To the Editor: Reticular dysgenesis (RD) is a rare form of autosomalrecessive severe combined immunodeficiency characterized by lack of circulating T lymphocytes, severe congenital neutropenia, and sensorineural deafness.1,2 The disease is caused by mutations in the gene encoding adenylate kinase 2 (AK2), a mitochondrial protein important for regulating intracellular levels of adenosine diphosphate and maintaining mitochondrial membrane potential. A similar function is also mediated by the cytoplasmic enzyme AK1. While most tissues express both AK1 and AK2 enzymes, neutrophils, T lymphocytes, and cells of the stria vascularis in the inner ear uniquely express AK2, thus explaining the RD phenotype.3,4 Omenn syndrome (OS) has been described in association with several genetic defects responsible for severe combined immunodeficiency and results from residual development of oligoclonal T lymphocytes that undergo peripheral expansion and infiltrate various tissues including the skin, gut, liver, and lymphoid organs.5 We report the first case of OS in a patient with RD.
A male infant of Kuwaiti origin was born to consanguineous parents who had a previously affected daughter with RD due to a homozygous missense AK2 mutation (c.524 G>A, p.R175Q). The sister’s first hematopoietic stem cell transplant from cord blood with reduced intensity conditioning failed, but she later had a successful matched related transplant with conditioning consisting of busulfan and cyclophosphamide.6 At birth, the infant was vigorous with an otherwise unremarkable physical examination; however, laboratory studies demonstrated leukopenia, absence of neutrophils, and undetectable IgA and IgM levels (Table I), and a chest radiograph lacked a thymic shadow. Immunologic evaluation at our institution at 6 weeks of age revealed undetectable T-cell receptor excision circles with detectable RNAse P, near-absence of natural killer cells, and B-cell lymphopenia. The T-cell count was slightly reduced, composed entirely of CD45RA-cells. T-lymphocyte proliferation to mitogens was decreased (Table I). Auditory brain stem response-evoked potential testing documented profound hearing loss. A diagnosis of RD was presumed, and mutation analysis by using genomic DNA derived from fibroblasts confirmed homozygosity for the same AK2 mutation as the infant’s sister. The patient was started on prophylactic antibiotics and antifungal medications along with intravenous gammaglobulin. At 8 weeks, the infant developed desquamative erythroderma, pachydermia (see Fig E1 in this article’s Online Repository at www.jacionline.org), diarrhea, and generalized lymphadenopathy. The CD3+ T-cell count increased to 10,032 cells/μL (Table I). Treatment with methylprednisolone 1 mg/kg given twice daily and cyclosporine was started; improvement was noted clinically, with laboratory studies documenting decreased circulating T lymphocytes (Table I). The infant underwent a 9/10 HLA-A mismatched unrelated donor bone marrow transplant at 3 months of age, with conditioning consisting of 4 days of busulfan given every 6 hours with area under the curve dosing targeted to 800 to 1200 μmol*min/L. cyclophosphamide 50 mg/kg daily for 4 days, and equine antithymocyte globulin 30 mg/kg daily for 3 days. Cyclosporine and 1 mg/kg per day of methylprednisolone were continued for graft versus host prophylaxis. There was no evidence for liver involvement secondary to the OS as the pretransplant liver function test results were normal. Vitamin E and ursodiol were administered for veno-occlusive disease prophylaxis. He achieved neutrophil engraftment on day +16, with 100% donor chimerism in the peripheral blood documented on day +21. The child unfortunately developed severe veno-occlusive disease with portal venous thrombosis and was treated with defibrotide. He subsequently developed progressive multiorgan dysfunction with fevers, respiratory insufficiency, hemodynamic instability, renal insufficiency, and coagulopathy. Similar to published cases of infants transplanted for immunodeficiency treated with corticosteroids,10 he developed cardiomyopathy with new severe concentric left ventricular hypertrophy. Cardiac biopsy on day +25 showed a mild T lymphocyte and histiocyte infiltrate and lack of myocyte hypertrophy. Rectal biopsy on day +28 demonstrated severe colitis consistent with acute graft versus host disease. Methylprednisolone was increased to 3 mg/kg per day, but despite this therapy, the patient died on day +31 after developing asystole. Postmortem examination was not performed.
TABLE I.
Hematologic and immunologic characteristics of the patient
| Birth | 6 wk | 8 wk | 10 wk s/p MP, CsA |
Normal values | |
|---|---|---|---|---|---|
| WBC (K cells/μL) | 0.350 | 3.36 | 8.33 | 1.43 | 7.20-18.00 |
| ANC (K cells/μL) | N/A | 0 | 0.08 | 0.09 | 2.57-7.54 |
| ALC (K cells/μL) | N/A | 3.16 | 7.96 | 1.43 | 3.40-7.60 |
| IgA (mg/dL) | 0 | <7 | - | - | 6-50 |
| IgM (mg/dL) | 0 | <5 | - | - | 15-70 |
| IgE (IU/mL) | - | 1 | 10 | - | 0-30 |
| CD3+ (cell/μL) (%) | - | 1918 (71) | 10032 (92) | 788 (86) | 2500-5500 (53-84) |
| CD4+ (cell/μL) (%) | - | 1414 (53) | 8094 (74) | 516 (56) | 1600-4400 (35-64) |
| CD8+ (cell/μL) (%) | - | 526 (19) | 2069 (19) | 293 (32) | 560-1700 (12-28) |
| CD16+56+ (cell/μL) (%) | - | 36 (1) | 75 (1) | 25 (3) | 170-1100 (4-18) |
| CD19+ (cell/μL) (%) | - | 703 (26) | 734 (7) | 93 (10) | 300-2000 (6-32) |
| Naive CD3+CD4+ (%) | - | 0 | - | - | 64-95 |
| Memory CD3+CD4+ (%) | - | 100 | - | - | 2-22 |
| Naive CD3+CD8+ (%) | - | 0 | - | - | 80-99 |
| Memory CD3+CD8+ (%) | - | 100 | - | - | 1-9 |
| PHA (counts/min) | 35205 | >27846* | |||
| TREC (copies/μL) | 0 | <252 | |||
| RNAse P (copies/μL) | 21359 |
ANC, Absolute neutrophil count; ALC, absolute lymphocyte count; CsA, cyclosporine A; MP, methylprednisolone; s/p, status post; TREC, T-cell receptor excision circle, measured as described from dried blood spot with RNAse P control gene determination7; WBC, white blood cell.
5th percentile for normal adult donors in this laboratory. The 50th percentile for normal adult donors in this laboratory is 141,254 counts/min.
We sought to confirm the diagnosis of RD by assessing AK2 protein expression and function. We also examined the etiology of this infant’s rash, diarrhea, and lymphadenopathy in the setting of the expanded CD3+ T-lymphocyte population, considering maternal engraftment or OS as possible causes of the presentation.
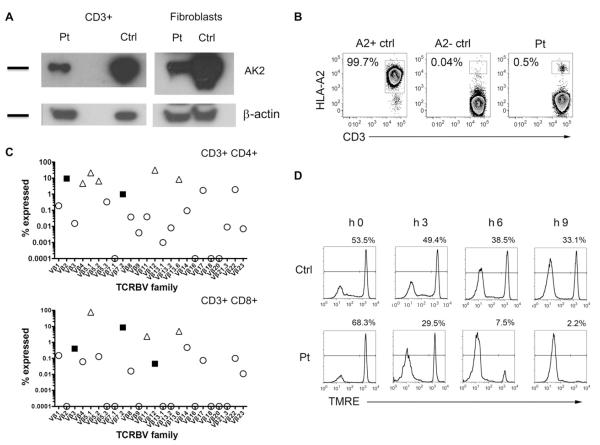
AK2 protein was detected at reduced levels in the patient’s CD3+ T cells and fibroblasts by Western blot (Fig 1, A). To determine whether this AK2 protein was derived from autologous or maternally engrafted T cells, we performed fluorescence in situ hybridization of peripheral blood cells (99% carried a Y chromosome), short tandem repeat analysis of purified CD3+ cells (0% maternal), and flow cytometry analysis (0.5% bearing the noninherited maternal HLA-A2 allele), thus ruling out maternal T-cell engraftment as the source of expanded T cells (Fig 1, B). Somatic reversion in T cells was ruled out by detecting the same AK2 mutation in purified CD3+ T lymphocytes (see Fig E2 in this article’s Online Repository at www.jacionline.org). OS is characterized by the production and expansion of oligoclonal T cells, and indeed, the infant’s CD3+, CD4+, and CD8+ T-cell receptor repertoire was highly oligoclonal, with only 5 of 24, 2 of 24, and 3 of 24 TCRß variable region families falling in the normal range, respectively (Fig 1, C). Purified CD3+ T lymphocytes showed susceptibility to apoptosis, evidenced by increased depolarization of the mitochondrial membrane potential at 3, 6, and 9 hours of incubation with staurosporin, indicating abnormal function of the mutant AK2 (Fig 1, D) (see additional information in this article’s Methods section in the Online Repository at www.jacionline.org).
FIG 1.
Characteristics of the infant’s presentation. A, Expression of AK2 protein detected in patient’s PBMC and fibroblasts (Pt) compared with normal control (Ctrl). Expression of β-actin is shown as a loading control. B, Maternal engraftment was excluded by flow analysis of the noninherited HLA-A2 allele. C, T-lymphocyte receptor oligoclonality was demonstrated by skewed expression of TCRVb families in the patient’s CD3+CD4+ and CD3+CD8+ cells. Open circle, below normal; closed square, within normal range; open triangle, above normal. D, Disruption of mitochondrial membrane potential in CD3+ cells incubated with staurosporin and detected by using tetramethylrhodamine ethyl ester (TMRE) dye.
This infant’s presentation of desquamative erythroderma, diarrhea, and generalized lymphadenopathy in the setting of an expanded and oligoclonal autologous CD3+ T-cell population is consistent with OS. This case indicates that at least some missense AK2 mutations may result in residual T-lymphocyte development, oligoclonal expansion, and symptoms of OS. Thus, AK2 should be added to the list of severe combined immunodeficiency–causing genes that may manifest as OS.
Supplementary Material
FIG E1. Patient exhibited desquamative erythroderma.
FIG E2. Analysis using genomic DNA from fibroblasts and CD3+ lymphocytes demonstrating a missense mutation in AK2 (c.524 G>A, p.R175Q).
Acknowledgments
This work was partly supported by a Translational Investigator Service Award (toS.-Y.P.), the Manton Foundation (a grant to L.D.N), the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant no. U54AI082973 to S.-Y.P. and L.D.N.), the Centers for Disease Control and Prevention (grant no. 1U01EH00362 to A.M.C.), and the Kuwait Foundation for Advancement of Sciences (grant no. 2010-1302-05 to W.A.-H.)
Footnotes
Disclosure of potential conflict of interest: F. A. Bonilla is a Board member for Green Cross; has consultancy arrangements with the Immune Deficiency Foundation, Springleaf Pharmaceuticals, and CSL Behring; has received one or more payments for lecturing from Baxter Healthcare Corporation; and has received royalties from UpToDate in Medicine. A. M. Comeau has been supported by one or more grants from and has received support for travel from the Centers for Disease Control and Prevention; has consultancy arrangements with Duke and ACMG; and is employed by the University of Massachusetts Medical School. L. A. Henderson is employed by Children’s Hospital Boston and has received one or more payments for travel/accommodations/meeting expenses from the Childhood Arthritis & Rheumatology Research Alliance. L. D. Notarangelo has received one or more grants from the National Institute of Allergy and Infectious Diseases/National Institutes of Health (grant no. U54-AI082973) and from the Manton Foundation; is a Board member for the Immune Disease Institute and for the Center for Chronic Immunodeficiencies, Freiburg (Germany); has received one or more grants from or has one or more grants pending with the March of Dimes; has received payments for lecturing and for travel/accommodations/meeting expenses from various institutions (nationally and internationally); and has received royalties from UpToDate. S.-Y. Pai has been supported by one or more grants from Boston Children’s Hospital’s Translational Investigator Service, funded in part by the Venture Philanthropy Network.
REFERENCES
- 1.de Vaal O, Seynhaeve V. Reticular dysgenesia. Lancet. 1959;2:1123–5. doi: 10.1016/s0140-6736(59)90105-9. [DOI] [PubMed] [Google Scholar]
- 2.Small TN, Wall DA, Kurtzberg J, Cowan MJ, O’Reilly RJ, Friedrich W. Association of reticular dysgenesis (thymic alymphoplasia and congenital aleukocytosis) with bilateral sensorineural deafness. J Pediatr. 1999;135:387–9. doi: 10.1016/s0022-3476(99)70141-1. [DOI] [PubMed] [Google Scholar]
- 3.Lagresle-Peyrou C, Six EM, Picard C, Rieux-Laucat F, Michel V, Ditadi A, et al. Human adenylate kinase 2 deficiency causes a profound hematopoietic defect associated with sensorineural deafness. Nat Genet. 2009;41:106–11. doi: 10.1038/ng.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pannicke U, Hönig M, Hess I, Friesen C, Holzmann K, Rump E-M, et al. Reticular dysgenesis (aleukocytosis) is caused by mutations in the gene encoding mitochondrial adenylate kinase 2. Nat Genet. 2009;41:101–5. doi: 10.1038/ng.265. [DOI] [PubMed] [Google Scholar]
- 5.Villa A, Notarangelo LD, Roifman CM. Omenn syndrome: inflammation in leaky severe combined immunodeficiency. J Allergy Clin Immunol. 2008;122:1082–6. doi: 10.1016/j.jaci.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 6.Straathof KC, Rao K, Eyrich M, Hale G, Bird P, Berrie E, et al. Haemopoietic stem-cell transplantation with antibody-based minimal-intensity conditioning: a phase 1/2 study. Lancet. 2009;374:912–20. doi: 10.1016/S0140-6736(09)60945-4. [DOI] [PubMed] [Google Scholar]
- 7.Gerstel-Thompson JL, Wilkey JF, Baptiste JC, Navas JS, Pai S-Y, Pass KA, et al. High-throughput multiplexed T-cell-receptor excision circle quantitative PCR assay with internal controls for detection of severe combined immunodeficiency in population-based newborn screening. Clin Chem. 2010;56:1466–74. doi: 10.1373/clinchem.2010.144915. [DOI] [PubMed] [Google Scholar]
- 8.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–80. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K, et al. Immunophenotyping of blood lymphocytes in childhood: reference values for lymphocyte subpopulations. J Pediatr. 1997;130:388–93. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- 10.Bulley SR, Benson L, Grunebaum E, Roifman CM. Cardiac chamber hypertrophy following hematopoietic stem cell transplantation for primary immunodeficiency. Biol Blood Marrow Transplant. 2008;14:229–35. doi: 10.1016/j.bbmt.2007.10.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIG E1. Patient exhibited desquamative erythroderma.
FIG E2. Analysis using genomic DNA from fibroblasts and CD3+ lymphocytes demonstrating a missense mutation in AK2 (c.524 G>A, p.R175Q).