Abstract
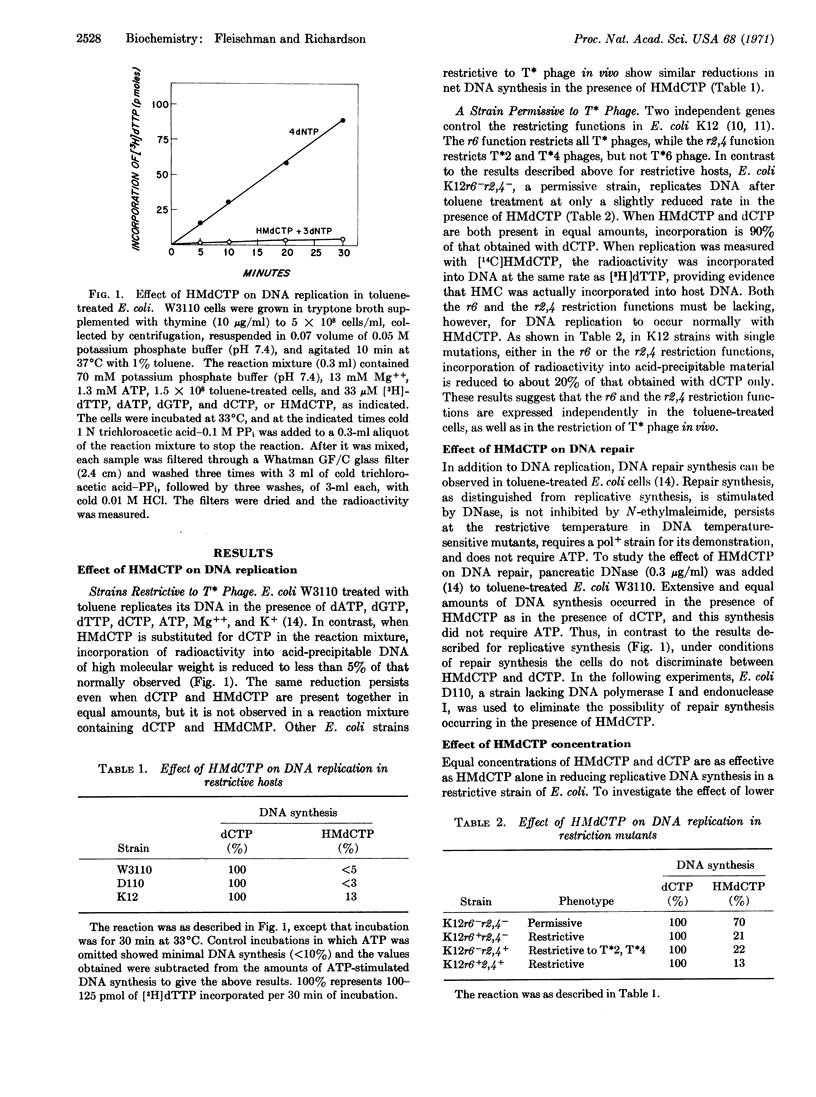
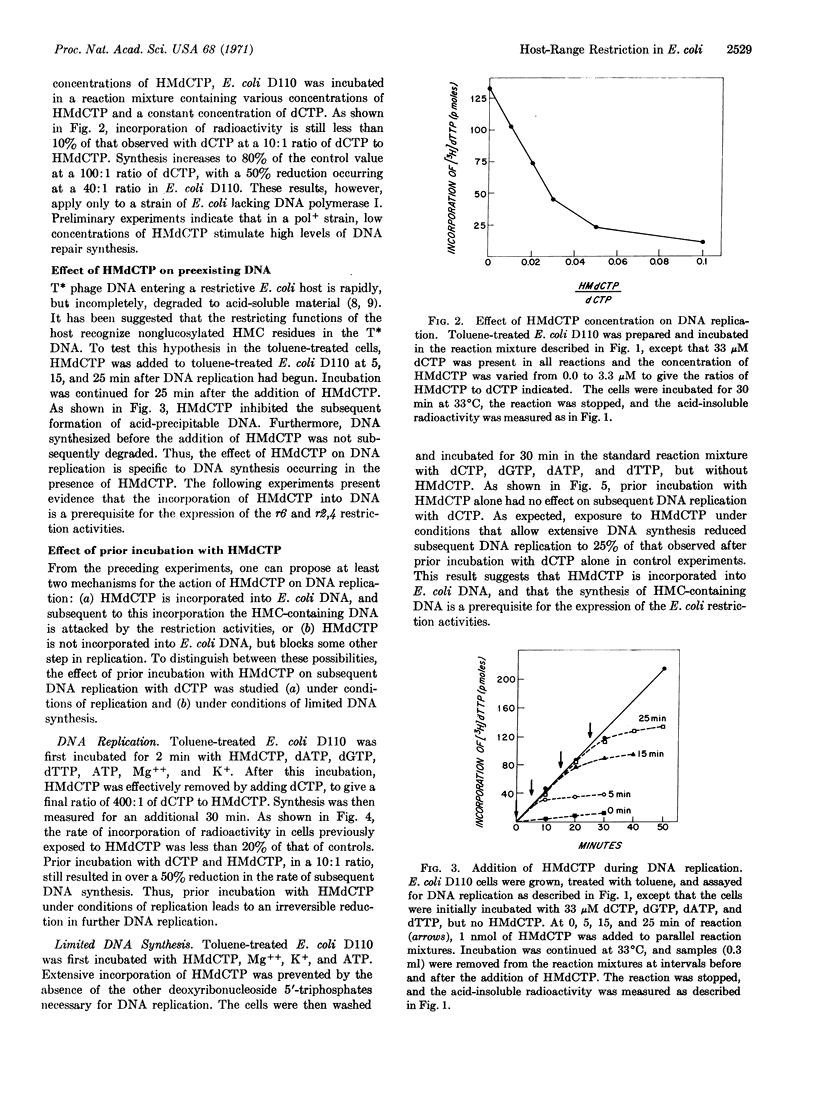
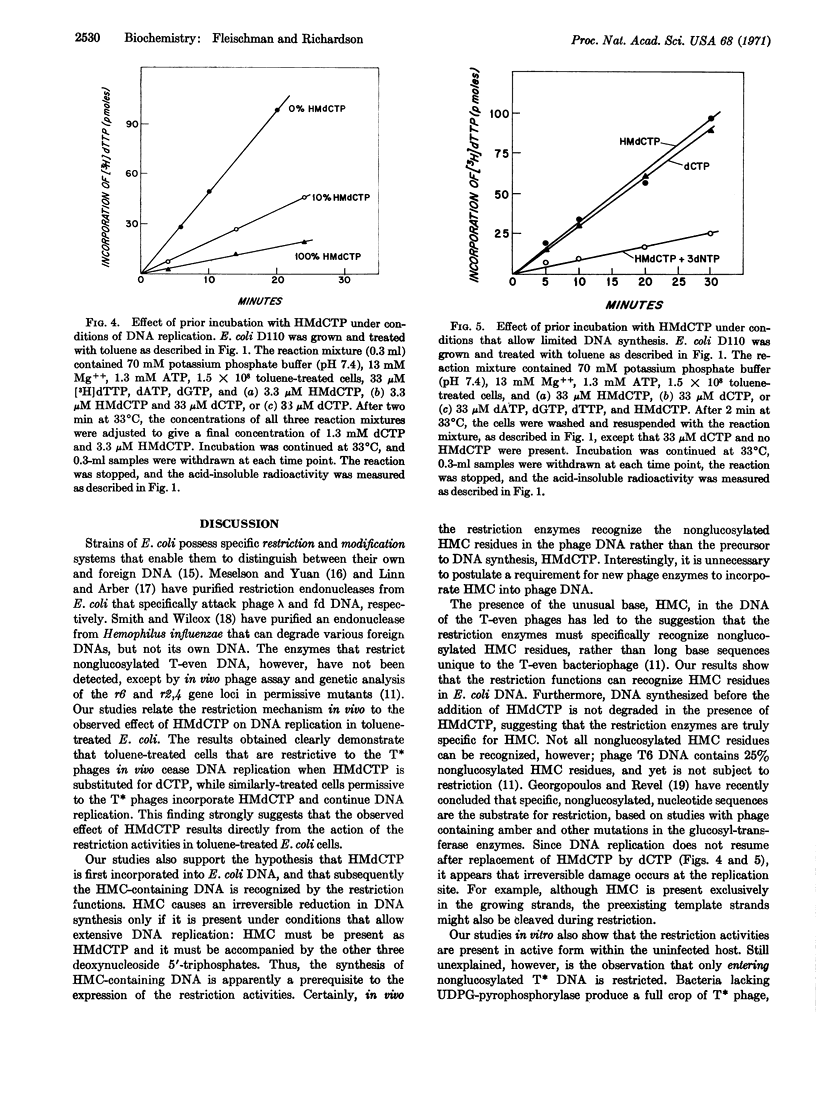
Escherichia coli cells treated with toluene replicate DNA when they are provided with deoxyribonucleoside 5′-triphosphates, ATP, Mg++, and K+. However, when deoxycytidine 5′-triphosphate is replaced by hydroxymethyl deoxycytidine 5′-triphosphate, incorporation of nucleotides into acid-precipitable material by toluenetreated strains restrictive to nonglucosylated T-even phage is reduced to less than 5% of that normally observed. Even when dCTP is present in the reaction mixture, a similar effect of the hydroxymethyl analogue on DNA replication is observed. In contrast, toluene-treated E. coli K12r6-r2,4-, a strain permissive to the nonglucosylated T-even phage, incorporates hydroxymethyl deoxycytosine into its DNA, and replication proceeds at only a slightly reduced rate in the presence of the hydroxymethyl deoxycytidine 5′-triphosphate. The presence of the hydroxymethyl deoxycytidine 5′-triphosphate in the reaction mixture does not lead to degradation of preexisting DNA of the restrictive host, but it does lead to an irreversible inhibition of further DNA replication; the inhibition is observed only when the hydroxymethyl deoxycytidine 5′-triphosphate is present during replication. Thus phage-specific enzymes are not necessary for the incorporation of hydroxymethylcytosine into phage DNA, and the restrictive mechanism, present in the host cell before infection, can recognize hydroxymethylcytosine residues in its own DNA, as well as the DNA of the T-even phage.
Keywords: hydroxymethylcytosine, hydroxymethylcytidine triphosphate, T-even phages, DNA replication
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W., Linn S. DNA modification and restriction. Annu Rev Biochem. 1969;38:467–500. doi: 10.1146/annurev.bi.38.070169.002343. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., SAITO S. THE COURSE OF INFECTION WITH T-EVEN PHAGES ON MUTANTS OF ESCHERICHIA COLI K12 DEFECTIVE IN THE SYNTHESIS OF URIDINE DIPHOSPHOGLUCOSE. J Mol Biol. 1964 Feb;8:175–183. doi: 10.1016/s0022-2836(64)80127-3. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T. THE COURSE OF INFECTION WITH ABNORMAL BACTERIOPHAGE T4 CONTAINING NON-GLUCOSYLATED DNA ON ESCHERICHIA COLI STRAINS. J Mol Biol. 1964 Aug;9:525–536. doi: 10.1016/s0022-2836(64)80224-2. [DOI] [PubMed] [Google Scholar]
- HATTMAN S., FUKASAWA T. HOST-INDUCED MODIFICATION OF T-EVEN PHAGES DUE TO DEFECTIVE GLUCOSYLATION OF THEIR DNA. Proc Natl Acad Sci U S A. 1963 Aug;50:297–300. doi: 10.1073/pnas.50.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATTMAN S. THE FUNCTIONING OF T-EVEN PHAGES WITH UNGLUCOSYLATED DNA IN RESTRICTING ESCHERICHIA COLI HOST CELLS. Virology. 1964 Nov;24:333–348. doi: 10.1016/0042-6822(64)90171-0. [DOI] [PubMed] [Google Scholar]
- Jackson R. W., DeMoss J. A. Effects of toluene on Escherichia coli. J Bacteriol. 1965 Nov;90(5):1420–1425. doi: 10.1128/jb.90.5.1420-1425.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG S. R., ZIMMERMAN S. B., KORNBERG A. Glucosylation of deoxyribonucleic acid by enzymes from bacteriophage-infected Escherichia coli. J Biol Chem. 1961 May;236:1487–1493. [PubMed] [Google Scholar]
- LEHMAN I. R., PRATT E. A. On the structure of the glucosylated hydroxymethylcytosine nucleotides of coliphages T2, T4, and T6. J Biol Chem. 1960 Nov;235:3254–3259. [PubMed] [Google Scholar]
- LURIA S. E., HUMAN M. L. A nonhereditary, host-induced variation of bacterial viruses. J Bacteriol. 1952 Oct;64(4):557–569. doi: 10.1128/jb.64.4.557-569.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn S., Arber W. Host specificity of DNA produced by Escherichia coli, X. In vitro restriction of phage fd replicative form. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1300–1306. doi: 10.1073/pnas.59.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REVEL H. R., HATTMAN S., LURIA S. E. MUTANTS OF BACTERIOPHAGES T2 AND T6 DEFECTIVE IN ALPHA-GLUCOSYL TRANSFERASE. Biochem Biophys Res Commun. 1965 Feb 17;18:545–550. doi: 10.1016/0006-291x(65)90788-6. [DOI] [PubMed] [Google Scholar]
- Revel H. R., Luria S. E. DNA-glucosylation in T-even phage: genetic determination and role in phagehost interaction. Annu Rev Genet. 1970;4(0):177–192. doi: 10.1146/annurev.ge.04.120170.001141. [DOI] [PubMed] [Google Scholar]
- Revel H. R. Restriction of nonglucosylated T-even bacteriophage: properties of permissive mutants of Escherichia coli B and K12. Virology. 1967 Apr;31(4):688–701. doi: 10.1016/0042-6822(67)90197-3. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Influence of glucosylation of deoxyribonucleic acid on hydrolysis by deoxyribonucleases of Escherichia coli. J Biol Chem. 1966 May 10;241(9):2084–2092. [PubMed] [Google Scholar]
- SHEDLOVSKY A., BRENNER S. A CHEMICAL BASIS FOR THE HOST-INDUCED MODIFICATION OF T-EVEN BACTERIOPHAGES. Proc Natl Acad Sci U S A. 1963 Aug;50:300–305. doi: 10.1073/pnas.50.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. W., Hanawalt P. C. Properties of the growing point region in the bacterial chromosome. Biochim Biophys Acta. 1967 Dec 19;149(2):519–531. doi: 10.1016/0005-2787(67)90180-3. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- WYATT G. R., COHEN S. S. The bases of the nucleic acids of some bacterial and animal viruses: the occurrence of 5-hydroxymethylcytosine. Biochem J. 1953 Dec;55(5):774–782. doi: 10.1042/bj0550774. [DOI] [PMC free article] [PubMed] [Google Scholar]



