Abstract
Non-cardiac chest pain (NCCP) is a common and distressing condition. Prior studies suggest that psychotropic medication or pain coping skills training (CST) may benefit NCCP patients. To our knowledge, no clinical trials have examined the separate and combined effects of CST and psychotropic medication in the management of NCCP. This randomized clinical trial examined the separate and combined effects of CST and antidepressant medication (sertraline) in participants with non-cardiac chest pain. A sample of individuals diagnosed with NCCP was randomly assigned to one of four treatments: (1) CST plus sertraline (CST + sertraline), (2) CST plus placebo (CST + placebo), (3) sertraline alone, or (4) placebo alone. Assessments of pain intensity, pain unpleasantness, anxiety, pain catastrophizing, depression, and physical disability were collected prior to treatment, and at 10- and 34-weeks following randomization. Data analyses revealed that CST and sertraline either alone or in combination significantly reduced pain intensity and pain unpleasantness. The combination of CST plus sertraline may have the greatest promise in that, when compared to placebo alone, it not only significantly reduced pain but also pain catastrophizing and anxiety. Overall, these findings support the importance of further research on the effects of CST and sertraline for non-cardiac chest pain.
Keywords: Non-cardiac chest pain, Pain, Pain catastrophizing, Coping skills training, Sertraline, Anxiety
1. Introduction
Over 50% of patients who are referred for evaluation of chest pain are diagnosed as having non-cardiac chest pain (NCCP) [4,36,39], i.e., angina-like chest pain in the absence of coronary artery disease [19,20]. Despite a negative work-up and an excellent medical prognosis, over 65% of NCCP patients report persistent pain [4,39,43,45]. Research has shown that these patients also report high levels of anxiety, depression, catastrophic thinking, and physical disability [17,65,69].
The conventional medical approach to NCCP consists of a negative medical evaluation coupled with reassurance that they do not have a cardiac condition. Many NCCP patients who receive this approach remain concerned and continue to report persistent and disabling pain [4,39,43,45]. Thus, there has been growing interest in novel treatment approaches. Two promising approaches that have been developed largely independent of each other are coping skills training (CST) and antidepressant medications. CST is based on cognitive–behavioral principles and views factors such as beliefs about pain as having a major impact on non-cardiac chest pain and distress [31,33,38,40,50,51,61,65,66]. CST protocols systematically teach patients cognitive and behavioral strategies for managing pain. CST trials for NCCP show promising results [16,26,27,33, 37,40,64–66]. Klimes et al. [33] found that NCCP patients who received CST showed significant decreases in pain, depression, and activity avoidance [33]. Other studies have reported that CST can produce significant decreases in pain and other outcome measures (anxiety, depression, well being, or activity limitations) [26,40, 65,66]. Although these studies suggest CST has promise in the treatment of NCCP, they generally are limited by small sample sizes, lack of long-term follow-up, and no control or active comparison groups.
Antidepressant medications have been shown to reduce pain in patients with NCCP. Cannon et al. [13] reported that NCCP patients who received imipramine reported significant decreases in chest pain [13]. However, because this drug can cause cardiac arrhythmias, its use in clinical management of NCCP is limited. Varia et al. [67] reported that sertraline produced significant reductions in pain, but not depressive symptoms [67]. More recently, Doraiswamy et al. [18] found that paroxetine led to significant improvements in physician assessments of the health of NCCP patients, but no improvement in pain [18]. However, this study was limited by a relatively small sample and no comparison to cognitive–behavioral strategies for pain control.
To our knowledge, no methodologically rigorous trials have examined the effects of both CST and antidepressant medication in the management of NCCP. This study had two major hypotheses. First, that active treatments – CST combined with sertraline, CST plus placebo, or sertraline alone – would be more effective than placebo alone in reducing pain, anxiety, pain catastrophizing, depression, and physical disability in NCCP patients. Second, that CST plus sertraline would be more effective than CST plus placebo or sertraline alone. A secondary aim was to examine the effects of the interventions on daily pain coping and daily perceived control over pain.
2. Methods
2.1. Participants
Participants were recruited from Duke University Medical Center, satellite clinics, and the community from February 2002 to October 2005. Eligibility criteria for study entry were (a) presented for medical care with complaints of chest pain in the previous 6 months, (b) received a negative stress test within the past 2 years, normal coronary angiogram within the past 2 years, or had a survival probability [2A7E]98% at 2 years (calculated from a prognostogram developed by statistical modeling from the Duke Cardiovascular Database) [33], (c) a low likelihood of significant coronary artery disease (<25%) on the National Cholesterol Education Program’s (NCEP) modification of the Framingham Risk Calculator (FRC), (d) able to swallow oral medication, and (e) age 18–85 years.
Exclusion criteria included other cardiac problems associated with chest pain, current use of antidepressant medications or medications significantly affecting pain, a history of drug or alcohol abuse within the past 6 months, pregnancy, severe psychopathology (i.e., suicidal patients, severe depression, or psychosis), or treatment with another antidepressant within a period of less than five times the half-life of the drug concerned (e.g., fluoxetine within 5 weeks of beginning double-blind therapy). Patients who required treatment with reserpine, methyldopa, guanethidine, clonidine or Type 1C antiarrhythmics were excluded. Also excluded were patients with sensitivity to sertraline or a significant active condition that could affect absorption, distribution, or metabolism of the study drug (e.g., inflammatory bowel disease, gastric or duodenal ulcers, or lactose intolerance). All participants agreed to wean completely off opioid therapy for the treatment of chest pain. All participants provided written informed consent, and all study procedures and materials were approved by and in compliance with the Duke University Medical Center institutional review board.
Participants included 38 (33%) men and 77 (67%) women. Participants ranged in age from 22 to 74 years (M = 48, SD = 12) and average education was 14 years (SD = 3, range 6–23). Fifty-eight (50%) participants were white, 50 (44%) participants were African-American, two (2%) participants were Pacific Islander, three (3%) participants self-identified as multiracial, and two (2%) participants self-identified as other race. Approximately half (53%) of the sample was married.
2.2. Study design and attrition
After completing the baseline assessment that included 1 week of pain diary recordings, a statistician not involved in the study generated a randomization table to randomly assign participants in blocks of four to one of four treatments (online Fig. 1): (1) coping skills training plus sertraline (CST + sertraline), (2) coping skills training plus placebo (CST + placebo), (3) sertraline alone, or (4) placebo alone. Medication and placebo was given in identical capsules blind to investigator and the participant. Treatment components were blinded only for the medication and not for the CST component. Participants in the CST conditions attended five sessions held bi-weekly for 10 weeks and six sessions held monthly for 6 months (i.e., treatment was conducted over a time span of 34 weeks). For drug administration and monitoring, participants in the sertraline alone and placebo alone conditions attended five medication visits held bi-weekly for 10 weeks and then six follow-up medication visits held monthly for 6 months. Participants completed evaluations at baseline (prior to randomization), and 10 weeks and 34 weeks following randomization. Daily pain diaries were completed 1 week prior to randomization and during all 34 weeks of treatment.
Participant flow through the trial is displayed in Fig. 1. Of the 2300 patients approached, 74% (n = 1699) agreed to be screened and 26% (n = 601) declined to be screened (65% were not interested in the study, 16% were too busy, and 19% did not want to take the study medications). Of the 1699 patients who agreed to be screened, 88% (n = 1496) were not eligible to participate. Reasons for ineligibility included having a cardiac problem (76%), medications (9%), having a diagnosis of a GI problem (7%), drug or alcohol abuse (6%), severe psychopathology (1%), or could not speak English (1%). Of the 203 eligible patients, 116 (57%) agreed to participate and 87 (43%) declined participation. Of these 87, 41% were no longer interested in the study, 30% were too busy, and 29% no longer wanted to take the study medication. One participant was excluded following randomization because the patient was diagnosed as having heart disease. At the 10-weeks assessment (following the initial treatment phase), attrition (n = 17) did not differ among the four treatment groups (p > 0.20); nine participants were lost to follow-up or could not be reached, five participants withdrew from the trial, and three participants discontinued participation due to treatment side effects. At the 34 week post-treatment assessment, there were no statistically significant differences in attrition (n = 14) across the four treatment groups (p > 0.20). Between the 10-weeks and 34-weeks assessments, seven participants were lost to follow-up or could not be reached, three participants withdrew from the trial, and four participants discontinued participation due to treatment side effects.
2.3. Coping skills training (CST)
Psychologists with experience in pain coping skills training administered CST. Each psychologist received training in the CST protocol, followed a detailed session by session outline, and was closely supervised. Therapists met weekly for supervision with a senior psychologist (DM) who reviewed audiotapes of the sessions and provided feedback regarding treatment quality and adherence to the study protocol. CST was delivered in 5, 60-min individual sessions held bi-weekly for 10 weeks and 6, 30-min individual follow-up sessions held monthly for 6 months. The CST intervention was designed to (1) decrease maladaptive pain catastrophizing, and (2) enhance patients’ ability to control and decrease pain by increasing use of adaptive coping strategies (e.g., distraction, relaxation, and changing activity patterns). To introduce CST, a simplified version of Melzack and Wall’s gate control model of pain [42] was used to show that pain is affected by thoughts, feelings, and behaviors. Pain coping strategies were described as skills that can be mastered through home practice. Patients were trained in three attention diversion methods: relaxation, imagery, and distraction. Activity–rest cycling [24,28] and pleasant activity scheduling [34] were used to reduce pain and enable patients to pace and increase their activity level. Cognitive-restructuring [7] was used to help patients recognize the relationships between thoughts, feelings and behavior. This technique was also used to identify pain catastrophizing and other maladaptive thoughts and to replace these with alternative, rational coping thoughts.
The six follow-up monthly sessions paralleled the format of the five bi-weekly sessions held during the initial phase of CST. Successes and problems in using the coping skills were reviewed, and difficulties encountered were identified. Methods for coping with common problems (e.g., helplessness in response to pain flare up) were reviewed and patients were assisted in developing a plan for coping with such problems. The follow-up sessions used two techniques that can play an important role in maintenance: (1) monitoring for early warning signs of setbacks in coping efforts and (2) problem solving. The follow-up sessions were designed to encourage patients to maintain use of learned coping skills and to assist them in coping with problems encountered in using these techniques.
2.4. Medication
Participants in the sertraline alone and placebo alone conditions attended five medication visits held bi-weekly for 10 weeks and then six follow-up medication visits held monthly for 6 months. Participants in the CST conditions were seen for drug administration and monitoring at the time of their CST sessions. Sertraline (or matched placebo) was started at 50 mg per day and the dose was titrated to a maximum of 200 mg over the course of the initial 10 weeks of treatment. Dosage was adjusted by the study psychiatrist based on clinical response. After the initial 10 weeks of treatment, the dose level was stabilized for the remaining 24 weeks of the study. At each visit, adverse events were evaluated and vital signs were taken by a study nurse blind to the patient’s randomization assignment. Information about concomitant illnesses and medications also was obtained, including diagnosis, dates of onset and remission, and names and doses of medications taken. Concomitant medication without psychoactive properties was permitted as needed. Over-the-counter medications were only allowed if they were without significant antidepressant activity. At each medication visit, the study psychiatrist assessed medication adherence using a patient interview and rated adherence on a three-point scale (1 = not at all, 2 = partial, or 3 = total).
2.5. Treatment credibility
Treatment outcome can be influenced by the degree to which patients perceive a treatment as credible and expect positive outcomes [21,54]. Thus, perceptions of treatment credibility were assessed using a five-item treatment credibility questionnaire that was adapted from Borkovec and Nau [11]. Participants rated each item on a 0 (not at all) to 10 (extremely) scale. Item responses were summed to create a total score with higher scores indicating greater treatment credibility. All participants completed a treatment credibility questionnaire for the study medication. Participants in the CST conditions completed an additional treatment credibility questionnaire for CST. Treatment credibility questionnaires were completed prior to the start of treatment.
For CST, overall perceptions of treatment credibility were high (M = 41.78, SD = 9.10, range 5–50). Perceptions of CST treatment credibility did not differ between the CST + sertraline and CST + placebo conditions (p = 0.53). For the study medication, perceptions of treatment credibility were moderate to high (M = 37.59, SD = 9.62, range = 8–50). Perceptions of treatment credibility for the study medication did not differ across the four treatment conditions (p = 0.27).
2.6. Measures
2.6.1. Chest pain intensity and unpleasantness
Chest pain scores were derived from pain diaries completed daily over the week prior to randomization and the 34 weeks of treatment. Use of daily pain ratings minimizes retrospective error by assessing pain experiences close to the real-time occurrence of pain [44,60]. Visual analog scales were used to assess daily chest pain intensity and unpleasantness. Visual analog scales have been shown to be reliable and valid methods of assessing pain [57] and have been found to be sensitive to changes in pain [1,29,30]. Participants rated chest pain intensity on a scale ranging from 0 (no pain) to 100 (pain as bad as it can be) and pain unpleasantness on a scale ranging from 0 (not at all unpleasant) to 100 (unpleasant as possible). Participants were instructed to complete pain ratings before retiring each night. For each week, an average pain intensity and pain unpleasantness score were computed for participants who completed four or more days of pain diaries for that particular week. Participants who reported no pain episodes and rated pain intensity and unpleasantness as zero on all days in a week were given scores of zero for average intensity and unpleasantness for that week. In the present study, the average week-to-week correlation of pain intensity scores was 0.74 and pain unpleasantness scores was 0.73.
2.6.2. Anxiety
Anxiety was assessed during the baseline, 10-weeks, and 34-weeks evaluations using the State-Trait Anxiety Inventory [STAI; 56]. While state anxiety is defined as an acute response to a threatening or challenging situation, trait anxiety is defined as a stable and enduring tendency to be anxious. The STAI is a 40 item self-report inventory measuring both state and trait anxiety. Each item is rated on a four-point scale (1 = almost never, 4 = almost always). Items are summed to create total state and trait anxiety scores (20 items on each scale) with higher scores indicating greater anxiety. The STAI, developed as a tool for investigating anxiety in normal adult populations, has been used to assess anxiety in neuropsychiatric, medical, and surgical patients [10,41]. The trait anxiety scale has high test–retest reliability ranging from 0.73 to 0.85 and α coefficients range from 0.83 to 0.92 indicating good internal consistency [56]. Internal consistency in this sample was high (state anxiety α = 0.89; trait anxiety α = 0.93).
2.6.3. Pain catastrophizing
Pain catastrophizing was assessed during the baseline, 10-weeks, and 34-weeks evaluations. The 13-item Pain Catastrophizing Scale [PCS; 58] was used to assess the degree to which participants experienced thoughts or feelings of helplessness (six items; e.g., “It’s awful and I feel that it overwhelms me.”), magnification (three items; e.g., “I wonder whether something serious will happen.”), and rumination (four items; e.g., “I keep thinking about how much it hurts.”) during pain. Each item is rated on a five-point scale (0 = not at all to 4 = always). Items were summed to create a total PCS score. The PCS total score was used because the helplessness, magnification, and rumination domains were highly correlated (r = 0.70–0.81). The PCS total score has high internal consistency [32] and has been found to contribute to variability in pain and pain-related disability ratings even after controlling for measures of anxiety, fear of pain, negative affect, and neuroticism [59]. The PCS total score demonstrated high internal consistency in the current sample (Cronbach’s α = 0.94) [49].
2.6.4. Depression
The Beck Depression Inventory [BDI; 8] was used to assess depression during the baseline, 10-weeks, and 34-weeks evaluations. The BDI is a 21-item self-report inventory that assesses affective, cognitive, and somatic symptoms associated with depression. The items consist of statements that are scored on a 0–3 scale depending on symptom severity. Items are summed to create a total score that ranges from 0 to 63 with higher scores indicating greater depressive symptoms [8]. The BDI has demonstrated high internal consistency and test–retest reliability in both clinical and non-clinical populations [6]. Internal consistency in this sample was high (α = 0.88).
2.6.5. Physical disability
The physical disability scale of the Sickness Impact Profile [SIP; 9] was used to measure physical disability. Participants completed the SIP during the baseline, 10-weeks, and 34-weeks evaluations. The SIP is a widely used measure of disability that indicates changes or limitations in a person’s behavior due to illness [9]. The SIP was specifically developed to measure physical limitations regardless of the patient’s medical condition or disorder [9]. The SIP has been broadly tested, has demonstrated high internal consistency, and has been found to be sensitive to change not only in patients with persistent pain but in patients having cardiovascular disease, chronic illnesses, and sleep problems [9,19,25,32,62,63]. The SIP physical disability score demonstrated high internal consistency (α = 0.91).
2.6.6. Pain coping
Daily pain coping was assessed using daily diaries completed over the week prior to randomization and the 34 weeks of treatment. Two items from Stone and Neale’s Daily Coping Inventory [57] were used: (a) taking action to reduce pain: “did something specific to try to reduce the pain,” and (b) seeking emotional support: “sought emotional support from loved ones, friends, or professionals concerning my pain.” For each day, participants indicated whether they used each coping strategy by checking yes or no (yes = 1, no = 0). For each strategy, the number of times a strategy was used per week was computed. The average week-to-week correlation for taking action to reduce pain was 0.73 and seeking emotional support was 0.79.
2.6.7. Perceived control over pain
Perceived control over pain scores were derived from daily diaries completed over the week prior to randomization and the 34 weeks of treatment. Two items from the Coping Strategies Questionnaire [49] were used to assess daily perceived pain control over pain: (a) “how much control do you feel that you had over the amount of pain you experienced today by doing all the things you did to cope with pain,” and (b) “how much were you able to decrease your pain today by doing all the things you did to cope with pain.” Each item was rated on a scale ranging from 0 (no control/cannot decrease pain at all) to 6 (complete control/can completely decrease pain). These two ratings were highly correlated (r = 0.92) in this sample. The two ratings were averaged to produce a daily perceived control over pain measure. Mean perceived control over pain scores were then computed for each week using the mean daily scores for participants who completed four or more days of diaries for that particular week. In the present study, the average week-to-week correlation for perceived control over pain scores was 0.88.
2.7. Statistical analyses
We first compared the four study conditions on baseline characteristics using chi-square analyses for categorical variables and analyses of variance (ANOVA) for continuous variables. This study was designed to (1) examine whether CST + sertraline, CST+ placebo, or sertraline alone were more effective than placebo alone in reducing the intensity and unpleasantness of pain, anxiety, pain catastrophizing, depression, and physical disability, and (2) test which active treatment (CST + sertraline, CST + placebo, or sertraline alone) was the most effective. Intention-to-treat analyses were employed [3,23]. A modified Bonferroni procedure was used to control the family wise type I error rate for the six a priori comparisons at 0.05 for each of the treatment outcomes [35,48]. This procedure improves upon other modified Bonferroni procedures by incorporating the magnitude of the largest p values in determining the critical values used.
To test the study hypotheses, multilevel modeling was used as the primary analytic strategy [47]. Multilevel models are particularly useful for the analyses of longitudinal data that have a hierarchical structure with both between- and within-subject predictors. This method uses all available repeated data and allows for randomly missing observations within a subject, which is typically present in longitudinal data sets. In addition, this data analytic method is appropriate for correlated data, such as repeated measures collected from an individual. Analyses were conducted in SPSS 15.0. Time was coded so that the intercept reflected the level of the outcome variable at the initial assessment (pre- randomization). Each model included fixed effects for initial status (intercept), time, treatment condition at baseline, and the effect of treatment condition on rate of change (treatment condition × time interactions). Each model included random effects for the intercept and time. The model specifications followed Singer and Willett’s [53] recommendations for identifying the best fitting model of the variances and covariances of the variables under study. Based on these recommendations and likelihood ratio tests (α = 0.05), the unstructured covariance structure was specified for all models which allowed the random terms (slope and intercept) to be correlated. For data collected via daily diaries, models were specified to allow for heteroscedastic error variances over time. As recommended [45], for daily diary data we also determined the general form of change that best fit the data, as it was possible to include higher-order polynomial terms (e.g., quadratic term) in the model. For example, using a likelihood ratio test, if the fit of the quadratic model was not significantly better (α = 0.05), the linear model was retained. Simple slopes analyses were conducted to examine whether the rate of change within each treatment condition differed from zero.
Although multilevel modeling is able to use all available repeated data (resulting in a full intent-to-treat sample), we used two strategies to examine whether missing data patterns or treatment dropout impacted the findings. First, we repeated the analyses only including those patients who did not drop out of the study prior to the end of treatment (i.e., a completer’s analysis). Second, using pattern-mixture models [22], we examined whether missing data patterns that differed between treatment conditions were associated with baseline values of the outcome variables (e.g., pain, anxiety), or were associated with the rate of change in outcome variables in each of the treatment conditions. Missingness may be informative since patients may self-select not to participate in sessions or dropout for reasons related to overall outcome (e.g., deteriorating clinical status, or conversely, a perceived lack of need for further treatment). Pattern-mixture modeling, as described by [22], was used to examine missing data patterns. For this strategy, participants are grouped into a small number of cohorts with different patterns of missing data. This method then models the typical trajectories of responses within each of these cohorts and combines results across cohorts to make overall comparisons between treatment conditions. Two patterns of missing data were examined: (1) missingness or dropout in the initial phase of treatment (0–10 weeks) and (2) missingness or dropout in the second phase of treatment (11–34 weeks).
Sensitivity analyses were conducted to examine the impact of extreme observations as defined by the magnitude of level-1 residuals in the models. For each model, the upper and lower 1% of extreme observations were identified by examining the magnitude of level-1 residuals. Extreme observations (upper and lower 1%) were then removed from the data and each model was rerun to examine whether removal of the extreme observations would impact the model results.
Finally, several of the outcome variables were identified as having non-normal distributions (skew > 1.5). We reran the models after transforming the outcome variables using a square root transformation. Model results were the same when including transformed variables. Because interpretation of outcomes is more difficult with transformed variables, results from models with original variables are presented.
2.8. Power
Power was calculated for comparing the effect of the four treatment conditions on the rate of change in outcomes over time (i.e., treatment condition × time effects). Power calculations were based on observed model parameters and were carried out using Power In Two-level designs version 2.11 [PINT; 12].The formulae used in PINT 2.11 are derived in [55]. We first calculated power for comparing the effect of CST + sertraline, CST + placebo, and sertraline alone vs. placebo alone. For measures collected via daily diaries, the current study had 0.80 power with two-tailed α = 0.05 to detect an average effect size of d = 0.20 (range across daily diary measures d = 0.15–0.22). For measures collected during the three assessments, the current study had 0.80 power with two-tailed α = 0.05 to detect an average effect size of d = 0.40 (range across assessment measures d = 0.29–0.60). We then calculated power for comparing the effects of the three active treatment conditions. For measures collected via daily diaries, the current study had 0.80 power with two-tailed α = 0.05 to detect an average effect size of d = 0.40 (range across daily diary measures d = 0.28–0.52). For measures collected during the three assessments, the current study had 0.80 power with two-tailed α = 0.05 to detect an average effect size of d = 0.63 (range across assessment measures d = 0.46–0.85).
3. Results
3.1. Descriptive statistics and baseline treatment condition comparability
Table 1 displays the means and standard deviations of the outcome measures by treatment condition at the baseline, 10-weeks, and 34-weeks assessments. For diary data, we provide the means and standard deviations for the weeks corresponding to each of the assessment periods. There were no statistically significant differences between treatment conditions at baseline on demographic characteristics, with the exception of a trend for marital status (χ2(3) = 7.32, p = 0.06). Participants in the CST + sertraline condition were more likely to be married compared to participants in the other treatment conditions. Thus, marital status was included as a control variable in all subsequent analyses. There were no statistically significant differences between treatment conditions on baseline values of the outcome variables. However, when comparing the CST + sertraline and sertraline alone conditions vs. the CST + placebo and placebo alone conditions there was a trend for higher baseline pain intensity in the Sertraline conditions (F(1, 112) = 2.94, p = 0.09). Baseline pain intensity was included as a control variable in all subsequent analyses (excluding analyses with pain intensity and pain unpleasantness as the dependent variables).
Table 1.
Descriptive statistics for outcome measures at initial, 10 weeks, and 34 weeks assessments by treatment condition.
| Measure | Possible score range | Placebo alone M (SD) | Sertraline alone M (SD) | CST + placebo M (SD) | CST + sertraline M (SD) |
|---|---|---|---|---|---|
| Pain intensity | 0–100 | ||||
| Initial | 17.96 (20.67) | 10.81 (15.09) | 19.28 (22.77) | 13.30 (23.23) | |
| 10 weeks | 11.66 (20.58) | 8.42 (15.20) | 15.13 (23.61) | 10.26 (19.86) | |
| 34 weeks | 10.02 (16.35) | 3.55 (9.82) | 8.57 (14.87) | 6.63 (11.92) | |
| Pain unpleasantness | 0–100 | ||||
| Initial | 18.75 (23.81) | 10.84 (15.01) | 20.25 (24.34) | 13.49 (23.08) | |
| 10 weeks | 11.03 (20.34) | 7.97 (14.52) | 14.49 (23.09) | 10.92 (20.53) | |
| 34 weeks | 10.36 (16.19) | 3.23 (8.80) | 8.15 (15.26) | 6.76 (11.80) | |
| State anxiety | 20–80 | ||||
| Initial | 34.42 (8.67) | 35.55 (9.67) | 37.24 (12.06) | 35.63 (8.14) | |
| 10 weeks | 31.89 (9.89) | 32.38 (7.90) | 33.96 (13.19) | 30.57 (8.75) | |
| 34 weeks | 34.54 (11.75) | 33.25 (12.91) | 34.33 (14.64) | 29.57 (7.07) | |
| Trait anxiety | 20–80 | ||||
| Initial | 39.30 (11.78) | 38.91 (9.20) | 41.37 (12.03) | 37.71 (9.45) | |
| 10 weeks | 36.05 (9.18) | 36.61 (9.29) | 38.17 (12.43) | 33.31 (8.54) | |
| 34 weeks | 38.33 (15.10) | 37.32 (11.25) | 36.63 (14.08) | 31.70 (7.64) | |
| Pain catastrophizing | 0–52 | ||||
| Initial | 13.03 (8.99) | 13.40 (9.43) | 16.90 (14.58) | 13.22 (12.55) | |
| 10 weeks | 6.95 (7.60) | 12.91 (11.77) | 11.74 (9.27) | 8.71 (8.05) | |
| 34 weeks | 12.81 (13.28) | 11.88 (11.62) | 9.90 (9.84) | 6.49 (7.32) | |
| Depression | 0–63 | ||||
| Initial | 8.96 (7.66) | 9.10 (6.36) | 10.21 (9.00) | 9.36 (8.19) | |
| 10 weeks | 5.16 (6.03) | 7.74 (6.95) | 7.26 (8.60) | 4.43 (3.52) | |
| 34 weeks | 4.48 (3.74) | 7.05 (8.45) | 7.05 (8.45) | 4.48 (3.74) | |
| Physical disability | 0–100 | ||||
| Initial | 6.26 (10.00) | 2.78 (5.24) | 7.02 (11.34) | 3.74 (7.16) | |
| 10 weeks | 1.95 (4.43) | 4.40 (7.11) | 2.59 (3.42) | 2.05 (3.79) | |
| 34 weeks | 4.46 (9.37) | 3.06 (7.72) | 3.64 (5.34) | 2.15 (3.78) | |
| Perceived control over pai | n 0–6 | ||||
| Initial | 2.86 (2.03) | 3.31 (2.18) | 2.97 (2.16) | 3.55 (2.14) | |
| 10 weeks | 3.02 (2.39) | 4.01 (2.22) | 2.95 (2.58) | 3.52 (2.24) | |
| 34 weeks | 3.08 (2.67) | 5.32 (1.11) | 3.29 (2.48) | 3.46 (2.29) | |
| Seeking emotional support | 0–7 | ||||
| Initial | 1.00 (1.61) | 0.46 (0.93) | 0.75 (1.85) | 0.92 (1.88) | |
| 10 weeks | 0.50 (1.87) | 0.58 (1.26) | 0.05 (0.22) | 0.67 (1.72) | |
| 34 weeks | 0.70 (1.89) | 0.24 (0.75) | 0.00 (0.00) | 0.88 (2.10) | |
| Taking action to reduce pain | 0–7 | ||||
| Initial | 1.44 (2.02) | 1.33 (1.58) | 1.71 (2.35) | 1.38 (2.12) | |
| 10 weeks | 0.88 (1.82) | 0.57 (1.25) | 0.70 (1.59) | 1.75 (2.83) | |
| 34 weeks | 0.70 (1.89) | 0.59 (1.66) | 0.54 (1.13) | 2.63 (3.38) |
Note: For all measures, higher scores indicate higher levels (e.g., more pain, more anxiety, more perceived control over pain, more taking action to reduce pain). Pain intensity and pain unpleasantness = daily pain ratings using Visual Analog Scales [VAS; 58], state anxiety and trait anxiety = State-Trait anxiety Inventory [STAI; 57], pain catastrophizing = Pain Catastrophizing Scale [PCS; 59], depression = Beck Depression Inventory [BDI; 8], physical disability = Sickness Impact Profile [SIP; 9], perceived control over pain and seeking emotional support = items from Daily Coping Inventory [58], taking action to reduce pain = items from Coping Strategies Questionnaire [CSQ; 49]. Average ratings for pain variables were computed using all days in a week including days with no pain (rating = 0). As would be expected, when pain variables were computed using only days when patients reported pain, the average pain levels were higher (e.g., pain intensity grand mean at baseline on only days with pain = 32.12 (SD = 19.73) vs. pain intensity grand mean at baseline for all days = 15.10 (SD = 18.63)).
3.2. Dosing and protocol adherence
At the end of the 10-weeks initial treatment phase, 88% (n = 51) of participants in the two sertraline conditions were taking the study medication. Dosing was as follows: 8 at 50 mg, 10 at 100 mg, 13 at 150 mg, and 20 at 200 mg. The median dose of sertraline was 150 mg. There were no significant differences in dosing between participants in the two sertraline conditions (p = 0.46). In the placebo conditions, 95% (n = 54) were taking the study medication at the end of the 10-weeks initial treatment phase. Dosing in the placebo conditions was as follows: 6 at 50 mg, 2 at 100 mg, 11 at 150 mg, and 35 at 200 mg. The median dose of placebo was 200 mg. There were no significant differences in dosing between participants in the two placebo conditions (p = 0.82). For all participants, dosage of the study drug remained stable from the 10-weeks assessment to the end of treatment (34 weeks).
Attendance at the sertraline and placebo medication visits for management of the study drug was high. On average, participants attended four of the five bi-weekly medication visits (SD = 1.36, range 1–5) and four of the six monthly medication visits (SD = 2.68, range 1–6). Attendance did not significantly differ by treatment condition (p = 0.28 for bi-weekly visits and p = 0.30 for monthly visits). At each medication visit, the study psychiatrist rated the participant’s adherence to the study protocol including adherence to taking the study medication, alcohol use, and use of concomitant medications that were not permitted by the study protocol. Over the five bi-weekly medication visits, the study psychiatrist rated 88% of participants as adherent to the study protocol, 9% of participants as partially adherent, and 3% as nonadherent. Over the six monthly medication visits, the study psychiatrist rated 80% of participants as adherent to the study protocol, 13% of participants as partially adherent, and 2% as nonad-herent. Adherence ratings given during the five bi-weekly medication visits did not significantly differ across the CST + placebo, sertraline alone, and placebo alone conditions. However, more participants were rated as partially adherent or nonadherent in the CST + sertraline condition as compared to the other treatment conditions (χ2(3) = 14.66, p = 0.002). During the six monthly medication visits, adherence ratings did not significantly differ across the four treatment conditions (χ2(3) = 5.95, p = 0.11).
Attendance at the CST sessions was also high, as participants attended an average of four out of the five bi-weekly sessions (SD = 1.6, range = 0–5) and four out of the six monthly sessions (SD = 2.79, range = 0–6). For the five bi-weekly sessions, attendance was higher (F(1, 55) = 4.41, p = 0.04) in the CST + placebo group (M = 4.28, SD = 1.46, range = 1–5) compared to the CST + sertraline group (M = 3.39, SD = 1.71, range = 0–5). For the six monthly sessions, attendance did not significantly differ by treatment condition (F(1, 55) = 3.10, p = 0.08). For each CST session, the study psychologist rated the participant’s adherence to the CST protocol including session participation and completion of home practice. Over the five bi-weekly sessions, the study psychologist rated 41% of participants as adherent to the study protocol, 41% of participants as partially adherent, and 8% as nonadherent. Over the six monthly sessions, the study psychologist rated 43% of participants as adherent, 25% as partially adherent, and 32% as nonadherent. There were no significant differences between adherence ratings for the two CST conditions (p = 0.83 for the bi-weekly sessions and p = 0.83 for the monthly sessions).
3.3. Adverse effects
There were no major adverse events associated with the study medication. Participants taking sertraline were more likely to report side effects compared to those taking placebo (χ2(3) = 9.38, p = 0.03): 26% of participants in the CST + sertraline condition, 33% in the sertraline alone condition, 24% in the CST + placebo condition, and 17% in the placebo alone condition. Common side effects included dry mouth (21%, n = 24), diarrhea (16%, n = 18), sexual side effects (14%, n = 16), nausea (12%, n = 14), and headache (11%, n = 12). Eighteen patients stopped taking the study medication due to side effects: CST + sertraline n = 7, sertraline alone n = 7, CST + placebo n = 2, and placebo alone n = 2.
3.4. Treatment outcomes
Table 2 displays the fixed effects for multilevel models examining whether CST + sertraline, CST + placebo, or sertraline alone were more effective than placebo alone. In these models, the intercept is the value of the outcome variable at the initial assessment in the placebo alone condition, and the time and time2 effects indicate the rate of change in the outcome per week in the placebo alone condition. The main effects for CST + sertraline, CST + placebo, and sertraline alone indicate the difference between the initial value of the outcome variable in each of these treatment conditions compared to the placebo alone condition. The treatment condition× time interaction terms indicate the difference between the rate of change in the CST + sertraline, CST + placebo, and sertraline alone conditions compared to the rate of change in the placebo alone condition. Simple slopes analyses tested whether the rate of change (i.e., slope) within each treatment condition significantly differed from zero.
Table 2.
Fixed effects for multilevel models examining the effects of CST + sertraline, CST + placebo, and sertraline alone compared to placebo alone on treatment outcome measures.
| Outcome | Fixed effect | B | SE | t | p | 95% CI | pr |
|---|---|---|---|---|---|---|---|
| Pain intensity | Intercept | 19.60 | 4.26 | 4.60 | <0.001 | 11.16, 28.04 | – |
| Time | −0.85 | 0.16 | −5.29 | <0.001 | −1.17, −0.54 | −0.46 | |
| Time2 (quadratic term) | 0.02 | 0.003 | 6.58 | <0.001 | 0.01, 0.03 | 0.55 | |
| CST + sertraline | 7.31 | 5.60 | 1.31 | 0.20 | 18.41, 3.79 | 0.13 | |
| CST + placebo | 1.33 | 5.43 | 0.25 | 0.81 | −9.43, 12.10 | 0.02 | |
| Sertraline alone | −9.33 | 5.34 | −1.75 | 0.08 | −19.93, 1.26 | −0.17 | |
| CST + sertraline ×time | 0.31 | 0.23 | 1.34 | 0.18 | −0.15, 0.76 | 0.11 | |
| CST + placebo × time | 0.23 | 0.21 | 1.09 | 0.28 | −0.19, 0.65 | 0.07 | |
| Sertraline alone × time | 0.58 | 0.21 | 2.72 | 0.01 | 0.16, 1.00 | 0.26 | |
| CST + sertraline × time2 | −0.013 | 0.004 | −2.47 | 0.01* | −0.02, −0.002 | −0.24 | |
| CST + placebo × time2 | −0.011 | 0.004 | −3.11 | 0.002* | −0.02, −0.002 | −0.29 | |
| Sertraline alone × time2 | −0.018 | 0.004 | −4.39 | <0.001* | −0.03, −0.01 | −0.40 | |
| Pain unpleasantness | Intercept | 19.67 | 4.31 | 4.56 | <0.001 | 11.13, 28.21 | – |
| Time | −0.95 | 0.16 | −5.81 | <0.001 | −1.27, −0.63 | −0.50 | |
| Time2 (quadratic term) | 0.02 | 0.003 | 6.77 | <0.001 | 0.02, 0.03 | 0.56 | |
| CST + sertraline | −7.71 | 5.72 | −1.35 | 0.18 | −19.05, 3.64 | −0.13 | |
| CST + placebo | 0.88 | 5.55 | 0.16 | 0.87 | −10.12, 11.89 | 0.02 | |
| Sertraline alone | −9.41 | 5.46 | −1.72 | 0.09 | −20.24, 1.42 | −0.17 | |
| CST + sertraline × time | 0.44 | 0.23 | 1.89 | 0.06 | −0.02, 0.90 | 0.18 | |
| CST + placebo × time | 0.32 | 0.22 | 1.49 | 0.14 | −0.10, 0.75 | 0.15 | |
| Sertraline alone×time | 0.63 | 0.22 | 2.88 | 0.004 | 0.19, 1.05 | 0.27 | |
| CST + sertraline × time2 | −0.013 | 0.004 | −2.83 | 0.005* | −0.02, −0.004 | −0.27 | |
| CST + placebo × time2 | −0.013 | 0.004 | −3.25 | 0.001* | −0.02, −0.005 | −0.31 | |
| Sertraline alone ×time2 | −0.017 | 0.004 | −4.25 | <0.00* | −0.03, −0.009 | −0.39 | |
| State anxiety | Intercept | 30.12 | 2.49 | 12.11 | <0.001 | 25.18, 35.06 | – |
| Time | 0.04 | 0.06 | 0.71 | 0.48 | −0.08, 0.17 | 0.09 | |
| CST + sertraline | 1.25 | 2.84 | 0.44 | 0.66 | −4.38, 6.89 | 0.04 | |
| CST + placebo | 3.05 | 2.72 | 1.12 | 0.27 | 2.35, 8.44 | 0.11 | |
| Sertraline alone | 3.98 | 2.71 | 1.47 | 0.15 | −1.39, 9.36 | 0.14 | |
| CST + sertraline × time | −0.23 | 0.09 | −2.56 | 0.01* | −0.41, −0.05 | −0.29 | |
| CST + placebo × time | −0.12 | 0.08 | −1.48 | 0.15 | −0.29, 0.04 | −0.18 | |
| Sertraline alone × time | −0.14 | 0.08 | −1.64 | 0.11 | −0.30, 0.03 | −0.19 | |
| Trait anxiety | Intercept | 37.95 | 2.80 | 13.56 | <.001 | 32.39, 43.50 | – |
| Time | −0.05 | 0.06 | −0.81 | 0.42 | −0.16, 0.07 | −0.09 | |
| CST + sertraline | −1.08 | 3.20 | −0.34 | 0.74 | −7.44, 5.28 | −0.03 | |
| CST + placebo | 0.89 | 3.07 | 0.29 | 0.29 | −5.19, 6.98 | 0.03 | |
| Sertraline alone | 0.41 | 3.05 | 0.14 | 0.89 | −5.65, 6.48 | 0.01 | |
| CST + sertraline × time | −0.17 | 0.08 | −2.03 | 0.05 | −0.34, −0.003 | −0.23 | |
| CST + placebo × time | −0.08 | 0.08 | −1.06 | 0.29 | −0.24, 0.07 | −0.12 | |
| Sertraline alone × time | −0.04 | 0.08 | −0.53 | 0.60 | −0.20, 0.11 | −0.06 | |
| Pain catastrophizing | Intercept | 8.80 | 2.31 | 3.80 | <.001 | 4.20, 13.39 | – |
| Time | −0.04 | 0.07 | −0.56 | 0.58 | −0.18, 0.10 | −0.06 | |
| CST + sertraline | 3.61 | 2.64 | 2.64 | 0.17 | −1.62, 8.84 | 0.14 | |
| CST + placebo | 3.49 | 2.53 | 1.38 | 0.17 | −1.53, 8.50 | 0.14 | |
| Sertraline alone | 2.49 | 2.51 | 0.99 | 0.33 | −2.50, 7.47 | 0.10 | |
| CST + sertraline × time | −0.24 | 0.10 | −2.31 | 0.02* | −0.45, −0.03 | −0.25 | |
| CST + placebo ×time | −0.13 | 0.10 | −1.31 | 0.20 | −0.32, 0.07 | −0.15 | |
| Sertraline alone × time | −0.04 | 0.10 | −0.40 | 0.69 | −0.23, 0.15 | −0.04 | |
| Depression | Intercept | 6.80 | 1.78 | 3.82 | <0.001 | 3.26, 10.33 | – |
| Time | −0.05 | 0.05 | −0.99 | 0.34 | −0.15, 0.05 | −0.11 | |
| CST + sertraline | −0.45 | 2.08 | −0.22 | 0.83 | −4.59, 3.69 | −0.02 | |
| CST + placebo | 0.80 | 2.00 | 0.40 | 0.69 | −3.16, 4.77 | 0.04 | |
| Sertraline alone | 0.41 | 1.99 | 0.21 | 0.84 | −3.53, 4.36 | 0.02 | |
| CST + sertraline × time | −0.05 | 0.07 | −0.69 | 0.50 | −0.20, 0.10 | −0.08 | |
| CST + placebo × time | −0.05 | 0.07 | −0.77 | 0.45 | −0.19, 0.08 | −0.09 | |
| Sertraline alone × time | −0.007 | 0.07 | −0.10 | 0.92 | −0.14, 0.13 | −0.01 | |
| Physical disability | Intercept | 3.85 | 1.60 | 2.41 | 0.02 | 0.68, 7.02 | – |
| Time | −0.06 | 0.04 | −1.59 | 0.11 | −0.13, 0.01 | −0.01 | |
| CST + sertraline | −0.79 | 1.86 | −0.43 | 0.67 | −4.47, 2.88 | −0.08 | |
| CST + placebo | −0.68 | 1.78 | −0.38 | 0.70 | −4.20, 2.84 | −0.07 | |
| Sertraline alone | −1.80 | 1.77 | −1.02 | 0.31 | −5.30, 1.71 | −0.18 | |
| CST + sertraline × time | 0.04 | 0.06 | 0.70 | 0.49 | −0.07, 0.15 | 0.005 | |
| CST + placebo × time | −0.002 | 0.05 | −0.03 | 0.98 | −0.10, 0.10 | −0.001 | |
| Sertraline alone ×time | 0.07 | 0.05 | 1.28 | 0.20 | −0.04, 0.17 | 0.01 | |
| Seeking emotional support | Intercept | 1.09 | 3.03 | 3.61 | <0.001 | 0.49, 1.69 | – |
| Time | −0.03 | 0.01 | −4.28 | <0.001 | −0.04, −0.01 | −0.47 | |
| CST + sertraline | −0.21 | 0.39 | −0.54 | 0.59 | −0.98, 0.56 | −0.05 | |
| CST + placebo | −0.50 | 0.38 | −1.34 | 0.18 | −1.24. 0.24 | −0.13 | |
| Sertraline alone | −0.70 | 0.37 | −1.88 | 0.06 | −1.43, 0.04 | −0.18 | |
| CST + sertraline × time | 0.02 | 0.009 | 2.66 | 0.01* | 0.006, 0.04 | 0.32 | |
| CST + placebo × time | 0.02 | 0.008 | 2.50 | 0.02* | 0.004, 0.04 | 0.31 | |
| Sertraline alone ×time | 0.03 | 0.009 | 3.26 | 0.002* | 0.01, 0.04 | 0.39 | |
| Taking action to reduce pain | Intercept | 1.42 | 0.39 | 3.64 | <0.001 | 0.65, 2.19 | – |
| Time | −0.03 | 0.01 | −2.61 | 0.01 | −0.05, −0.007 | −0.28 | |
| CST + sertraline | −0.01 | 0.49 | −0.02 | 0.99 | −0.97, 0.96 | 0.01 | |
| CST + placebo | 0.10 | 0.47 | 0.21 | 0.84 | −0.84, 1.03 | 0.02 | |
| Sertraline alone | −0.44 | 0.47 | −0.96 | 0.34 | −0.003, 0.06 | −0.09 | |
| CST + sertraline × time | 0.03 | 0.02 | 1.81 | 0.08 | −0.03, 0.03 | 0.21 | |
| CST + placebo × time | −0.001 | 0.01 | −0.01 | 0.99 | −0.03, 0.03 | −0.01 | |
| Sertraline alone × time | 0.02 | 0.01 | 1.16 | 0.25 | −0.01, 0.04 | 0.13 | |
| Perceived control over pain | Intercept | 2.75 | 0.51 | 5.38 | <0.001 | 1.73, 3.76 | – |
| Time | 0.009 | 0.01 | 0.69 | 0.49 | −0.02, 0.03 | 0.08 | |
| CST + sertraline | 0.84 | 0.63 | 1.33 | 0.19 | −0.42, 2.09 | 0.13 | |
| CST + placebo | 0.14 | 0.61 | 0.22 | 0.82 | −1.08, 1.36 | 0.02 | |
| Sertraline alone | 0.96 | 0.60 | 1.59 | 0.12 | −0.24, 2.16 | 0.15 | |
| CST + sertraline × time | 0.006 | 0.02 | 0.35 | 0.73 | −0.03, 0.04 | 0.04 | |
| CST + placebo × time | 0.01 | 0.02 | 0.66 | 0.51 | −0.02, 0.04 | 0.07 | |
| Sertraline alone × time | 0.01 | 0.02 | 0.75 | 0.46 | −0.02, 0.05 | 0.08 |
Note: CI = confidence interval; pr = partial correlation; all models controlled for marital status. Models for anxiety, pain catastrophizing, depression, physical disability, perceived control over pain, seeking emotional support, and taking action to reduce pain controlled for initial level of chest pain. Random effects for intercept and slope were included in all models.
Pain intensity and pain unpleasantness = daily pain ratings using Visual Analog Scales [VAS; 58], state anxiety and trait anxiety = State-Trait Anxiety Inventory [STAI; 57], pain catastrophizing = Pain Catastrophizing Scale [PCS; 59], depression = Beck Depression Inventory [BDI; 8], physical disability = Sickness Impact Profile [SIP; 9], perceived control over pain and seeking emotional support = items from Daily Coping Inventory [58], taking action to reduce pain = items from Coping Strategies Questionnaire [CSQ; 49].
3.4.1. Pain intensity and unpleasantness
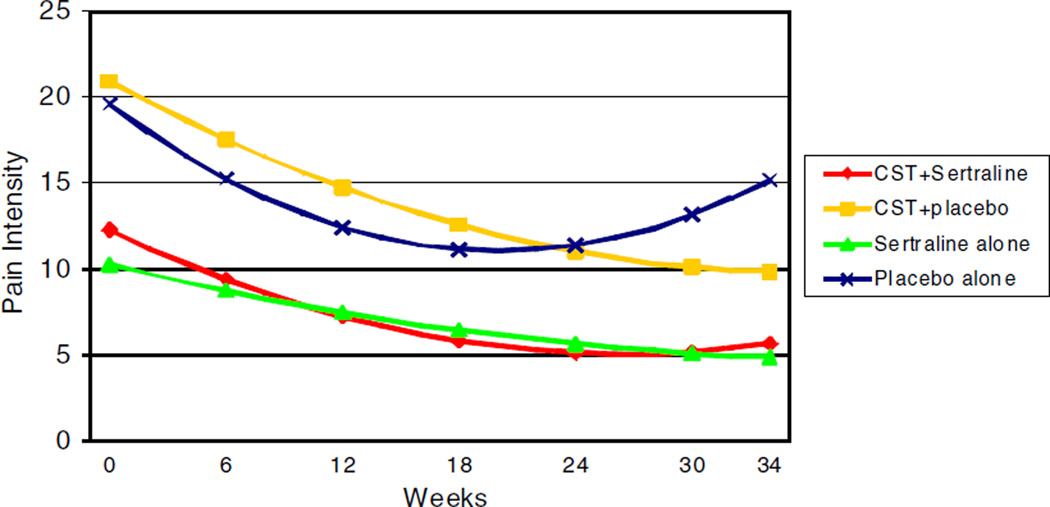
Findings were similar for pain intensity and unpleasantness. For both measures, the quadratic form of change best fit the data. The overall treatment × time2 interaction was significant for both pain intensity [F(3, 941) = 6.51, p< 0.001] and unpleasantness [F(3, 870) = 6.21, p < 0.001]. For comparisons with placebo alone, treatment × time2 interactions were significant indicating that CST + sertraline, CST + placebo, and sertraline alone resulted in greater reductions in pain intensity and unpleasantness compared to placebo alone (p values ≤ 0.001). For illustration, Fig. 2 displays the model-implied estimated trajectories of pain intensity scores over time by treatment condition. Because the quadratic time effect indicated that the amount of change in pain varied over each unit of time, we used the procedures outlined by Aiken and West [2] for probing curvilinear relationships. Simple slopes analyses were conducted at multiple timepoints: week 1 (the initial week of treatment), week 5 (mid-point of bi-weekly sessions), week 10 (end of bi-weekly sessions), week 22 (mid-point of monthly sessions), and week 34 (end of monthly sessions). Consistent with Fig. 2, results showed that pain intensity and unpleasantness significantly decreased in the CST + sertraline, CST + placebo, and sertraline alone conditions in weeks 1, 5, 10, and 22 (p values < 0.05). In contrast, while participants in the placebo alone condition showed a significant decrease in pain intensity and pain unpleasantness in weeks 1, 5, and 10 (p values < 0.05), their pain intensity and unpleasantness significantly increased in weeks 22 and 34 (p values < 0.05). Thus, the three active treatments produced initial and sustained decreases in pain intensity and unpleasantness while the placebo produced only initial decreases followed by a significant increase in these pain measures. Analyses were then conducted to examine whether there were differences in the effects of CST + sertraline, CST + placebo, or sertraline alone for reducing pain intensity and unpleasantness. These comparisons found no significant differences between the three active treatment conditions (p values > 0.50).
Fig. 2.
Model-implied estimated trajectories of pain intensity scores over time by treatment condition.
3.4.2. Anxiety
For state anxiety, there was a trend for the overall treatment × time interaction [F(3, 67) = 2.25, p = 0.09]. For comparisons with placebo alone, the treatment × time interaction for CST + sertraline was significant indicating that CST + sertraline resulted in greater reductions in anxiety over time compared to placebo alone (p = 0.01). Treatment × time interactions for CST + placebo and sertraline alone were not significant (p values [2A7E] 0.10). Simple slopes analyses showed that state anxiety significantly decreased over time in the CST + sertraline condition (B = −0.19, SE = 0.07, t= −2.84, p = 0.006, 95% CI = −0.32, −0.06), but state anxiety did not change over time in the other three treatment conditions (p values > 0.10). Analyses were then conducted to examine whether there were differences in the effects of CST + sertraline, CST + placebo, or sertraline alone for reducing state anxiety. These comparisons found no significant differences between the three active treatment conditions (p values > 0.20).
The overall treatment × time interaction was not significant for trait anxiety [F(3, 72) = 1.52, p = 0.22]. Results suggested that the CST + sertraline, CST + placebo, and sertraline alone conditions did not differ from the placebo alone condition on trait anxiety. Analyses examining whether CST + sertraline, CST + placebo, or sertraline alone was most effective for reducing trait anxiety found no significant differences between these three treatment conditions (p values > 0.10).
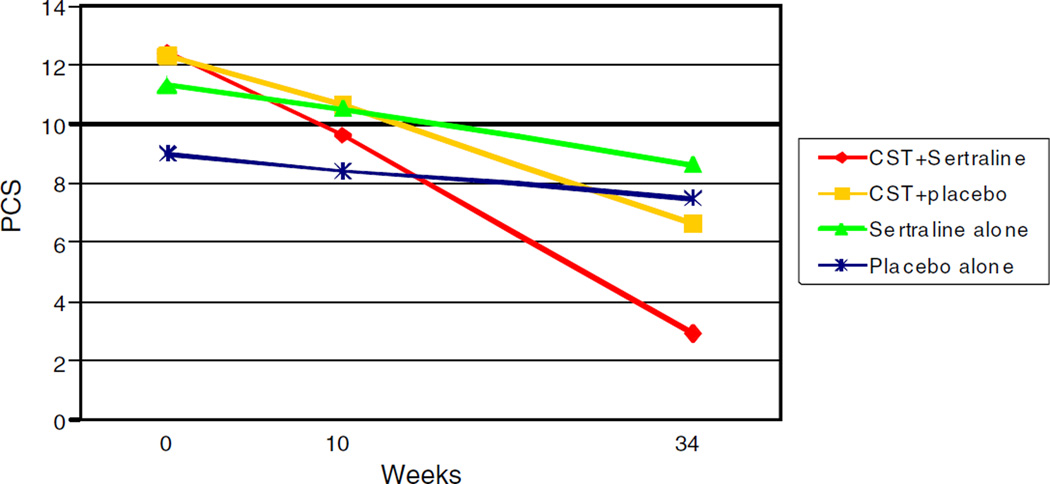
3.4.3. Pain catastrophizing
For pain catastrophizing, there was a trend for the overall treatment × time interaction [F(3, 86) = 2.21, p = 0.09]. For comparisons with placebo alone, the treatment × time interaction for CST + sertraline was significant (p = 0.02) indicating that CST + sertraline resulted in greater reductions in pain catastrophizing than placebo alone. For illustration, Fig. 3 displays the model-implied estimated trajectories of pain catastrophizing over time by treatment condition. Simple slopes analyses showed that pain catastrophizing significantly decreased over time in the CST + sertraline condition (B = −0.28, SE = 0.08, t=−3.58, p = 0.001, 95% CI = −0.43, −0.12), but did not change over time in the sertraline alone or placebo alone conditions (p values > 0.20). While the simple slope for the CST+ placebo condition significantly differed from zero (B = −0.17, SE = 0.07, t = −2.44, p = 0.02, 95% CI = −0.30, −0.03), the rate of change in the CST + placebo condition did not differ from the placebo alone condition (p = 0.20 for the CST + placebo × time effect). Analyses were then conducted to examine whether there were differences in the effects of CST + sertraline, CST + placebo, or sertraline alone for reducing pain catastrophizing. These comparisons found no significant differences between the three active treatment conditions (p values [2A7E] 0.05).
Fig. 3.
Model-implied estimated trajectories of pain catastrophizing scores over time by treatment condition.
3.4.4. Depression
The overall treatment × time interaction was not significant for depression [F(3, 77) = 0.33, p = 0.81]. Results suggested that the CST + sertraline, CST + placebo, and sertraline alone conditions did not differ from the placebo alone condition on depression. Analyses testing whether the effects of CST + sertraline, CST + placebo, or sertraline alone differed found no significant differences between these three treatment conditions on depression (p values > 0.40).
3.4.5.Physical disability
The overall treatment × time interaction was not significant for physical disability [F(3, 172) = 0.83, p = 0.48]. Analyses testing whether the effects of CST + sertraline, CST + placebo, sertraline alone, or placebo differed found no significant differences between the treatment conditions on physical disability (p values > 0.40).
3.4.6. Pain coping and perceived control over pain
For the pain coping strategy of obtaining emotional support, the linear form of change best fit the data. The overall treatment × time interaction was significant [F(3, 52) = 3.96, p = 0.01]. For comparisons with placebo alone, treatment × time interactions comparing the impact of CST + sertraline, CST + placebo, and sertraline alone vs. placebo alone on obtaining emotional support were significant (see Table 2, p values [2A7D] 0.02). Simple slopes analyses showed that use of emotional support significantly decreased over time in the placebo alone condition (B = −0.03, SE = 0.0007, t =−4.28, p< 0.001, 95% CI = −0.4, −0.01), but use of emotional support did not change over time in the other three treatment conditions (p values > 0.15). Analyses were then conducted to examine whether there were differences in the effects of CST + sertraline, CST + placebo, or sertraline alone for reducing pain coping by use of emotional support. These comparisons found no significant differences between the three active treatment conditions (p values > 0.40).
The overall treatment × time interactions were not significant for taking action to reduce pain [F(3, 61) = 1.77, p = 0.16] and perceived control over pain [F(3, 70) = 0.22, p = 0.88]. As shown in Table 2, analyses testing whether the effects of CST + sertraline, CST + placebo, sertraline alone, or placebo differed found no significant differences between the treatment conditions (p values > 0.05).
3.5. Treatment completer analyses and pattern-mixture models for missing data patterns
Analyses including only completers (i.e., patients who did not drop out of treatment prior to the end of the study) corroborated the results found with the intention-to-treat analyses for all treatment outcomes. Results were similar for all time, treatment, and treatment × time effects.
Pattern-mixture models examined whether the analyses were influenced by two missing data patterns: (1) missing data in the initial 0–10 weeks treatment phase and (2) missing data in the second 11–34 weeks treatment phase. Preliminary analyses of missing data patterns found no significant differences in the occurrence of missing data patterns across treatment conditions (χ2(6) = 6.57, p = 0.36). Pattern-mixture models indicated that there were no significant differences between participants with complete data and those with missing data on baseline levels of pain intensity, pain unpleasantness, perceived pain control, pain coping strategies, state anxiety, pain catastrophizing, or disability (p values for all missing data and missing data × treatment effects >0.10). However, having missing data in the second phase of treatment (11–34 weeks) was associated with higher levels of baseline depression and trait anxiety (p values for missing data effects < 0.05). Results for all missing data × time and missing data × treatment × time effects indicated that missing data patterns were not associated with the rate of change in the outcome variables (p values > 0.10).
3.6. Sensitivity analyses
Sensitivity analyses were conducted by removing the upper and lower 1% of extreme observations based on the magnitude of level-1 residuals and then rerunning the analyses to examine whether removal of the extreme observations would impact the model results. The results of the models after excluding extreme observations corroborated the results found with the full dataset for all treatment outcomes. Results were similar for all time, treatment, and treatment × time effects. Thus, sensitivity analyses suggested that study findings were not due to the presence of extreme observations.
4. Discussion
To our knowledge, this is the first study to compare the effects of both CST and sertraline for non-cardiac chest pain. The results suggest that these treatments can decrease the intensity and unpleasantness of NCCP patients’ pain, reduce their anxiety, and decrease the use of pain catastrophizing.
One of the most interesting findings of this study was that the three active treatments (i.e., CST + sertraline, sertraline alone, CST + placebo) had similar effects on daily diary ratings of pain. In contrast, patients who received placebo treatment showed an initial reduction in pain that was not maintained. Patients in the three active treatment groups demonstrated reductions in pain intensity, which is usually considered as measuring the evaluative component of pain. They also showed reductions in pain unpleasantness, which is considered as measuring the affective component of pain. The fact that these treatments showed effects on both pain intensity and unpleasantness suggests that these interventions may alter multiple dimensions of the pain experience. These findings suggest that CST or sertraline, whether alone or in combination, can yield both initial and sustained decreases in pain intensity and unpleasantness.
With regard to decreasing pain catastrophizing, this study found that CST + sertraline and CST + placebo were the only two conditions that produced significant decreases in pain catastrophizing during the intervention. Furthermore, of these two CST conditions, only CST + sertraline produced significantly greater changes in pain catastrophizing than placebo. These results suggest that CST may be a necessary component of interventions for NCCP, specifically to decrease pain catastrophizing. CST, which offers methods for training to identify, challenge, and manage overly negative thoughts, may be particularly effective when combined with a medication that can provide pain relief (e.g., sertraline). To put these findings into a clinical context, based on the beta weights displayed in Table 2, over the 34 weeks of treatment, participants in the CST + sertraline intervention showed an average decrease in pain catastrophizing scores of 9.52 while the participants in the CST + placebo group showed an average decrease in pain catastrophizing scores of 5.87. In contrast, the placebo alone arm showed an average change of 1.36. The fact that the CST + sertraline treatment combination had a significant effect on pain catastrophizing is important. Pain catastrophizing is believed to be a characteristic, maladaptive response to non-cardiac chest pain [59] and one which may contribute substantially to the pain and psychological distress observed in these patients [52].
This study found that only the CST + sertraline condition produced significant decreases in state anxiety during the intervention. Specifically, participants in the CST + sertraline group demonstrated significant reductions in state anxiety when compared to placebo alone. Research demonstrates that NCCP patients experience high levels of anxiety and show a high prevalence of anxiety disorders [69]. Carter et al. [15] have proposed that the high levels of anxiety experienced by NCCP patients may be related to abnormal activation of somatosensory areas in limbic regions such as the insular cortex [15]. An interesting direction for future research would be to examine the effects of CST and sertraline on brain regions related to the sensory and affective processing of pain stimuli.
Contrary to our hypotheses, none of the treatments had a significant effect on depression or physical disability. In this sample, the baseline depression measure indicated that on average, these participants had moderate levels of depressive symptoms (i.e., BDI mean = 9.28, SD = 7.58). This is not surprising given that patients with major depression were excluded from the trial. Future studies should test the effects of these interventions in NCCP patients with higher levels of depression. The BDI may also have limitations in this sample because: (1) it was designed for clinical samples and NCCP patients often report relatively low levels of clinical depression [69] and (2) it has several items with somatic content which are often highly endorsed in pain samples. A depression measure designed for use in community samples (e.g., Center for Epidemiological Studies Depression Scale [CES-D]; [46]) may have provided a greater range of scores and yielded additional information. The physical disability measure used in this study (Sickness Impact Profile) focuses on higher levels of disability related to problems with mobility and activities of daily living and did not seem to capture the types of problems experienced by these patients. This disability measure, thus, may not have been sensitive to the changes that may have occurred over the course of treatment [5] and future work should consider measures that might be more sensitive to clinically estimated improvements [e.g., Medical Outcomes Study Short-Form 36 Health Survey [SF-36; 68].
We initially hypothesized that the combined CST + sertraline condition would be more effective than either condition alone. The three active treatment conditions showed strikingly similar effects on pain intensity and unpleasantness. As hypothesized, the CST + sertraline condition demonstrated a unique ability to produce changes in anxiety and pain catastrophizing when compared to placebo alone. However, the effects of CST + sertraline on these outcomes did not differ significantly from CST + placebo or sertraline alone. It is likely that we did not find a significant difference between the three active treatment conditions for pain catastrophizing and state anxiety because the study was not powered to detect the smaller difference between these three active groups. For example, our results regarding anxiety (i.e., simple slopes analyses) showed that the CST + sertraline group was the only condition that significantly declined over time. Based on the beta weights displayed in Table 2, over the 34 weeks of treatment, participants in the CST + sertraline intervention showed an average decline in state anxiety scores of 6.46 while the participants in the CST + placebo and sertraline alone group showed declines of 2.72 and 3.40, respectively. This may suggest that there are differences between the three active treatment groups that could be detected with a larger sample size.
This study has several clinical implications. While sertraline alone produced improvements in pain intensity and unpleasantness when compared to placebo, it did not have a significant impact on other important outcomes (i.e., anxiety, pain catastrophizing). Compared to placebo alone, significant improvements in these outcomes were only obtained when patients received an intervention that combined sertraline with CST. These results suggest that a combined treatment CST plus sertraline approach might be particularly useful in clinical practice. Some NCCP patients may not be amenable to CST. In such instances, it could be useful to institute an initial course of sertraline (to produce pain relief) which is subsequently combined with CST (to produce effects on anxiety and pain catastrophizing). Future studies should explore the optimal sequencing of protocols that combine CST and sertraline.
This study had several limitations. First, over the course of the study, approximately 30% of patients dropped out. More attrition was noted in patients receiving sertraline, likely due to medication side effects with attrition somewhat higher in the sertraline alone group and CST + sertraline group. Nevertheless, both intent-to-treat analyses and completer analyses of treatment effects yielded the same results. Further, pattern-mixture models were used to examine whether the results were influenced by two missing data patterns (missing data during the initial 0–10 weeks treatment phase vs. missing data during the second 11–34 weeks treatment phase). Our analyses indicated that the patterns of missing data (i.e., missing data by time and missing data by treatment effects) were not associated with the rate of change in the outcomes. However, the pattern-mixture models showed that higher levels of baseline depression and trait anxiety were associated with having missing data near the end of treatment (e.g., 11 through 34 weeks). It will be important in future work to understand why these factors (i.e., depression, trait anxiety) are related to missing data. Second, of the participants who agreed to be screened and met study criteria, 57% agreed to participate and 43% reported they did not wish to participate. Thus, the final sample may have differed from the general population of patients with non-cardiac chest pain. While a strength of the study was that it was diverse in terms of ethnic/racial background (i.e., 44% African-American), it is also important to note that there may be differences between races in the metabolism of medications as well as response to psychological treatments [14,41]. The study sample size does not permit us to reliably assess race by treatment interactions. This is a limitation and should be considered in future work.
In summary, this study suggests that CST and sertraline either alone or in combination can be effective in reducing pain intensity and unpleasantness in non-cardiac chest pain patients. These findings have several clinical implications. First, patients who do not wish to take medications may benefit from a course of CST. Second, patients who cannot participate in a series of sessions for training in pain CST may benefit from sertraline. Our findings also suggest that the combination the CST + sertraline may have the greatest promise in that, when compared to placebo alone, it not only significantly reduced pain but also pain catastrophizing and anxiety. Taken together, these findings suggest that further research is warranted on the effects of CST and sertraline for non-cardiac chest pain.
Supplementary Material
Acknowledgements
The authors wish to thank Pfizer for providing the study medications used in this trial. This study was supported by a grant from NIMH (R01 MH63429).
Footnotes
Note: The study medication and placebo were provided by Pfizer.
Conflict of interest statement
The authors on this manuscript report no conflict of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.pain.2010.08.040.
References
- 1.Affleck G, Urrows S, Tennen H, Higgins P. Daily coping with pain from rheumatoid arthritis: patterns and correlates. Pain. 1992;51:221–229. doi: 10.1016/0304-3959(92)90263-B. [DOI] [PubMed] [Google Scholar]
- 2.Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 3.Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, Gotzsche PC, Lang T. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 4.Bass C, Mayou RA. Chest pain and palpitations. In: Mayou RA, Bass C, Sharpe M, editors. Treatment of functional somatic symptoms. Oxford: Oxford University Press; 1995. pp. 328–352. [Google Scholar]
- 5.Beaton DE, Bombardier C, Hogg-Johnson S. Choose your tool: a comparison of the psychometric properties of five generic health status instruments in workers with soft tissue injuries. Qual Life Res. 1994;3:50–56. [Google Scholar]
- 6.Beck AT, Beamesderfer KA. Assessment of depression: the depression inventory. In: Pichot P, editor. Modern problems in pharmacopsychiatry. Basel, Switzerland: Karger; 1974. pp. 15–169. [DOI] [PubMed] [Google Scholar]
- 7.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy and depression. New York: The Guilford Press; 1979. [Google Scholar]
- 8.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 9.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The sickness impact profile: development and final revision of a health status measure. Med Care. 1981;19:787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Bieling PJ, Antony MM, Swinson RP. The state-trait anxiety inventory, trait version: structure and content re-examined. Behav Res Ther. 1998;36:777–788. doi: 10.1016/s0005-7967(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 11.Borkovec TD, Nau CD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry. 1972;3:257–260. [Google Scholar]
- 12.Bosker RJ, Snijders TAB, Guldemond H. PINT (Power in Two-Level Designs): estimating standard errors of regression coefficients in hierarchical linear models for power calculations, user’s manual; 2003. Available from http://stat.gamma.rug.nl/snijders/ [retrieved February 2004]
- 13.Cannon 3rd RO, Quyyumi AA, Mincemoyer R, Stine AM, Gracely RH, Smith WB, Geraci MF, Black BC, Uhde TW, Waclawiw MA, Maher K, Benjamin SB. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. 1994;330:1411–1417. doi: 10.1056/NEJM199405193302003. [DOI] [PubMed] [Google Scholar]
- 14.Chen ML. Ethnic or racial differences revisited: impact of dosage regimen and dosage form on pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2006;45:957–964. doi: 10.2165/00003088-200645100-00001. [DOI] [PubMed] [Google Scholar]
- 15.Carter CS, Servan-Schreiber D, Perlstein WM. Anxiety disorders and the syndrome of chest pain with normal coronary arteries: prevalence and pathophysiology. J Clin Psychiatry. 1997;58:70–73. discussion 74-5. [PubMed] [Google Scholar]
- 16.Clark DM. A cognitive approach to panic. Behav Res Ther. 1986;24:461–470. doi: 10.1016/0005-7967(86)90011-2. [DOI] [PubMed] [Google Scholar]
- 17.Dammen T, Arnesen H, Ekeberg O, Husebye T, Friis S. Panic disorder in chest pain patients referred for cardiological outpatient investigation. J Intern Med. 1999;245:497–507. doi: 10.1046/j.1365-2796.1999.00447.x. [DOI] [PubMed] [Google Scholar]
- 18.Doraiswamy PM, Varia I, Hellegers C, Wagner HR, Clary GL, Beyer JL, Newby LK, O’Connor JF, Beebe KL, O’Connor C, Krishnan KR. A randomized controlled trial of paroxetine for noncardiac chest pain. Psychopharmacol Bull. 2006;39:15–24. [PubMed] [Google Scholar]
- 19.Esler JL, Bock BC. Psychological treatments for noncardiac chest pain: recommendations for a new approach. J Psychosom Res. 2004;56:263–269. doi: 10.1016/S0022-3999(03)00515-4. [DOI] [PubMed] [Google Scholar]
- 20.Fass R. Chest pain of esophageal origin. Curr Opin Gastroenterol. 2002;18:464–470. doi: 10.1097/00001574-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Goossens ME, Vlaeyen JW, Hidding A, Kole-Snijders A, Evers SM. Treatment expectancy affects the outcome of cognitive-behavioral interventions in chronic pain. Clin J Pain. 2005;21:18–26. doi: 10.1097/00002508-200501000-00003. discussion 69–72. [DOI] [PubMed] [Google Scholar]
- 22.Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Method. 1997;2:64–78. [Google Scholar]
- 23.Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ. 1999;319:670–674. doi: 10.1136/bmj.319.7211.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houpt JL, Keefe FJ, Snipes MT. The clinical specialty unit: the use of the psychiatry inpatient unit to treat chronic pain syndromes. Gen Hosp Psychiatry. 1984;6:65–70. doi: 10.1016/0163-8343(84)90061-6. [DOI] [PubMed] [Google Scholar]
- 25.Jensen MP, Strom SE, Turner JA, Romano JM. Validity of the sickness impact profile roland scale as a measure of dysfunction in chronic pain patients. Pain. 1992;50:157–162. doi: 10.1016/0304-3959(92)90156-6. [DOI] [PubMed] [Google Scholar]
- 26.Jones H, Cooper P, Miller V, Brooks N, Whorwell PJ. Treatment of non-cardiac chest pain: a controlled trial of hypnotherapy. Gut. 2006;55:1403–1408. doi: 10.1136/gut.2005.086694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katon W, Egan K, Miller D. Chronic pain: lifetime psychiatric diagnoses and family history. Am J Psychiatry. 1985;142:1156–1160. doi: 10.1176/ajp.142.10.1156. [DOI] [PubMed] [Google Scholar]
- 28.Keefe FJ. Behavioral assessment and treatment of chronic pain: current status and future directions. J Consult Clin Psychol. 1982;50:896–911. doi: 10.1037//0022-006x.50.6.896. [DOI] [PubMed] [Google Scholar]
- 29.Keefe FJ, Block AR. Development of an observation method for assessing pain behavior in low back pain patients. Behav Ther. 1982;13:363–376. [Google Scholar]
- 30.Keefe FJ, Caldwell DS. Cognitive behavioral control of arthritis pain. Med Clin North Am. 1997;81:277–290. doi: 10.1016/s0025-7125(05)70515-0. [DOI] [PubMed] [Google Scholar]
- 31.Keefe FJ, Ward JA, Lefebvre JC. Intervention specific response rates in behavioral treatments for chronic pain. Mind/Body Med. 1997;2:190–196. [Google Scholar]
- 32.Kerns RD, Jacob MC. Assessment of the psychosocial context of the experience of chronic pain. In: Turk DC, Melzack R, editors. Handbook of pain assessment. New York: Guilford Press; 1992. [Google Scholar]
- 33.Klimes I, Mayou RA, Pearce MJ, Coles L, Fagg JR. Psychological treatment for atypical non-cardiac chest pain: a controlled evaluation. Psychol Med. 1990;20:605–611. doi: 10.1017/s0033291700017116. [DOI] [PubMed] [Google Scholar]
- 34.Lewinsohn PM. The behavioral study and treatment of depression. In: Hersen M, Eisler RM, Miller PM, editors. Progress in behavior modification, vol. 1. New York: Academic Press; 1975. pp. 19–65. [Google Scholar]
- 35.Li J. A two-step rejection procedure for testing multiple hypotheses. J Statistical Planning Inference. 2008;138:1521–1527. [Google Scholar]
- 36.Mayou R. Invited review: atypical chest pain. J Psychosom Res. 1989;33:393–406. doi: 10.1016/0022-3999(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 37.Mayou R. Chest pain, palpitations and panic. J Psychosom Res. 1998;44:53–70. doi: 10.1016/s0022-3999(97)00209-2. [DOI] [PubMed] [Google Scholar]
- 38.Mayou RA, Bass CM, Bryant BM. Management of non-cardiac chest pain: from research to clinical practice. Heart. 1999;81:387–392. doi: 10.1136/hrt.81.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayou R, Bryant B, Forfar C, Clark D. Non-cardiac chest pain and benign palpitations in the cardiac clinic. Br Heart J. 1994;72:548–553. doi: 10.1136/hrt.72.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayou RA, Bryant BM, Sanders D, Bass C, Klimes I, Forfar C. A controlled trial of cognitive behavioural therapy for non-cardiac chest pain. Psychol Med. 1997;27:1021–1031. doi: 10.1017/s0033291797005254. [DOI] [PubMed] [Google Scholar]
- 41.McIlyan JM, Baker TA, Mingo CA, Haley WE. Are behavioral interventions for arthritis effective with minorities? Addressing racial and ethnic diversity in disability and rehabilitation. Arthritis Rheum. 2008;59:1512–1518. doi: 10.1002/art.24117. [DOI] [PubMed] [Google Scholar]
- 42.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 43.Papanicolaou MN, Califf RM, Hlatky MA, McKinnis RA, Harrell FE, Jr, Mark DB, McCants B, Rosati RA, Lee KL, Pryor DB. Prognostic implications of angiographically normal and insignificantly narrowed coronary arteries. Am J Cardiol. 1986;58:1181–1187. doi: 10.1016/0002-9149(86)90378-4. [DOI] [PubMed] [Google Scholar]
- 44.Parker JC, Smarr KL, Buescher KL, Phillips LR, Frank RG, Beck NC, Anderson SK, Walker SE. Pain control and rational thinking. Implications for rheumatoid arthritis. Arthritis Rheum. 1989;32:984–990. doi: 10.1002/anr.1780320807. [DOI] [PubMed] [Google Scholar]
- 45.Potts SG, Bass CM. Psychological morbidity in patients with chest pain and normal or near-normal coronary arteries: a long-term follow-up study. Psychol Med. 1995;25:339–347. doi: 10.1017/s0033291700036242. [DOI] [PubMed] [Google Scholar]
- 46.Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 47.Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 48.Rom DM. A sequentially rejective test procedure on a modified Bonferroni inequality. Biometrika. 1990;77:663–665. [Google Scholar]
- 49.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 50.Salkovskis PM. Psychological treatment of noncardiac chest pain: the cognitive approach. Am J Med. 1992;92:114S–121S. doi: 10.1016/0002-9343(92)80066-9. [DOI] [PubMed] [Google Scholar]
- 51.Sanders D, Bass C, Mayou RA, Goodwin S, Bryant BM, Tyndel S. Non-cardiac chest pain: why was a brief intervention apparently ineffective? Psychol Med. 1997;27:1033–1040. doi: 10.1017/s0033291797005266. [DOI] [PubMed] [Google Scholar]
- 52.Shelby RA, Somers TJ, Keefe FJ, Silva SG, McKee DC, She L, Waters SJ, Varia I, Riordan YB, Knowles VM, Blazing M, Blumenthal JA, Johnson P. Pain catastrophizing in patients with noncardiac chest pain: relationships with pain, anxiety, and disability. Psychosom Med. 2009;71:861–868. doi: 10.1097/PSY.0b013e3181b49584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 54.Smeets RJ, Beelen S, Goossens ME, Schouten EG, Knottnerus JA, Vlaeyen JW. Treatment expectancy and credibility are associated with the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. Clin J Pain. 2008;24:305–315. doi: 10.1097/AJP.0b013e318164aa75. [DOI] [PubMed] [Google Scholar]
- 55.Snijders TAB, Bosker RJ. Standard errors and sample sizes for two-level research. J Educational Statist. 1993;18:237–259. [Google Scholar]
- 56.Spielberger CD. Manual for the state-trait anxiety inventory (Form Y) Palo Alto, CA: Mind Garden; 1983. [Google Scholar]
- 57.Stone A, Neale J. New measure of daily coping: development and preliminary results. J Personal Soc Psychol. 1984;46:892–906. [Google Scholar]
- 58.Sullivan M, Bishop S, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 59.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Tennen H, Suls J, Affleck G. Personality and daily experience: the promise and the challenge. J Personal. 1991;59:892–906. [Google Scholar]
- 61.Turk DC, Meichenbaum D, Genest M. Pain and behavioral medicine: a cognitive–behavior perspective. New York: The Guilford Press; 1983. [Google Scholar]
- 62.Turner JA, Clancy S. Comparison of operant behavioral and cognitive-behavioral group treatment for chronic low back pain. J Consult Clin Psychol. 1988;56:261–266. doi: 10.1037//0022-006x.56.2.261. [DOI] [PubMed] [Google Scholar]
- 63.Turner JA, Clancy S, McQuade KJ, Cardenas DD. Effectiveness of behavioral therapy for chronic low back pain: a component analysis. J Consult Clin Psychol. 1990;58:573–579. doi: 10.1037//0022-006x.58.5.573. [DOI] [PubMed] [Google Scholar]
- 64.Tyrer P, Lee I, Alexander J. Awareness of cardiac perception in patients with panic attacks: a field study. Behav Res Ther. 1991;29:137–145. doi: 10.1016/0005-7967(91)90042-2. [DOI] [PubMed] [Google Scholar]
- 65.Van Peski-Oosterbaan AS, Spinhoven P, Van der Does AJ, Bruschke AV, Rooijmans HG. Cognitive change following cognitive behavioural therapy for non-cardiac chest pain. Psychother Psychosom. 1999;68:214–220. doi: 10.1159/000012335. [DOI] [PubMed] [Google Scholar]
- 66.Van Peski-Oosterbaan AS, Spinhoven P, van Rood Y, van der Does JW, Bruschke AV, Rooijmans HG. Cognitive-behavioral therapy for noncardiac chest pain: a randomized trial. Am J Med. 1999;106:424–429. doi: 10.1016/s0002-9343(99)00049-2. [DOI] [PubMed] [Google Scholar]
- 67.Varia I, Logue E, O’Connor C, Newby K, Wagner HR, Davenport C, Rathey K, Krishnan KR. Randomized trial of sertraline in patients with unexplained chest pain of noncardiac origin. Am Heart J. 2000;140:367–372. doi: 10.1067/mhj.2000.108514. [DOI] [PubMed] [Google Scholar]
- 68.Ware JE, Sherbourne CD. The MOS36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 69.White KS, Raffa SD, Jakle KR, Stoddard JA, Barlow DH, Brown TA, Covino NA, Ullman E, Gervino EV. Morbidity of DSM-IV axis I disorders in patients with noncardiac chest pain: psychiatric morbidity linked with increased pain and health care utilization. J Consult Clin Psychol. 2008;76:422–430. doi: 10.1037/0022-006X.76.3.422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.