Abstract
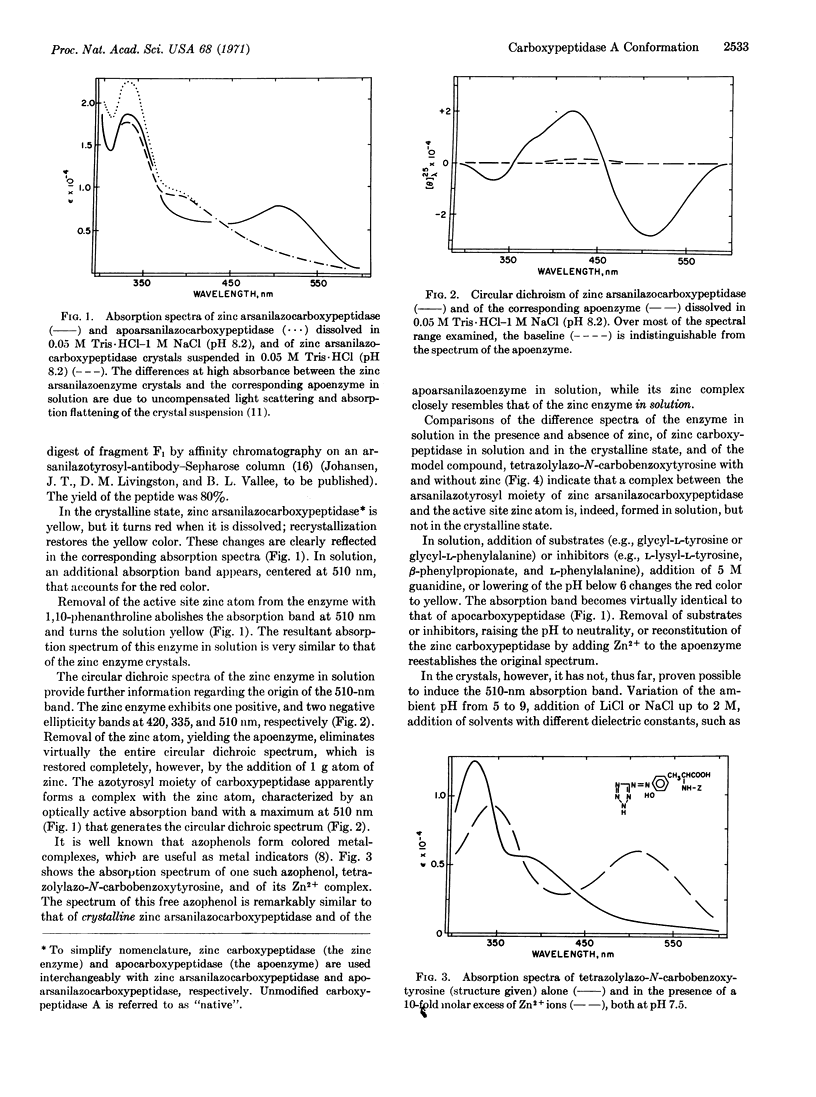
Coupling of carboxypeptidase A crystals with diazotized arsanilic acid specifically labels tyrosine 248, an active-site residue of the enzyme. Many azophenols are yellow and their zinc complexes are red; the “yellow” absorption spectrum of zinc arsanilazocarboxypeptidase crystals is characteristic of the arsanilazotyrosyl group, not of the zinc complex. This is consistent with the interpretation of x-ray data on native crystals of carboxypeptidase A, indicating that tyrosine 248 and the zinc atom are too far apart to form a complex. However, the enzyme in solution is red, denoting the formation of a complex between zinc and arsanilazotyrosine 248. The most likely interpretation of the data is that the orientation of arsanilazotyrosine 248 in solution and in the crystal is different. If the unlabeled tyrosine 248 of native carboxypeptidase undergoes similar changes, these data may bear upon the low activity of the enzyme in the crystalline state and on the catalytic mechanism of the enzyme based on the crystal structure. The opportunities for analogous spectrochemical studies of other, similar systems are pointed out.
Keywords: circular dichroism, azoprobe, visible spectrum
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Nickless G. Heterocyclic azo dyestuffs in analytical chemistry. A review. Analyst. 1967 Apr;92(93):207–238. doi: 10.1039/an9679200207. [DOI] [PubMed] [Google Scholar]
- Arnone A., Bier C. J., Cotton F. A., Day V. W., Hazen E. E., Jr, Richardson D. C., Yonath A., Richardson J. S. A high resolution structure of an inhibitor complex of the extracellular nuclease of Staphylococcus aureus. I. Experimental procedures and chain tracing. J Biol Chem. 1971 Apr 10;246(7):2302–2316. [PubMed] [Google Scholar]
- Bradshaw R. A., Ericsson L. H., Walsh K. A., Neurath H. The amino acid sequence of bovine carboxypeptidase A. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1389–1394. doi: 10.1073/pnas.63.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUYSENS L. N. The flattening of the absorption spectrum of suspensions, as compared to that of solutions. Biochim Biophys Acta. 1956 Jan;19(1):1–12. doi: 10.1016/0006-3002(56)90380-8. [DOI] [PubMed] [Google Scholar]
- Fairclough G. F., Jr, Vallee B. L. Characteristic extrinsic Cotton effects of azo proteins. Biochemistry. 1970 Oct 13;9(21):4087–4094. doi: 10.1021/bi00823a009. [DOI] [PubMed] [Google Scholar]
- Fretto L., Strickland E. H. Effect of temperature upon the conformations of carboxypeptidase A (Anson), A gamma Leu, A gamma Val, AND A alpha + beta. Biochim Biophys Acta. 1971 Jun 16;235(3):473–488. doi: 10.1016/0005-2744(71)90289-0. [DOI] [PubMed] [Google Scholar]
- Kagan H. M., Vallee B. L. Environmental sensitivity of azo chromophores in arsanilazocarboxypeptidase. Biochemistry. 1969 Nov;8(11):4223–4231. doi: 10.1021/bi00839a001. [DOI] [PubMed] [Google Scholar]
- LEVIN Y., PECHT M., GOLDSTEIN L., KATCHALSKI E. A WATER-INSOLUBLE POLYANIONIC DERIVATIVE OF TRYPSIN. I. PREPARATION AND PROPERTIES. Biochemistry. 1964 Dec;3:1905–1913. doi: 10.1021/bi00900a021. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- Lipscomb W. N., Reeke G. N., Jr, Hartsuck J. A., Quiocho F. A., Bethge P. H. The structure of carboxypeptidase A. 8. Atomic interpretation at 0.2 nm resolution, a new study of the complex of glycyl-L-tyrosine with CPA, and mechanistic deductions. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):177–214. doi: 10.1098/rstb.1970.0020. [DOI] [PubMed] [Google Scholar]
- Neurath H., Bradshaw R. A., Ericsson L. H., Babin D. R., Petra P. H., Walsh K. A. Current status of the chemical structure of bovine pancreatic carboxypeptidase A. Brookhaven Symp Biol. 1968 Jun;21(1):1–23. [PubMed] [Google Scholar]
- Nomoto M., Srinivasan N. G., Bradshaw R. A., Wade R. D., Neurath H. The amino acid sequence of bovine carboxypeptidase A. I. Preparation and properties of the fragments obtained by cyanogen bromide cleavage. Biochemistry. 1969 Jul;8(7):2755–2762. doi: 10.1021/bi00835a010. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Reeke G. N., Hartsuck J. A., Ludwig M. L., Quiocho F. A., Steitz T. A., Lipscomb W. N. The structure of carboxypeptidase a, vi. Some results at 2.0-a resolution, and the complex with glycyl-tyrosine at 2.8-a resolution. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2220–2226. doi: 10.1073/pnas.58.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovsky M., Vallee B. L. The reaction of diazonium-1H-tetrazole with proteins. Determination of tyrosine and histidine content. Biochemistry. 1966 Nov;5(11):3574–3581. doi: 10.1021/bi00875a028. [DOI] [PubMed] [Google Scholar]
- TABACHNICK M., SOBOTKA H. Azoproteins. II. A spectrophotometric study of the coupling of diazotized arsanilic acid with proteins. J Biol Chem. 1960 Apr;235:1051–1054. [PubMed] [Google Scholar]
- VALLEE B. L. ACTIVE CENTER OF CARBOXYPEPTIDASE A. Fed Proc. 1964 Jan-Feb;23:8–17. [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F. Chemical approaches to the mode of action of carboxypeptidase A. Brookhaven Symp Biol. 1968 Jun;21(1):91–119. [PubMed] [Google Scholar]
- Wilchek M., Bocchini V., Becker M., Givol D. A general method for the specific isolation of peptides containing modified residues, using insoluble antibody columns. Biochemistry. 1971 Jul 20;10(15):2828–2834. doi: 10.1021/bi00791a004. [DOI] [PubMed] [Google Scholar]



