostic profile of pulmonary hypertension in Veteran patients.
| Patient Characteristics | |
| Men (%) | 332 (97.6) |
| Deceased (%) | 150 (44.1) |
| Age in years (range) | 77 (52-100) |
| PH Diagnosed in the Medical Record (%) | 59 (17.3) |
| Patient Comorbidities (range) | 13.8 (0-40) |
| Cardiovascular Comorbidities (range) | 5.3 (0-16) |
| Pulmonary Comorbidities (range) | 1.3 (0-6) |
| Days from Diagnosis (range) | 832 (0-4,817) |
The clinical characteristics of patients from two major Veterans Affairs hospitals undergoing transthoracic echocardiography demonstrating a pulmonary artery systolic pressure ≥60 mmHg were analyzed. Values are presented as N (%) for the categories: Men, Deceased, and Pulmonary hypertension (PH) diagnosed in the medical record. Values are presented as mean (range) for the categories: Age, Patient comorbidities, and Days from diagnosis. For each category, the total number of patients included in the analysis was N=340, except the category Days from diagnosis (N=82), which indicates the time elapsed between initial detection of severe PH of any severity by echocardiography and patient death.
Diagnostic tests recommended to evaluate the etiology of PH that were performed in this cohort are illustrated in Figure 2. Among the entire cohort (N=340), a minority of patients underwent pulmonary function testing with lung diffusion capacity (40.5%), right heart catheterization (35.8%), or pulmonary thromboembolic assessment with V/Q scan or thoracic computed tomographic angiography (25.8%). For patients with a clinical and cardiopulmonary hemodynamic profile that was compatible with chronic thromboembolic pulmonary hypertension (CTEPH) (i.e., N=18), a V/Q scan to diagnose CTEPH occurred in only 3 (16%) patients. Seventeen patients meeting hemodynamic criteria for WHO Group 1 PH (i.e., PAH) had an appropriate diagnostic evaluation including an echocardiogram and pulmonary function test. Of these, four patients did not have any evidence of significant cardiopulmonary disease (i.e., LV ejection fraction <0.50, significant left-sided valvular disease, ratio of forced expiratory volume-1 second to forced vital capacity [FEV1/FVC] <0.7, or pulmonary fibrosis) and, thus, may represent WHO Group 1 PH.
Figure 2. The distribution of tests used to diagnose pulmonary hypertension (PH) etiology and severity.
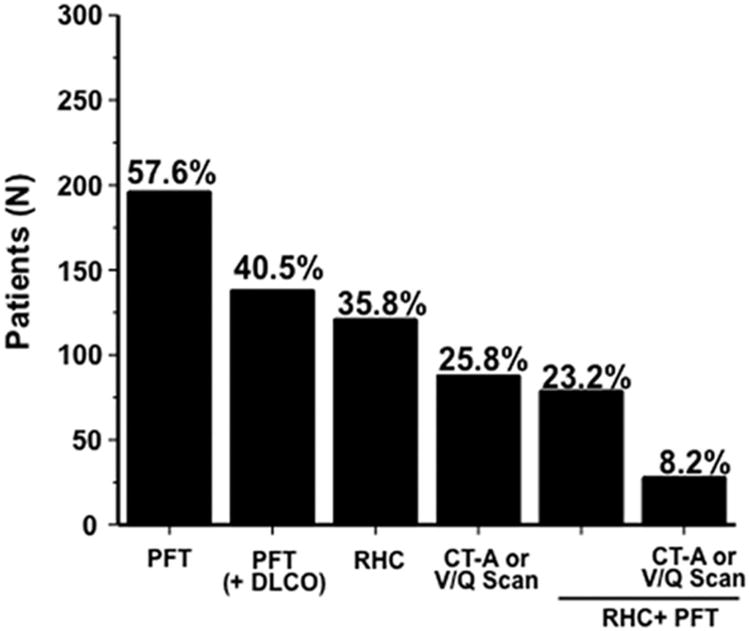
The electronic medical record for each patient with echocardiographically assessed moderate or severe PH (N=340) was analyzed to identify patients undergoing specific test(s) that are important for establishing PH disease etiology as well as disease severity. PFT, pulmonary function test (spirometry only); DLCO, lung diffusion capacity to carbon monoxide; RHC, right heart catheterization; CT-A, thoracic computed tomographic angiography; V/Q, ventilation/perfusion.
Of the ten most prevalent comorbidities in the overall PH cohort, nine were primary diseases of the cardiovascular or pulmonary system (Figure 3), which is reflected in the distribution of patients' medications that emphasize pharmacotherapies commonly used for the treatment of systemic hypertension, congestive heart failure, coronary artery disease, and/or chronic obstructive lung disease (Figure 4). Pulmonary circulation-specific pharmacotherapies were reported for 8 (2.3%) and 3 (0.8%) patients prescribed a phosphodiesterase type-V-inhibitor or endothelin receptor antagonist, respectively.
Figure 3. Cardiovascular or pulmonary co-morbidites reported for the patient cohort.
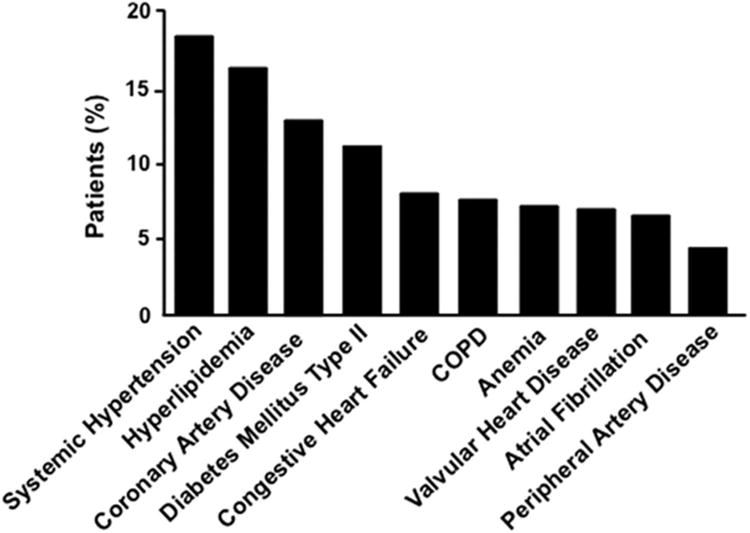
Primary cardiovascular or pulmonary disease accounted for nine of the ten most common comorbidites reported for the patient cohort, with osteoarthritis as the single non-cardiopulmonary condition (data not shown). COPD, chronic obstructive pulmonary disease.
Figure 4. Selected patient medications recorded at the time of data analysis.
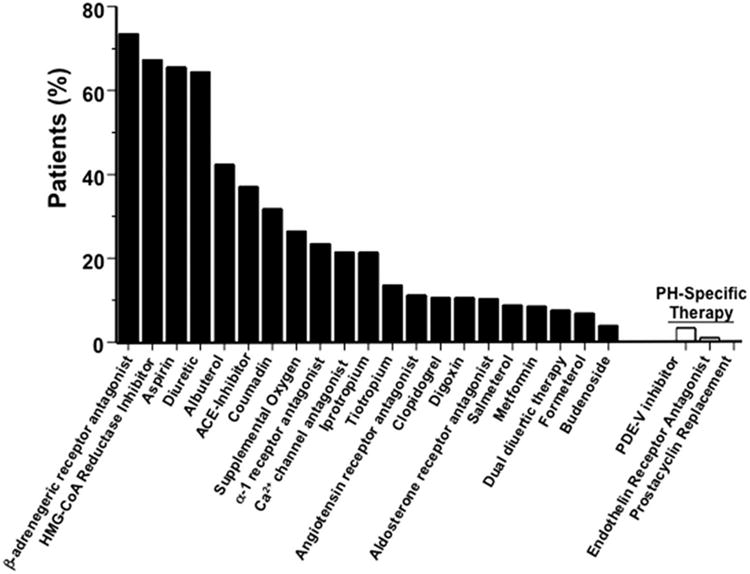
ACE, angiotensin converting enzyme; PDE-V, phosphodiesterase type-V; HMG-CoA reductase inhibitor (‘statin’).
Echocardiographic and Hemodynamic Characteristics
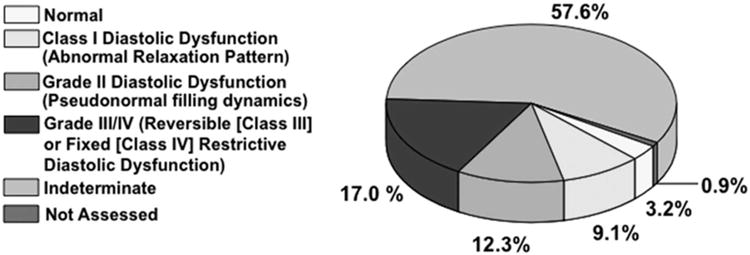
Analysis of echocardiography data in this cohort demonstrated a mean PASP of 69.4 ± 10.5 mmHg without evidence of substantial left heart remodeling: the mean LV ejection fraction was 0.50 ± 0.16%, intraventricular septal thickness was 1.1 ± 0.4 cm, LV end-diastolic dimension was 5.0 ± 0.9 cm, and left atrial dimension was 4.4 ± 0.7 cm (Table 2). This degree of left atrial enlargement has been previously identified as a maker of left atrial hypertension-associated PH.10 Therefore, the distribution of diastolic function in our cohort was assessed next. Interestingly, although class III (reversible restrictive) or class IV (fixed restrictive) diastolic dysfunction was present in 17.0% of patients, diastolic function was reported to be indeterminate in the majority of the cohort (57.6%) (Figure 5), indicating a potential limitation of this echocardiographic application for diagnosing PH due to diastolic dysfunction-induced left atrial hypertension in this patient population.
Table 2. Echocardiographic and pulmonary function test data for U.S. veteran patients with severe pulmonary hypertension.
| Echocardiography and Pulmonary Function Test Characteristics | |
| Estimated PASP (mmHg)(N=340) | 69.4±10.5 |
| TR Velocity (m/s)(N=340) | 3.78±34.8 |
| Left Atrial Dimension (cm)(N=333) | 4.4±0.7 |
| LV End-Diastolic Dimension (cm)(N=332) | 5.0±0.9 |
| Intraventricular Septal Thickness (cm)(N=330) | 1.1±0.4 |
| LV Ejection Fraction (N=340) | 0.50±0.16 |
| Estimated Right Atrial Pressure (mmHg) (N=340) | 11.8±5.3 |
| FEV-1% (N=188) | 63.5±22.7% |
| FEV-1/FVC (N=188) | 75.8±16.1% |
| DLCO (N=138) | 48.9±18.8% |
Data are expressed as value ± SD. For FEV-1, FEV-1/FVC, and DLCO, values are expressed as percent of predicted. PASP, pulmonary artery systolic pressure; TR, tricuspid valve regurgitation jet; LV, left ventricle; FEV-1, forced expiratory volume in one second; FVC, forced vital capacity; DLCO, diffusion lung capacity for carbon monoxide.
Figure 5. Distribution of diastolic filling patterns for Veteran patients with severe pulmonary hypertension as assessed by tissue Doppler echocardiography (N=340).
Complete hemodynamic data were recorded for 90 patients (Table 3). The mPAP was 40.5 ± 11.4 mmHg and PCWP was 22.6 ± 6.5 mmHg; despite elevations in PCWP, the mean PVR was 4.6 ± 2.9 Wood units. Compared to patients not undergoing cardiac catheterization (N=218), patients in whom cardiac catheterization was performed (N=122) had a greater cardiovascular disease burden (4.9 ± 2.6 vs. 5.5 ± 2.9 diagnosed cardiovascular comorbidities, p<0.01) and evidence of more severe left heart remodeling, including decreased LV ejection fraction (0.51 ± 0.16 vs. 0.47 ± 0.17, p<0.03), increased LV end-diastolic dimension (4.9 ± 0.9 vs. 5.2 ± 0.8 cm, p<0.01), as well as more severe pulmonary hypertension assessed by PASP on echocardiography (67.5 ± 9.8 vs. 72.0 ± 12.4 mmHg, p<0.002) (Table 4). By contrast, there was no difference between groups in pulmonary disease burden or performance on pulmonary function testing.
Table 3. The cardiopulmonary hemodynamic profile of pulmonary hypertension in Veteran patients.
| Cardiac Catheterization Characteristics | |
| Right Atrial Pressure (mmHg) | 14.2±6.5 |
| PASP (mmHg) | 64.7±17.6 |
| Pulmonary Artery Diastolic Pressure (mmHg) | 25.8±8.1 |
| mPAP (mmHg) | 40.5±11.4 |
| Pulmonary Capillary Wedge Pressure (mmHg) | 22.6±8.9 |
| Cardiac Output (L/min) | 4.5±1.3 |
| Cardiac Index (L/min/m2) | 2.2±0.5 |
| Pulmonary Vascular Resistance (Wood units) | 4.6±2.9 |
Data are expressed as value ± SD. PASP, pulmonary artery systolic pressure; mPAP, mean pulmonary artery pressure. N=90 for each variable.
Table 4. Clinical profile of patients with echocardiographically assessed moderate or severe pulmonary hypertension according to history of invasive hemodynamic monitoring by right heart catheterization (RHC).
| Characteristic | RHC (N=122) | Non-RHC (N=218) | P-Value |
|---|---|---|---|
|
| |||
| Comorbidities | |||
| Total | 15.5 (7.0) | 12.9 (5.3) | <0.001 |
| Cardiovascular | 5.5 (2.9) | 4.9 (2.6) | <0.01 |
| Pulmonary | 1.4 (1.4) | 1.2 (1.3) | 0.07 |
| Echocardiography | |||
| LV Ejection Fraction | .47 (.17) | .51 (.16) | <0.03 |
| LA Size (cm) | 4.6 (0.8) | 4.3 (0.7) | <0.01 |
| IVS (cm) | 1.2 (0.6) | 1.1 (0.2) | 0.81 |
| LV EDD (cm) | 5.2 (0.8) | 4.9 (0.9) | <0.01 |
| TR Velocity (cm/s) | 3.85 (0.38) | 3.73 (0.38) | <0.02 |
| RA Pressure (mmHg) | 12.3 (5.3) | 11.5 (5.2) | 0.15 |
| PASP (mmHg) | 72.0 (12.4) | 67.5 (9.8) | <0.002 |
| Pulmonary Function Test | |||
| FEV-1/FVC (%) | 75.0 (15.1) | 76.3 (16.8) | 0.81 |
| FEV-1 (%) | 61.5 (20.7) | 64.9 (24.1) | 0.32 |
| FVC (%) | 71.2 (20.2) | 68.5 (18.0) | 0.71 |
| DLCO (%) | 48.3 (16.7) | 49.4 (22.0) | 0.76 |
For Pulmonary Function Test data, results are expressed as percent of predicted. LV, left ventricle; LA, left atrium; IVS, intraventricular septum; EDD, end-diastolic dimension; TR tricuspid valve regurgitation; RA, right atrium; FEV-1, forced expiratory volume in one second; FVC, forced vital capacity; DLCO, diffusion lung capacity for carbon monoxide, PASP, pulmonary artery systolic pressure; mPAP, mean pulmonary artery pressure.
Discussion
We report that the prevalence of PH in an unselected cohort of Veteran patients is ∼14%, which compares favorably to rates of PH reported previously for populations characterized by a high burden of co-morbid diseases known to promote pulmonary vascular dysfunction.11-13 Our data demonstrate that despite the well-established relationship between PH and cardiovascular morbidity and mortality,14,15 this disease remains substantially under recognized in clinical practice. Evidence in support of this assertion was that less than one-fifth of patients with echocardiographically-assessed PASP ≥60 mmHg had PH listed in the problem list in the medical record, and, when ultimately established, the diagnosis was delayed and associated with substantial mortality.
Expert consensus guideline statements recommend a multi-disciplinary strategy to assess PH classification and prognosis,9,16 and that the identification of one pathophysiological mechanism accounting for patients' PH phenotype does not exclude alternative mediators of disease expression.9 This is particularly salient to our study population, in which multiple cardiovascular, pulmonary, and hematological co-morbidities were present that adversely affect pulmonary artery pressure. Nevertheless, we observed that only a minority of patients with evidence of PH on echocardiography underwent at least one consensus guideline-recommended test9 to assess disease etiology or severity. For example, despite sound clinical evidence indicating that PH due to chronic thromboembolic disease is a treatable form of PH,17-18 evaluating for this was uncommon in our study cohort.
Although the available cardiac catheterization and pulmonary function test data indicate that PH due to left atrial hypertension (WHO Group 2 PH) and/or chronic lung disease (WHO Group 3) was well represented in this study cohort, the high proportion of patients in whom a PH diagnostic evaluation was incomplete confounded an accurate determination of PH prevalence by disease mechanism. Interestingly, findings demonstrating that right heart catheterization tended to occur in patients with more severe left heart remodeling on echocardiography suggests that this, rather than PH severity per se, may be a key factor in the decision to undergo diagnostic right heart catheterization.
Despite the elderly nature of our cohort, we identified 4 patients with a clinical profile consistent with PAH. This observation is in concert with reports indicating that PAH is increasingly diagnosed initially in older patients.19 Indeed, the mean age of our PAH patients (68 years) is well within the age range reported for other cohorts in which this form of PH was diagnosis was late in life.20
Findings from this study are in agreement with previous reports9,21 indicating the limitations to echocardiography as a central method by which to assess the extent and cause of PH and that this may be of particular importance to the Veteran patient population. For example, our observation that diastology was indeterminate in a majority of the cohort despite enlargement of the left atrium illustrates a potential limitation to the effectiveness of using this echocardiographic strategy to implicate impaired diastolic function as a pathophysiological mechanism by which to account for PH. Obesity, atrial fibrillation, and mitral valvular disease, which are established confounders to the echocardiographic assessment of diastolic function and PASP, and which were present in a sizeable proportion of our cohort, are likely to have hindered the diagnostic utility of echocardiography for these purposes. Collectively, these observations provide support to future endeavors utilizing novel echocardiography scoring systems that do not rely solely on PASP or left atrial dimension for predicting PH severity.10
This study has numerous limitations that merit consideration when interpreting our findings. First, selection and referral bias, which are inherent to our retrospective study design, are likely to have confounded the accurate determination of PH prevalence in Veteran patients. Secondly, the identification of an appropriate control group necessary to accurately assess the consequences of PH on key outcome measures such as hospitalization or survival was not possible due to unavailability of these data in Veteran patients without PH or only mild PH. Thirdly, our study was not designed to assess appropriateness of treatment, and, therefore, any speculation relating to the quality of care for PH within the VA healthcare system cannot be supported. Fourth, the possibility exists that certain PH diagnostic tests, particularly right heart catheterization, were deferred due to clinically sound reasons (e.g., test contraindication(s), patient refusal, etc.). Thus, although the implementation of appropriate diagnostic testing was suboptimal for some forms of PH, such as CTEPH, the generalizability of this trend to all WHO PH Groups cannot be determined definitively from results in the current study.
In conclusion, our data support recently published findings from others22,23 suggesting that in at-risk patients, PH is prevalent yet largely under-diagnosed. While it is unresolved if pulmonary vasodilator therapy alters morbidity or mortality in forms of PH other than PAH,24-26 our work highlights the need for enhanced awareness among the practicing cardiovascular community regarding contemporary strategies for identifying treatable forms of PH, particularly CTEPH, or assessing PH severity.
Clinical Summary.
Pulmonary hypertension (PH) is associated with increased morbidity across the spectrum of cardiopulmonary diseases. Yet, despite the large population of patients at increased risk for PH, reports are lacking on the epidemiology, clinical profile, and extent to which guideline-based recommendations for the diagnosis of PH are implemented into clinical practice. In this study, we report that among an unselected population of at-risk patients, the prevalence of PH is 14%. However, even in patients with severe PH (3.2% of population), the diagnosis of PH is largely under-recognized, and, when ultimately established, is delayed and associated with substantial mortality. We observed that practice patterns for the diagnosis of PH are variable and often incomplete: a minority of patients with echocardiographically assessed severe PH underwent pulmonary function testing, right heart catheterization, ventilation-perfusion scan, or thoracic computed tomography to determine the etiology of pulmonary vascular disease, despite guideline-based recommendations indicating appropriateness of these tests. Shortcomings in the determination of PH etiology are particularly evident for chronic thrombembolic pulmonary hypertension (CTEPH), which is a form of pulmonary vascular disease that is potentially amenable to treatment. Among patients with a clinical profile compatible with CTEPH, the diagnosis was pursued appropriately in only 16% of patients. Overall, our work highlights the need for enhanced awareness among the practicing cardiovascular community regarding contemporary strategies for identifying treatable forms of PH, particularly CTEPH, as well as assessing PH severity.
Acknowledgments
Sources of Funding: This work was supported in part by the American Heart Association (11POST6720000), US National Institutes of Health (1K08HL111207-01A1), and the Lerner and Klarman Foundation at Brigham and Women's Hospital to B.A.M.; VA Merit Award (IBX000711A) from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development: Biomedical Laboratory Research and Development Service to G.C..
Footnotes
Disclosures: Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: Medscape Cardiology; Board of Directors: Boston VA Research Institute, Society of Chest Pain Centers; Chair: American Heart Association Get With The Guidelines Science Subcommittee; Honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Duke Clinical Research Institute (clinical trial steering committees), Slack Publications (Chief Medical Editor, Cardiology Today Intervention), WebMD (CME steering committees); Other: Senior Associate Editor, Journal of Invasive Cardiology; Research Grants: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, The Medicines Company; Unfunded Research: FlowCo, PLx Pharma, Takeda.
Disclaimer: The contents of this scientific manuscript are the work of the listed authors, and do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Shah SJ. Pulmonary hypertension. JAMA. 2012;308:1366–1374. doi: 10.1001/jama.2012.12347. [DOI] [PubMed] [Google Scholar]
- 2.Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ, White M, Aban IB, Mujib M, Dell'Italia LJ, Ahmed A. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010;121:252–258. doi: 10.1161/CIRCULATIONAHA.109.887570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu W, Ravelo A, Wagner TH, Phibbs CS, Bhandari A, Chen S, Barnett PG. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60:146S–167S. doi: 10.1177/1077558703257000. [DOI] [PubMed] [Google Scholar]
- 4.Lanzarini L, Fontana A, Campana C, Klersy C. Two simple echo-Doppler measurements can accurately identify pulmonary hypertension in the large majority of patients with chronic heart failure. J Heart Lung Transplant. 2005;24:745–754. doi: 10.1016/j.healun.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodenheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 7.Ryan JJ, Rich JD, Thiruvoipati T, Swamy R, Kim SH, Rich S. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J. 2012;163:589–594. doi: 10.1016/j.ahj.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Maron BA, Bhatt DL, Nykiel M, Kinlay S, Waxman AB. Protocol for vasoreactivity testing with epoprostenol in pulmonary hypertension. Crit Pathw Cardiol. 2012;11:40–2. doi: 10.1097/HPC.0b013e3182480725. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J. American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Opotowsky AR, Ojeda J, Rogers F, Prasanna V, Clair M, Moko L, Vaidya A, Afilalo J, Forfia PR. Circ Cardiovasc Imaging. 2012;5:765–775. doi: 10.1161/CIRCIMAGING.112.976654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128:2393–399. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 12.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevelance and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129:746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 13.Oudiz RJ. Pulmonary hypertension associated with left-sided heart disease. Clin Chest Med. 2007;28:333–41. doi: 10.1016/j.ccm.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Thenappan T, Ryan JJ, Archer SL. Evolving epidemiology of pulmonary arterial hypertension. Am J Respir Care. 2012;186:707–709. doi: 10.1164/rccm.201207-1266ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ, White M, Aban IB, Mujib, Dell'Italia LJ, Ahmed A. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010;121:252–258. doi: 10.1161/CIRCULATIONAHA.109.887570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Hearth and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 17.Jamieson SW, Kapelanski DP, Sakakibara N, Manecke GR, Thistlethwaite PA, Kerr KM, Channick RN, Fedullo PF, Auger WR. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thoracic Surg. 2003;76:1457–62. doi: 10.1016/s0003-4975(03)00828-2. discussion 1462-4. [DOI] [PubMed] [Google Scholar]
- 18.Suntharalingam J, Treacy CM, Doughty NJ, Goldsmith K, Soon F, Toshner MR, Sheares KK, Hughes R, Morrell NW, Pepke-Zaba J. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest. 2008;134:229–236. doi: 10.1378/chest.07-2681. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro BP, McGoon MD, Redfield MM. Unexplained pulmonary hypertension in elderly patients. Chest. 2007;131:94–100. doi: 10.1378/chest.06-1571. [DOI] [PubMed] [Google Scholar]
- 20.Hoeper MM, Huscher D, Ghofani HA, Delcroix M, Distler O, Schweiger C, Grunig, Staehler G, Rosenkranz S, Halank M, Held M, Grohé C, Lange TJ, Behr J, Klose H, Wilkens H, Filusch A, Germann M, Ewert R, Seyfarth HJ, Olsson KM, Opitz CF, Gaine SP, Vizza CD, Vonk-Noordegraaf A, Kaemmerer H, Gibbs JS, Pittrow D. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: Results from the COMPERA registry. Int J Cardiol. 2012 Nov 16; doi: 10.1016/j.ijcard.2012.10.026. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988–993. doi: 10.1378/chest.10-1269. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin VV, Langer A, Tan M, Clements PJ, Oudiz RJ, Tapson VF, Channick RN, Rubin LJ. Pulmonary Arterial Hypertension-Quality Enhancement Research Initiative. Contemporary trends in the diagnosis and management of pulmonary arterial hypertension: an initiative to close the care gap. Chest. 2013;143:324–332. doi: 10.1378/chest.11-3060. [DOI] [PubMed] [Google Scholar]
- 23.Deaño RC, Glassner-Kolmin C, Rubenfire M, Frost A, Visovatti S, McLaughlin VV, Gomberg-Maitland M. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: The Multicenter RePHerral Study. JAMA Intern Med. 2013;173:887–93. doi: 10.1001/jamainternmed.2013.319. [DOI] [PubMed] [Google Scholar]
- 24.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–62. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 26.Higenbottom T. Pulmonary hypertension and chronic obstructive pulmonary disease: a case for treatment. Proc Am Thorac Soc. 2005;2:12–19. doi: 10.1513/pats.200411-053SF. [DOI] [PubMed] [Google Scholar]