Abstract
Minimizing and alleviating pain and distress in laboratory mice without compromising the methodologic integrity of research is a crucial goal. However, current methods for welfare assessment in mice are not well suited to cageside checks. In the present study, we developed a simple assessment tool—the time-to-integrate-to-nest test (TINT)—and evaluated its ability to identify mice with compromised welfare. To conduct the TINT, a nominal amount of nesting material is added to a mouse cage, and the nesting behaviors that occur immediately thereafter are observed. The TINT yields a positive result when a mouse integrates the new nesting material into the main nest site within 10 min; failure to interact with the nesting material is defined as a negative TINT. Our first experiment examined whether genetic background and sex are associated with differences in the likelihood of a positive TINT in unmanipulated mice. A significant effect related to mouse strain was found: C3H/HeNCrl had the lowest positive TINT rate among the 10 strains evaluated. A second experiment assessed whether results of the TINT would be altered after a painful surgical procedure, such as carotid artery injury. Despite all mice having received buprenorphine as analgesia at the time of surgery, significantly more mice had a negative TINT for 2 d after surgery than before surgery. Based on the results of the current study, additional work is needed to specifically validate the TINT in injured and noninjured subjects.
Abbreviations: CAI, carotid artery injury; TINT, time-to-integrate-to-nest test
Evaluation of pain in laboratory animals typically incorporates the measurement of physiologic and behavioral indicators. To date, the assessment of pain in laboratory mice has involved the evaluation of one of more of the following: locomotor activity, food and water consumption, body weight gain or loss, fur quality, threshold or latency to stimulus testing, posture evaluation, presence of vocalization, temperament changes, biochemical changes, level of self-administration of analgesics, and other behavioral measures.7,12,15,21,29 Each of these methods has limitations. Many of them require handling, sample analysis, or concurrent controls and therefore are not useful for clinical evaluation. In addition, single physiologic parameters are not specific to pain and do not fully characterize the experience in the way that behavioral observation does.14,22 Effects of pain on locomotor activity have been studied, but locomotion deficits can either be a side effect of anesthesia and analgesia or a sign of pain.29 Furthermore, rodents have been suggested to display few, if any, overt behaviors indicative of ongoing pain.19 These and other hurdles have made effective pain assessment and management in laboratory mice onerous.
An objective, simple, and valid diagnostic screening tool for pain or distress in laboratory mice is needed. Observation of activity levels, general appearance, temperament, changes in feeding and surgical site evaluation may all play a role in determining whether mice are experiencing pain.29 For example, the Mouse Grimace Scale is a useful and increasingly validated technique for the scientific study of pain that focuses on capturing facial grimaces by using video footage for subsequent scoring and assessment.15,19 Using the Mouse Grimace Scale in mice may provide a specific and sensitive detection method for significant acute pain, but it currently appears to require investments of equipment and time to train and score individual animals.16 To provide a practical, straightforward assessment tool for mice, we can examine mouse behavioral time budgets by focusing on the behaviors that this species is most motivated to perform. If a mouse is unable to perform motivated behaviors or acquire commodities that are most directly related to fitness and survival, then one can surmise that its welfare is affected.6
The nests of mice have been described as “the central location for a mouse's activities”.17 The functions of a nest for mice are many: thermoregulation, rearing and maternal activity, avoidance of predators, cover from harsh lighting conditions, and protection from other external environmental variations.3,17,25,31 Mice that have nests have improved conversion of calories because of their ability to regulate their body temperature and maintain homeostatic control.9,25 Differences in nesting behavior between strains largely revolve around the ability to construct more or less sophisticated nests rather than in the basic ability or motivation to nest.4,8 In fact, all mice are highly motivated to construct and use nests.11,25,30,33 Through both preference assessment and operant conditioning methods, mice have been shown to work very hard to gain access to nesting material.31
Therefore, reasonable evidence supports the conclusion that nest construction is a species-specific behavior that is a direct behavioral strategy to improve fitness and survival. Normal, noninjured healthy mice should be intensely motivated to rapidly perform nesting behavior when presented with a small amount of fresh nesting material. Our novel assessment tool—the time-to-integrate-to-nest test (TINT)—produces a binary outcome. A mouse that integrates the new nesting material into the main nest site within 10 min is considered to have a positive TINT. Failure to retrieve the nesting material in 10 min is scored as a negative TINT. To make use of this tool across a variety of laboratory conditions, using various strains of laboratory mice, we must verify that normal, noninjured healthy mice routinely exhibit this nesting behavior. We hypothesize that genetic background and sex will not affect the TINT in healthy laboratory mice. We also hypothesize that the TINT will be altered when a laboratory mouse undergoes a painful surgical procedure.
Materials and Methods
Experiment 1: Determination of duration of TINT and effects of genetic background and sex.
The Charles River IACUC approved all housing and experimental procedures described. Charles River is AAALAC-accredited. The mice were free of commonly excluded organisms.24
Animals and housing.
Ten inbred mouse strains were studied at Charles River (Wilmington, MA): BALB/cAnNCrl, 129S2/SvPasCrl, FVB/NCrl, C57BL/6NCrl, C3H/HeNCrl, CB17/Icr-Prkdcscid/IcrIcoCrl, NOD.CB17-Prkdcscid/NCrCrl, CB17.Cg/ Prkdcscid Lystbg/Crl, C57BL/6NTac, and C57BL/6NJ. Mice were matched for age and sex and housed by strain in groups of 5 (2 cages per sex per strain; total of 40 cages) in solid-bottomed cages in flexible-film isolators. Cages were bedded with kiln-dried hardwood shavings (Nepco, Warrensburg, NY), and mice were provided with nesting material (Enviro-Dri, Shepherd Specialty Papers, Watertown, TN). Because this experiment was an opportunistic observational study of mice enrolled in another study, bedding and nesting material were not standardized between cages. The amount of nesting material was weighed at the end of the study and ranged from 3.8 to 39.5 g. Food (Lab Diet 5L79; Purina Mills, Richmond, IN) and water were available ad libitum. The average temperature in the room was 20.2 °C ± 1.2. The room was on a 12:12-h light:dark cycle.
Experimental design.
A factorial design (2 sexes × 10 strains × 2 replicates = 40 cages) was used to maximize power and reduce sample size by incorporating, accounting for, and eliminating unwanted variance.10 A random-number generator was used to select 4 cages among the 20 within the isolator. The selected cages were balanced for location within the isolator (top, bottom, left, and right) and sex (male, female). TINT administration was conducted over 3 consecutive days, for a total of 3 TINT observations per cage. A cage's time (in seconds) to first integration of nesting material was averaged over the 3 tests.
Nest quality scoring.
The naturalistic scoring method was used to score each main nest site from 0 to 5 immediately prior to the TINT.13 Unmanipulated material with no central nest site received a score of 0; manipulated material with no central nest site received a score of 1; 2 described a flat nest; 3 was a cupped nest; 4 was a nest that had an incomplete dome; and 5 indicated a complete and enclosed dome with an internal cavity.
TINT procedure.
Four individual strips of 3-ply nesting material (Enviro-Dri, Shepherd Specialty Papers) were separated to standardize the amount of substrate used for each TINT. These 4 individual strips then were crumpled to make a ball. The cage top was opened and forceps were used to clear or compress an area in the corner of the cage opposite the main nest site. Some of the cages were turned around completely or positioned to the side if this area was not easily visible to the TINT administrator. All reoriented cages were documented and included in the analysis as a blocking factor. Forceps then were used to place the TINT substrate into the corner of the home cage. Mice were observed for 10 min, and the time to first interaction with the novel material and time to integration were recorded. Two observers participated in this experiment, and each observed 2 cages within each isolator. Observers stood in front of the isolator, about 3 ft from the individual mouse cages. All TINT observations in this study were made within 3 h of light onset.
Statistical analysis.
All analyses used a general linear model (JMP version 10, SAS Institute, Cary, NC). The first model, used to determine appropriate TINT timing, incorporated genetic background and sex to examine the square-root–transformed latency to first integration. The assumptions of the model (linearity, homogeneity of variance, and normality of error) were confirmed graphically post hoc.10 Statistical significance was determined post hoc by using Tukey pairwise comparisons. All analyzed data are represented as least-square means ± SE. Effect of genetic background and sex on TINT outcome could not be analyzed due to too few cages failing the TINT (that is, 9 of 120 tests conducted over the 3 testing periods).
Experiment 2: Use of the TINT during potentially painful surgical procedures.
The Tufts Medical Center IACUC approved all housing and experimental procedures described. Tufts Medical Center is AAALAC-accredited.
Animals and housing.
Inbred male mice (n = 27; age, 16 wk) were studied at the Molecular Cardiology Research Institute at Tufts Medical Center (Boston, MA). Mice were MRf/f/SMA-Cre-ERT2-negative (Cre–) and MRf/f/SMA-Cre-ERT2-positive (Cre+) littermates (Jackson Laboratories, Bar Harbor, ME).20 Mice were housed as follows: 5 mice were singly housed; 5 cages of 2 mice; 1 cage of 3 mice; 1 cage of 4 mice; and 1 cage of 5 mice. Social environment was accounted for in the statistical analysis. Mice were housed in microisolation caging and provided corncob bedding (Bed-O'cobs, PharmaServ, Framingham, MA) with a square of nesting material (Nestlet, Ancare, Bellmore, NY) and food (TD8604 Teklad rodent diet, Harlan Laboratories, Madison, WI) and water ad libitum. Bedding was changed twice each week, unless otherwise specified. Room temperature ranged between 19 to 23 °C and humidity was 30% to 70%, with an approximately 14:10 light:dark cycle.
Experimental design and CAI surgical procedure.
At 1 to 3 mo before our study began, mice received tamoxifen (0.1 mg IP daily for 5 d; Sigma Aldrich, St Louis, MO) injections at 5 to 8 wk of age. All mice had at least 3 d of TINT experience prior to recording of baseline measures. On day −1, mice underwent baseline TINT analysis prior to replacement of an osmotic minipump (Alzet, Cupertino, CA) via subcutaneous dorsal incision under isoflurane anesthesia. Pumps contained either vehicle (11% ethanol in saline) or aldosterone (6 μg/kg/d; Sigma Aldrich). Buprenorphine (0.05 mg/kg SC) was given to the mice postoperatively. On day 0 (that is, 24 h after pump implantation), mice were again assessed by the TINT procedure and then underwent isoflurane anesthesia, carotid artery injury (CAI) surgery, and placement of a second infusion pump containing bromodeoxyuridine (25 mg/kg/d; Sigma-Aldrich). CAI surgery involved a ventral midline neck incision, with wire insertion into the left carotid artery to the level of the aortic arch. Buprenorphine (0.05 mg/kg SC) was given to the mice postoperatively. No further analgesia was given. TINT outcomes were recorded within 3 h of lights-on for 3 consecutive days after CAI surgery. A total of 5 TINT observations per cage were done over the course of this experiment.
TINT procedure.
The TINT procedure used in experiment 2 was identical to that used in experiment 1 except that a different nesting material was used (Nestlets, Ancare) as the testing material.. A 2-in. square of cotton nesting material was cut into quarters to obtain four 1-in testing squares. This change was made because this product was the standard nesting material used by this laboratory. A 10-min cut-off time for the TINT to be considered positive was chosen on the basis of substantial pilot work and results from experiment 1, during which mice integrated TINT substrate into the home nest within 7 min or less on average.
Statistical analysis.
Binary logistic regression in JMP statistical software (version 10, SAS Institute), using the Firth adjusted maximum likelihood estimation method, was performed to determine the likelihood ratios of a cage of mice that yielded a negative TINT. To accommodate repeated measures, the analysis was blocked by cage and nested within social treatment (solitary or group housed). Minipump drug treatment (from the first implanted osmotic pump), Cre status (+, −, or mixed cage), and the interaction between time point and social treatment were not significant and were removed from the final analysis. Bonferroni-corrected custom contrasts were used post hoc to determine differences between variables in the model. All data are reported as least-square means ± SE.
Results
Experiment 1.
Mouse strains showed a significant difference in mean latency to integrate the TINT substrate (F9, 29 = 5.6314; P = 0.0002); C3H/HeNCrl mice were significantly slower than were all other strains. Four of the 10 strains tested had an average TINT substrate integration time of less than 1 min, and 8 of 10 strains were within 2 min. All strains tested had an average TINT substrate integration time of less than 7 min. Sex did not significantly contribute to the latency to retrieve the TINT substrate (F1, 29 = 0.18; P > 0.05). Over the 3 testing sessions, only 9 cages failed the TINT (Table 1), and 6 of those failures occurred on the first day of testing. C3H mice were the only strain to have any failures during the 2nd and 3rd testing sessions.
Table 1.
Influence of sex on the rate of positive TINT results among mice
| No. of positive TINT tests among 4 cages of mice |
|||
| Mouse strain | Trial 1 | Trial 2 | Trial 3 |
| 129S2/SvPasCrl | 4 | 4 | 4 |
| BALB/c AnNCrl | 4 | 4 | 4 |
| C57BL/6NJ | 3 | 4 | 4 |
| C57BL/6NTac | 2 | 4 | 4 |
| C57BL/6NCrl | 4 | 4 | 4 |
| C3H/HeNCrl | 2 | 2 | 3 |
| FVB/NCrl | 3 | 4 | 4 |
| NOD.CB17-Prkdcscid/NCrCrl | 4 | 4 | 4 |
| CB17/Icr-Prkdcscid/IcrIcoCrl | 4 | 4 | 4 |
| CB17.Cg/ Prkdcscid Lystbg/Crl | 4 | 4 | 4 |
Experiment 2.
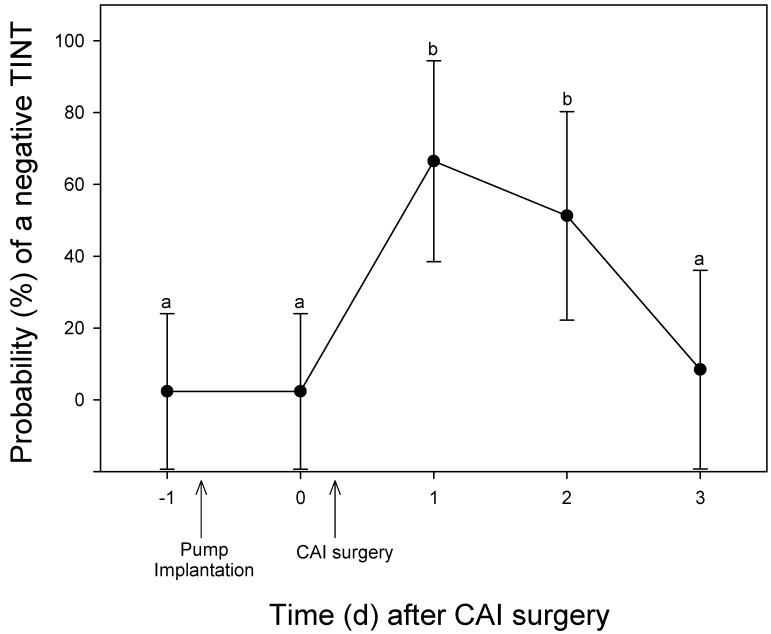
Social condition was associated with a significant difference in the TINT outcome (χ2 = 24.34; P < 0.0001). Regardless of surgical treatment, singly housed mice were more likely to fail the TINT than were mice housed with at least one conspecific. The TINT outcome also changed over the course of the experiment. Specifically, TINT outcome was similar to baseline levels after mini-pump surgery. Failing the TINT was more likely during the first 2 d after the CAI surgery than at any of the other time points (χ2 = 22.27; P < 0.0002; Figure 1).
Figure 1.
Probability of TINT failure before and after a CAI procedure. Data are given as least-squares means ± SE, and different letters indicate significant (P < 0.05; posthoc Tukey) differences between values.
Discussion
We hypothesized that the TINT outcome would be positive regardless of genetic background and sex because nesting is a highly motivated behavior in laboratory mice and is unlikely to be eliminated from their behavioral time-budget under normal laboratory conditions. Our results indicate that strain may moderately influence the TINT in unmanipulated mice. For example, C3H/HeNCrl mice only achieved a positive TINT in 7 of 12 trials, a rate that was lower than those of the other strains studied. The fact that strain differences may influence the TINT is not surprising, given that genetic background informs behavioral phenotype. Specifically, nesting behavior variations have been linked to environmental, genetic, and genotype-by-environment interactions.3,18,33 We noted behavioral differences in the way C3H/HeNCrl mice processed the TINT substrate during this study. Other studies comparing this strain to FVB/NCrl mice in performance tests suggest that they are behaviorally different.23 A previous study on nesting behavior noted that C3HeB/FeJ mice were indifferent to each other and nesting material.17 Their ability to gather and construct a cohesive nest was described as poor; the mice simply constructed temporary sleeping spots with the nesting material provided17—we observed this same behavior in our study. Gathering and carrying nesting substrate are the key to achieving a positive TINT. Strains or stocks that are less likely to perform this behavior may be less likely to have a positive TINT. However with increased experience, it appeared that even the low-performing C3H/HeNCrl strain improved its TINT outcome.
This finding underscores the importance of establishing a baseline TINT outcome in unmanipulated mice before the TINT is used as a screening tool for pain and distress, to avoid the possibility of a type 1 error. Our baseline data suggest that this test is more likely to fail due to type 1 error than Type 2. In other words, the TINT is more likely to identify a nonpainful mouse with a negative TINT than it is to fail to identify a painful mouse with a positive TINT. Given that our goal is to design a simple method by which laboratory animal professionals can detect postsurgical or other types of pain easily, we believe that this tradeoff is appropriate. However, given that small differences between strains were found, we believe an inhouse baseline should be established before the TINT is used in a widespread manner.
Various inherited traits could affect nesting behavior indirectly. For example, C3H/HeNCrl carry an allele (Pde6brd1) that causes retinal degeneration and blindness. This defect could pose a challenge to their ability to retrieve a small sample of nesting material. However, FVB/NCrl mice carry the same allele and achieved positive TINT outcomes at a rate consistent with those of other sighted strains. Blindness, therefore, may not be the specific reason why the C3H/HeNCrl mice did not reliably have positive TINT outcomes. We did not examine the behavior of various outbred stocks in this experiment, and additional work is needed to evaluate the suitability of this test for outbred mice.
The amount of nesting material given to some cages in experiment 1 was substantial in comparison to the standard provided in many facilities There was also a wide range (3.8 to 39.5 g) in the amount of nesting material distributed to each cage; these data were collected, consistent with 3Rs guidelines, as an add-on to an ongoing experiment in which these parameters were not standardized. The wide range in amounts of nesting material could have influenced the overall TINT outcome. However we do not believe this influence occurred, because mice actually had fewer negative TINT outcomes as they gained TINT experience and material. We believe that the TINT is a useful assessment tool for many different laboratory environments, because it does not require either a large investment of nesting material and because it can be used with strains that are not regarded as excellent nesters. Future investigations should consider the questions of how developmental and lifetime experience with nesting material interacts with the TINT and whether varying types of nesting material affects TINT outcome.
The majority of mice in experiment 1 carried the new TINT substrate to their home nest within 2 min; in fact, we observed that once mice become accustomed to the daily dose of additional nest material, they tend to leave the main nest rapidly to retrieve the new material. This pattern sheds light on just how motivated mice are to perform nesting behaviors. The 10-min timeframe within which the TINT can be conducted has been shown to be more than enough time for unaltered mice to complete the TINT. Husbandry technicians are not required to observe mice continuously during TINT administration, making it a reliable, easy, and inexpensive test to administer.
Our results also draw attention to the fact that practices in the laboratory have an effect on the reliability and outcome of the TINT. In experiment 1, some cages were turned 90° to assist with TINT substrate delivery and observation. The novelty of having their home cage in a different direction may have affected the behavioral repertoire, awakened sleeping mice, or perhaps caused them to be more vigilant of their environment, ultimately making them more likely to achieve a positive TINT outcome. Due to the low number of overall failures, these data could not be analyzed statistically to determine whether turning the cage affected the overall outcome. Differential housing has been shown to influence the health and behavioral outcomes of studies focused on infectious disease, cancer, and insulin-dependent diabetes mellitus.1 The shelf levels in the cited study1 correlated with disease development. Although we did not notice an effect of cage location on TINT outcome, these factors could be a challenge to implementing the TINT in a vivarium with individually ventilated caging, which requires additional manipulation to administer the test.
Housing-related behavioral differences between mice housed in groups and those housed singly are well documented.27,28,32 It was interesting to note that singly housed mice were more likely to have a negative TINT than were group-housed mice. The physiologic and behavioral effects of single housing on mice, and experimental results, are an important consideration. Individually housed mice have higher heart rates and have more, but shorter, periods of inactivity, and experience disruptions to their normal circadian sleep cycle.28 In addition, individual housing alters food intake, organ weight, metabolism, tumor growth, and immune responses.28 Mice are social animals, and group housing has been shown to reduce anxious behavior,5 postoperative recovery, and pain.26 In preference studies, mice are significantly more likely to spend time in inhabited cages than uninhabited ones.33 In the same studies, mice that had a choice between an inhabited cage and a cage with nesting material all chose the cage with nesting material to sleep in.32 The reasons for variations in behavior between individually and group-housed mice are beyond the scope of this study and are a potential future direction of this research. Our data provide further evidence to the already solid foundation of studies suggesting that solitary housing in mice is an important, independent variable in biomedical and behavioral research.
In our surgical experiment, we hypothesized that pain as a result of a moderately invasive surgical procedure would affect the outcome of the TINT. Minipump implantation surgery did not appear to affect TINT results, yet mice were more likely to fail the TINT the morning after CAI surgery. Mice required 2 full days to return to baseline TINT levels. This lag may be due to the fact that minipump implantation surgery is adequately treated with a single dose of buprenorphine whereas CAI surgery is not; alternative explanations can be surmised, such as the combined effect of 2 anesthetic and surgical procedures within days and that CAI surgery causes nonpainful distress (for which buprenorphine was not an adequate treatment). It is important to remain open to the fact that the TINT may be affected by diverse negative experiences, not just pain. These preliminary results suggest that the TINT could be a useful method by which to quickly assess wellbeing in mice. A previous study noted that home nest sophistication was altered when surgical conditions produced sufficient pain, although the observation was not formally tested.2 This result lends support to our conclusion that more work is needed to validate the TINT in known painful subjects and nonpainful controls.
We acknowledge certain shortcomings in experiment 2. For example, in an attempt to reduce the number of animals subjected to potentially painful procedures, we tested mice that were used for other studies at the Molecular Cardiology Research Institute at Tufts University. As a result, we did not have full control over surgical protocols, husbandry conditions, or animal numbers and were not able to design an experiment that appropriately balanced painful and nonpainful subjects. Similarly, it is unclear what effects buprenorphine, used as an analgesic at the time of surgery, had on their behavior and motor function. This medication could have produced sufficient derangement in behavioral motivation and locomotion to produce a TINT failure in the face of appropriate pain relief.
However, this foundational study did provide baseline information on which additional work to validate the TINT can be based. The goal of using the TINT as a tool is to be able to rapidly screen mice, whether singly or group housed, to detect when they are in a behaviorally abnormal state. Gradations of distress will certainly be encountered, and mice may perform this nesting behavior until a threshold when it becomes no longer possible. TINT is not designed to specifically detect the difference between pain and nonpain distress, and the extent to which mice are affected when they are TINT-negative remains to be seen. Similarly, the TINT may be limited in its ability to detect distress in a single mouse housed within a group of otherwise nondistressed mice that perform nesting behavior readily. Appropriately controlled studies may determine whether this potential limitation to the TINT is real. Nevertheless, this test has the potential to be a fast and simple cage-side screening technique that can be used by investigators, veterinarians, and technicians charged with caring for laboratory mice, regardless of the users’ ability to identify more subtle mouse behaviors. The actual cause or treatment for TINT-negative mice may require additional evaluation. The TINT can be studied for its ability to determine a threshold where analgesia is insufficient, or significant distress is present, for purposes of treatment or predicting humane endpoints.
References
- 1.Ader DN, Johnson SB, Huang SW, Riley WJ. 1991. Group size, cage shelf level, and emotionality in nonobese diabetic mice: impact on onset and incidence of IDDM. Psychosom Med 53:313–321 [DOI] [PubMed] [Google Scholar]
- 2.Arras M, Rettich A, Cinelli P, Kasermann HP, Burki K. 2007. Assessment of postlaparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. BMC Vet Res 3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broida J, Svare B. 1982. Strain typical patterns of pregnancy-induced nest-building in mice: maternal and experiential influences. Physiol Behav 29:153–157 [DOI] [PubMed] [Google Scholar]
- 4.Bult A, Lynch CB. 1997. Nesting and fitness: lifetime reproductive success in house mice bidirectionally selected for thermoregulatory nest-building behavior. Behav Genet 27:231–240 [DOI] [PubMed] [Google Scholar]
- 5.Chourbaji S, Zacher C, Sanchis-Segura C, Spanagel R, Gass P. 2005. Social and structural housing conditions influence the development of a depressive-like phenotype in the learned-helplessness paradigm in male mice. Behav Brain Res 164:100–106 [DOI] [PubMed] [Google Scholar]
- 6.Dawkins MS. 1988. Behavioral deprivation: a central problem in animal welfare. Appl Anim Behav Sci 20:209–225 [Google Scholar]
- 7.Flecknell PA. 1994. Refinement of animal use: assessment and alleviation of pain and distress. Lab Anim 28:222–231 [DOI] [PubMed] [Google Scholar]
- 8.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. 2012. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS ONE 7:e32799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. 2013. Impact of nesting material on mouse body temperature and physiology. Physiol Behav 110–111:87–95 [DOI] [PubMed] [Google Scholar]
- 10.Grafen A, Hails R. 2002. Modern statistics for the life sciences. Oxford (UK): Oxford University Press [Google Scholar]
- 11.Gross AN-M, Engel AKJ, Würbel H. 2011. Simply a nest? Effects of different enrichments on stereotypic and anxiety-related behaviour in mice. Appl Anim Behav Sci 134:239–245 [Google Scholar]
- 12.Hawkins P. 2002. Recognizing and assessing pain, suffering, and distress in laboratory animals: a survey of current practice in the UK with recommendations. Lab Anim 36:378–395 [DOI] [PubMed] [Google Scholar]
- 13.Hess SE, Rohr S, Dufour BD, Gaskill BN, Pajor EA, Garner JP. 2008. Home improvement: C57BL/6J mice given more naturalistic nesting materials make better nests. J Am Assoc Lab Anim Sci 47:25–31 [PMC free article] [PubMed] [Google Scholar]
- 14.Karas AZ, Danneman PJ, Cadillac JM. 2008. Strategies for assessing and minimizing pain. In: Fish R, Brown M, Danneman PJ, Karas AZ. Anesthesia and analgesia in laboratory animals. San Diego (CA): Academic Press [Google Scholar]
- 15.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, LaCroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg A, Ferrari MD, Craig KD, Mogil JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7:447–449 [DOI] [PubMed] [Google Scholar]
- 16.Leach MC, Klaus K, Miller AL, di Perrotolo MS, Sotocinal SG, Flecknell PA. 2012. The assessment of postvasectomy pain in mice using behaviour and the Mouse Grimace Scale. PLoS ONE 7:e35656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CT. 1972. Development of nest-building behavior in inbred mice. J Gen Psychol 87:13–21 [Google Scholar]
- 18.Lynch CB. 1980. Response to divergent selection for nesting behavior in Mus musculus. Genetics 96:757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, Zaloum A, King OD, Mogil JS. 2012. Using the mouse grimace scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci 51:42–49 [PMC free article] [PubMed] [Google Scholar]
- 20.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. 2012. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 18:1429–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller AL, Flecknell PA, Leach MC, Roughan JV. 2011. A comparison of a manual and an automated behavioural analysis method for assessing postoperative pain in mice. Appl Anim Behav Sci 131:138–144 [Google Scholar]
- 22.Mogil JS, Crager SE. 2004. What should we be measuring in behavioral studies of chronic pain in animals? Pain 112:12–15 [DOI] [PubMed] [Google Scholar]
- 23.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. 2007. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res 176:4–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicklas W, Baneux P, Boot R, Decelle T, Deeny AA, Fumanelli M, Illgen-Wilcke B, Monitoring FWGH. 2002. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab Anim 36:20–42 [DOI] [PubMed] [Google Scholar]
- 25.Olsson IAS, Dahlborn K. 2002. Improving housing conditions for laboratory mice: a review of environmental enrichment. Lab Anim 36:243–270 [DOI] [PubMed] [Google Scholar]
- 26.Pham TM, Hagman B, Codita A, Van Loo PLP, Strommer L, Baumans V. 2010. Housing environment influences the need for pain relief during postoperative recovery in mice. Physiol Behav 99:663–668 [DOI] [PubMed] [Google Scholar]
- 27.Sherwin CM. 2003. Social context affects the motivation of laboratory mice, Mus musculus, to gain access to resources. Anim Behav 66:649–655 [Google Scholar]
- 28.Spani D, Arras M, Konig B, Rulicke T. 2003. Higher heart rate of laboratory mice housed individually vs in pairs. Lab Anim 37:54–62 [DOI] [PubMed] [Google Scholar]
- 29.Stasiak KL, Maul D, French E, Hellyer PW, Vandewoude S. 2003. Species-specific assessment of pain in laboratory animals. Contemp Top Lab Anim Sci 42:13–20 [PubMed] [Google Scholar]
- 30.Van de Weerd HA, Van Loo PLP, Van Zutphen LFM, Koolhaas JM, Baumans V. 1997. Nesting material as environmental enrichment has no adverse effects on behavior and physiology of laboratory mice. Physiol Behav 62:1019–1028 [DOI] [PubMed] [Google Scholar]
- 31.Van de Weerd HA, Van Loo PLP, Van Zutphen LFM, Koolhaas JM, Baumans V. 1998. Strength of preference for nesting material as environmental enrichment for laboratory mice. Appl Anim Behav Sci 55:369–382 [DOI] [PubMed] [Google Scholar]
- 32.Van Loo PLP, Van de Weerd HA, Van Zutphen LFM, Baumans V. 2004. Preference for social contact versus environmental enrichment in male laboratory mice. Lab Anim 38:178–188 [DOI] [PubMed] [Google Scholar]
- 33.Van Oortmerssen GA. 1971. Biological significance, genetics and evolutionary origin of variability in behaviour within and between inbred strains of mice (Mus musculus). Behaviour 38:1–92 [DOI] [PubMed] [Google Scholar]