Abstract
Educational institutions maintain group-housed mice of both sexes for training veterinarians and technicians in husbandry, medication, and sampling procedures. Mice kept in all-male groups may experience poor welfare due to fighting. Castrated mice may be used to replace gonadally intact males for such training programs. In this prospective cohort study, 80 castrated and 80 control (intact) male mice were studied over 3 mo to monitor aggression frequency and injury levels. Behavioral observations were performed twice weekly by using an all-occurrences sampling method to quantify behavioral events and the number and severity of bite wounds. Under these housing conditions, group-housed male mice castrated postpubertally exhibited significantly less aggression than did intact male mice. Castration therefore improves welfare in group-housed male mice and thus provides a husbandry alternative to individually housing animals in nonstudy situations.
Laboratory mice and rats are the most commonly used animals in biomedical research, comprising 95% of all animals used.27 Accordingly, knowledge of and familiarity with these species are major foci of training for biomedical technicians and laboratory animal veterinarians. To meet these training needs, tertiary education providers may maintain housed mice for prolonged periods over an academic year. Typically, in our institution, mice are kept in large single-sex groups in open-top conventional cages. This housing arrangement is influenced by the need to provide for social enrichment and by budgetary restrictions. However, maintaining all-male groups of 8 or more mice leads to more aggression than that of those kept in groups of 3.28,29 Institutional animal ethical committees have an expectation that mice will be group-housed, with enriched environments and reduced stocking densities, as specified in revised national codes.21 However, various enrichment strategies and materials have been shown to accentuate aggression in group-housed intact male mice of different inbred strains,8-10 including the provision of running-wheel igloos for outbred CD1 mice.10 In addition, the optimal space requirement per mouse and group size remain unclear.32
Aggression within socially stable groups of wild mice may assist selection for fitness. Although intermale aggression may represent normal behavior in captivity, aggression itself as well as subsequent injury and mortality can threaten the welfare of group-housed mice.29 Social instability in rodents, particularly unstable dominance hierarchies in large groups of male mice, has been causally linked with immune disorders and disease susceptibility.1,25,28 A prerequisite to the development of a stable social system is the ability to establish and maintain dominance relationships.1 It is the loss of position in a hierarchy that is inherently stressful. A defeated wild rodent is unlikely to remain in the territory of an aggressive dominant animal and is more likely to die as a result of cold, predators, or food shortage rather than social stress. The captive defeated rodent, however, remains in an abnormal social environment and suffers the consequences of exposure to social stressors. Evidence indicates that rodents are unlikely to adapt to such stressors.1 Such chronic exposure may be sufficient to translate the stress response into a pathologic condition.1
Management systems that maintain animals in chronic social stress conditions are in conflict with welfare models such as the ‘Five Freedoms’ of the UK Farm Animal Welfare Council30 and the 5 domains of animal welfare compromise described by Mellor and Reid17 and further refined by Mellor.15,18 Creating conditions in which animals live in stable social groups with opportunities to experience positive welfare states is consistent with current welfare concepts.4,16
Previous studies have used castrated mice as controls in endocrinologic and behavioral research. One study investigated the development of neonatal organization of androgen-dependent aggressive behavior and discovered that this behavior terminated between 2 and 6 d of age in male mice.23 Another study showed that the familiarity conveyed by urine odor plays an important role in the control of territorial aggression.5 There appear, however, to be few studies that have investigated the welfare benefits of castration as a husbandry tool. One recent study that investigated such benefits used health-surveillance CD1 mice to demonstrate a significant reduction in ‘fighting’ from 64% in intact male mice to 0% in mice castrated before they were 1 mo old.14 In addition to determining the effect of postpubertal castration on aggression incidence, our current study builds on this previous study14 by quantifying agonistic and defensive behaviors and examining their temporal distribution.
Here we sought to demonstrate that postpubertal castration of Swiss ARC;ARC(S) group-housed male mice improves welfare outcomes for mice through reduction of aggressive behavior and injury rates.
Materials and Methods
Animals.
Outbred Swiss ARC;ARC(S) breeding stock were obtained from a barrier-maintained SPF commercial mouse production facility (University of Adelaide Laboratory Animal Services, Adelaide, Australia). These mice were free of parasites and negative for viral and bacterial mouse pathogens including mouse hepatitis virus, minute virus of mice, mouse parvovirus, and rotavirus. Experimental male mice (n = 160) were bred inhouse from these breeding stock in open-top conventional cages in a barrier facility. At weaning, the mice were placed randomly into groups of 10 in polycarbonate open-top rat cages (590 mm × 385 mm × 200 mm; Tecniplast, Rydalmere, New South Wales, Australia). Enrichment materials consisted of tissues, cardboard rolls, egg cartons, and a running wheel hung from the top of each cage. Enrichment materials were standardized between cages.
The facility in which the mice were kept was maintained on a 12:12-h light:dark cycle, with a humidity of 40% to 70%, temperature range of 20 to 24 °C, and 15 to 20 air changes hourly. Bedding consisted of recycled paper pellets (Animal Bedding, Fibrecycle, Yatala, Queensland, Australia), and fixed formulation cubes (Rat and Mouse Cubes, Specialty Feeds, Glen Forrest, Western Australia, Australia) were provided free-choice from wire hoppers on cage tops. All bedding and feed was sterilized by autoclaving before being unpacked in the barrier facility. Water was provided in glass bottles from the potable main supply and changed weekly.
The project was approved by the Institutional Animal Ethics Committee of Technical and Further Education South Australia according to the provisions of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.22
Experimental design.
Each of the 16 cages was randomly assigned to 1 of 2 groups by an investigator not involved in performing behavioral observations. The first group of 80 mice was castrated between the ages of 6 and 9 wk according to the procedure described following. Behavior observations began 1 mo after surgery and were conducted twice weekly over 3 mo (25 observations). Observations consisted of 5-min periods during the light phase (not associated with cage cleaning). Other workers in this field have similarly conducted aggression tests during the light period.25 Veterinary technology students, who were blind to the mice's treatment status, supervised by experienced technical staff performed the observations, which were recorded manually from a distance of 2 m.
Behavioral categories are shown in Figure 1. These involved previously described ritualized patterns of aggression and defense, including offensive-sideways (Figure 2), chase, bite, defensive-upright, crouch or freeze, and flee behaviors.7 Observations during the 5-min observation periods were recorded and quantified by an all-occurrences sampling method. After each observation period, the number of behavioral occurrences was tallied, mice were examined, and injuries recorded and scaled on clinical record sheets. The record included information on the number of mice with bites in each cage, the total number of bites per cage, and the number of mice euthanized due to injury. Animals were euthanized when there was evidence of body cavity penetration, obvious subcutaneous swelling, 3 or more nonhealing bites, and scab or skin excoriation larger than 1 cm2. The injury scoring system used 3 classifications—minor, moderate, and severe. Minor injury was deemed to consist of bites or scratches without skin penetration. Moderate injury consisted of skin penetration with slight redness or swelling but without functional impedance. Severe injury was classified as skin penetration with inflammation or discharge and functional impedance. Mice with minor or moderate wounds were left in the project untreated. Separating mice from all-male groups, treating them and reintroducing them later was deemed likely to destabilize group hierarchies and constitute a confounding variable. We found that superficial and moderate bite injuries in the mice healed very rapidly, whereas severe wounds did not. Therefore, instead of separating and treating mice, strict euthanasia criteria were adhered to, such that all mice showing signs of severe wounds were euthanized. In these cases, veterinary examination was performed to confirm the need for euthanasia.
Figure 1.
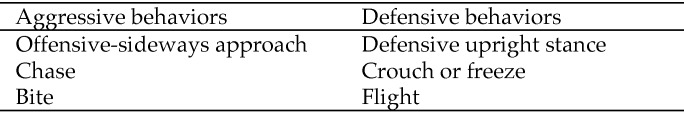
Measured behavior categories.
Figure 2.
Offensive sideways approach with defensive upright stance.
Surgery and anesthesia.
Mice were anesthetized with medetomidine hydrochloride (0.75 mg/kg IP; Domitor, 1 mg/mL, Zoetis Australia, West Ryde, New South Wales, Australia) and ketamine hydrochloride (100 mg/kg IP; Parnell Ketamine Injection, 100 mg/mL, Parnell Laboratories, Alexandria, New South Wales, Australia). Oxygen at 100% was supplied throughout by a nonrebreathing modified T piece. All surgeries were undertaken with full aseptic procedure. Castrations were accomplished by making a midline abdominal incision according to an approach described for vasectomy surgery.20 Testicular arteries were crushed and cauterized; the linea alba was repaired by using simple interrupted sutures (5-0 Dexon S, Covidien, Dublin, Ireland), with wound clips (9-mm Reflex Wound Clips, CellPoint Scientific, Gaithersburg, MD) to complete the repair. Analgesia was provided by using butorphanol tartrate (1.5 mg/kg SC; Torbugesic, 10 mg/mL, Fort Dodge Australia, Baulkham Hills, New South Wales, Australia) just prior to surgery and meloxicam (1.5 mg/kg SC; Ilium Meloxicam, 5 mg/mL, Ilium Veterinary Products, Smithfield, New South Wales, Australia) at the conclusion of surgery. Medetomidine sedation was reversed by using atipamezole hydrochloride (1 mg/kg SC; Antisedan, 5 mg/mL, Zoetis Animal Health). Mice recovered on thermostatically controlled heated pads until ambulatory and then were provided with water-soaked cubes in their cages for 18 h after surgery. Mice were monitored individually daily for 7 d after surgery by using clinical record sheets, and veterinarians were consulted when anomalies were detected. Wound clips were removed at 7 d.
Statistical analysis.
The number of behavioral and injury events were summed for each cage over the 25 observations, and means were derived for both the castrated mice and the intact controls for the period of the study. The cage was considered to be the experimental unit (n = 8 for each treatment). Differences between the 2 treatment groups were compared, and the P values of differences between means were calculated by using the Wilcoxon–Mann–Whitney test. Repeated-measures ANOVA using the Kruskal–Wallis test of aggressive, defensive/avoidance events and the number of bites per cage for the intact animals over the duration of the study was performed. Nonparametric tests were used because some of the data were not normally distributed. The Wilcoxon–Mann–Whitney and Kruskal–Wallis tests were done by using the Megastat Excel Add-In (version 10.2, McGraw-Hill Higher Education, New York, NY). Differences between means were determined to be significant when the P value was less than or equal to 0.05.
Results
In the castrated group, one postoperative complication (wound dehiscence) necessitated euthanasia. All other mice recovered well and required no additional rescue analgesia.
Data regarding behaviors in each treatment group are shown in Table 1 demonstrate significant (P ≤ 0.05) differences between castrated and control mice for all variables. Similarly, bite injury data showed significant differences between treatment groups for all parameters measured. For the intact controls, the number of mice with bites (mean ± SEM) was 34.8 ± 5, with 49.3 ± 8 bites per cage. Injuries were categorized as minor, moderate, or severe, corresponding to 19.6 ± 3.7, 8.4 ± 1.5, and 4.5 ± 1.3, respectively, for the control mice, with 4.6 ± 1.3 mice euthanized due to injuries. Among the castrated mice, the values for all of these measures were 0.
Table 1.
No. of incidents of measured behaviors per cage (mean ± SEM) for each treatment group over the 3-mo observation period
| Behavior criteria | Castrated mice | Intact (control) mice | |
| Aggressive behaviors | |||
| Offensive-sideways | 1.3 ± 0.4 | 31.2 ± 4.4 | |
| Chase | 1.9 ± 0.8 | 51.4 ± 6.5 | |
| Bite | 1.9 ± 1.2 | 60.3 ± 7.0 | |
| Defensive avoidance behaviors | |||
| Defensive-upright | 1.9 ± 0.8 | 39.0 ± 4.1 | |
| Crouch or freeze | 0.8 ± 0.4 | 10.4 ± 1.8 | |
| Flee | 0.8 ± 0.5 | 31.1 ± 4.7 |
Differences between groups are significant (P < 0.05) for all behaviors.
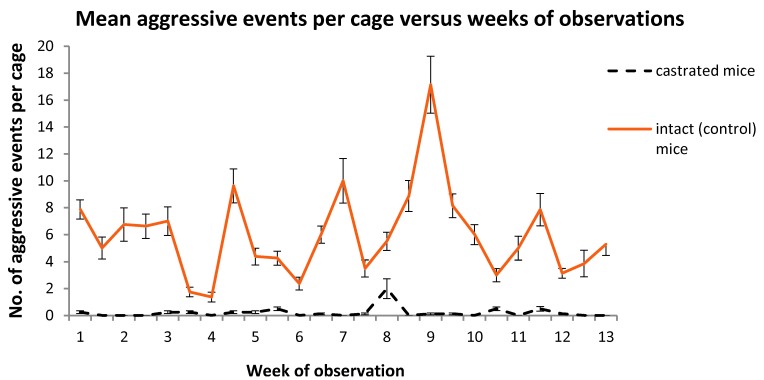
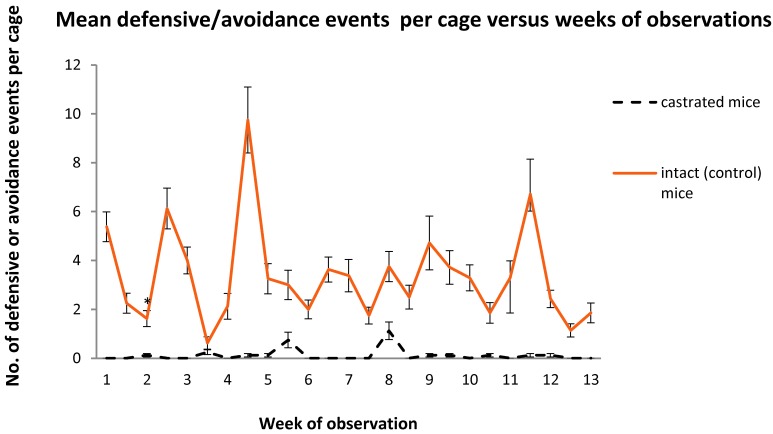
The serial time charts (Figures 3 through 5) illustrate differences over the weeks of observations for the collated variables used to assess aggression between the cages of intact and castrated animals. The time relationships between spikes and troughs in events for the cages housing intact mice were present for aggressive events (Figure 2), defensive–avoidance events (Figure 3), and bite injuries (Figure 4). Repeated-measures ANOVA of aggressive and defensive–avoidance events data for the intact mice over the duration of the study revealed that time was not a statistically significant variable. However, in intact mice, time was a significant (P < 0.05; repeated-measures ANOVA) variable for number of bites per cage and was associated with a decrease in the number of injuries as the study progressed.
Figure 3.
Number of aggressive events per cage (mean ± SEM) for each treatment group over the 3-mo observation period. Differences between groups are significant (P ≤ 0.05) at all time points.
Figure 4.
Number of defensive events per cage (mean ± SEM) for each treatment group over the 3-mo observation period. Differences between groups are significant (P ≤ 0.05) at all time points.
Discussion
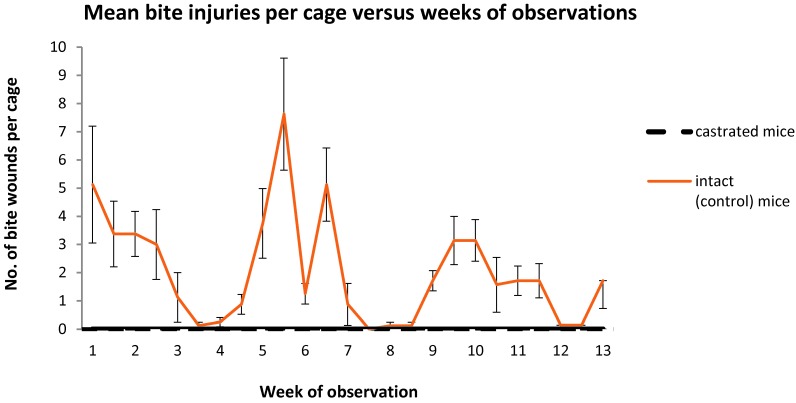
The results demonstrate a substantial decrease in agonistic and defensive behaviors among group-housed castrated male mice compared with intact controls. In addition, castration reduced the actual injuries sustained to 0 (Figure 5). This result concurs with the findings of a previous study, which similarly demonstrated a zero incidence of fighting in prepubertally castrated mice.14 The work of other colleagues also demonstrated that castration eliminated aggression, due to androgen reduction, in mice.19 However, that study19 focused on the time of castration and its effect on aggressive behavior and did not include intact animals as controls.19 Conversely, other work determined that castration of mice after 6 d of age, with subsequent treatment with exogenous testosterone propionate as adults, did not reduce the incidence or severity of fighting below that seen in neonatally sham-operated or intact controls.23 However, making direct comparisons between this previous study23 and ours is problematic due to the experimental design, in that the previous study incorporated testosterone treatments in animals in an attempt to elucidate androgen responsiveness and its age of onset. Age of castration in early life probably has little effect on an aggression response to other male mice, although it does influence response to exogenous androgen administration.5,19,23 In one previous study,14 mice were castrated when prepubertal, but in our current study, in which we castrated postpubertal mice,26 a significant difference in aggression was still obtained. This finding concurs with the earlier assertion that age of castration has little effect on development of aggressive behavior. Unfortunately, androgen reduction through castration has been shown to cause effects in diverse parameters of interest in many research applications, including tissue mass changes,6 increases in immunologic reactivity,2 altering differentiation of glandular secretory cells3 and decreasing plasma concentrations of hormones such as insulin and leptin.12 Nevertheless, a knowledge of the changes that result from castration in the animal model of interest will reduce the deleterious nature of this procedure on the interpretation of research results. Such characterization is therefore essential before a castrated animal model could have widespread application in biomedical research. Additional considerations could support the use of castrated mice in research studies after determination of their research effects. For example, housing in social isolation, suffering injury as a result of aggression, and the chronic social stress incurred from social defeat all lead to significant behavioral and physiologic sequelae.1,25,28,29 Therefore stable social housing of male mice, made possible through castration, may actually decrease confounding variables in research.
Figure 5.
Number of bite injuries per cage (mean ± SEM) for each treatment group over the 3-mo observation period. Differences between groups are significant (P ≤ 0.05) at all time points.
Androgens modulate the urinary volatiles that directly contribute to aggressive behavior.11 When a male mouse sprays urine into the environment, urinary proteins act as long-lasting depots that release volatile agents, including 2-sec-butyl-4,5-dihydrothiazole (‘thiazole’) and 2,3-dehydro-exo-brevicomin (‘brevicomin’). In addition to these androgen-dependant urinary chemicals is a group of chemicals called the farnesenes, which are produced by the preputial glands. Farnesene production is partially suppressed in subordinate males when housed in all-male groups.11 This finding hints at the potential effect of farnesenes on cage aggression through their role in signaling social status and establishment of the dominance hierarchy. However, whether direct manipulation of the production pathway of these volatiles would still influence aggression in the absence of an altered androgen concentration is unknown. Such a manipulation might be brought about through preputial gland ablation. If successful, this technique would provide an aggression-modulating strategy that might avoid the potential detrimental effects on research that are associated with castration. This topic would be an interesting area of additional investigation.
The presence of fight-induced injuries in male mice is a frequent observation in laboratory animal establishments and a major cause of poor welfare. A high proportion of these mice will be euthanized on humane grounds. As a result, many researchers predominately use female mice in their studies, necessitating the euthanasia of numerous male mice. This figure might be as high as 65% in preweaning animals alone, creating a substantial ethical issue relating to animal wastage.13 However, the injury itself is not the only welfare concern in these animals. The negative emotional experience that results from aggressive encounters is likely to be a source of chronic stress to these mice and could impair immune function and increase disease susceptibility.1 Therefore, quantification of these encounters is important in making welfare assessments. Nevertheless castration surgery and the associated recovery do impose an added cost to wellbeing, which needs to be factored into a cost–benefit analysis on an individual-case basis. In our study, all castrated mice received pre- and postoperative analgesia and recovered well (with a single exception). We used a midline ventral abdominal surgical approach rather than the scrotal approach preferred by other colleagues.14 The scrotal approach was argued to be less traumatic than is the abdominal, because the scrotal approach does not expose a body cavity.14 However given the presence of open inguinal canals in mice, an open scrotal approach is equivalent to incising the peritoneum. Therefore, we chose a midline abdominal incision, which requires a single closure. In determining cost–benefit, an erroneous conclusion could be drawn if only the immediate effects of invasive procedures are considered. If the principal of ‘whole-of-life animal welfare’ is applied,24 we consider that the benefits resulting from castration, in terms of reduced aggression, outweigh the additional wellbeing cost incurred by surgery, especially in long-term group-housed mice.
The time-series data illustrated in Figure 3 through 5 show a series of peaks and troughs for the measured criteria which, when analyzed by using repeated-measures ANOVA, revealed a decrease in bite injuries in the intact mice over the course of the study. There was no significant change in aggressive and defensive–avoidance events over the same time period. This finding is in contrast to results of colleagues who studied BALB/c intact male mice, in which the frequency and duration of aggression increased with increasing age of the mice.28 However, that study used different behavioral criteria and statistical analysis methods from ours. In addition, the strict humane end-point criteria used in our study may have contributed to the lack of escalation in injuries by removing mice and contributing to more stable hierarchies. This topic could be a future area for investigation.
Our study showed a temporal relationship between peak agonistic and defensive behaviors during the observation periods, such that an almost 1:1 relationship existed. This result is to be expected, given that an aggressor requires a target for aggression. The factors that may have contributed to the aggression peaks seen periodically among the intact mice are unknown. These peaks could have occurred in response to the weekly cage cleaning, but this notion is not borne out by the data. This finding would be an interesting area for additional exploration to determine and possibly refine husbandry interventions that are heightening factors for aggression.
Constraints of the current study include the limited number of observations undertaken, the presence of human observers, and the recording of data during the mice's inactive phase (light period). In future studies, we hope to make experimental refinements to include the use of video behavioral recording during all phases of the mice's circadian rhythm. In addition, this methodology will allow results to be verified by review of the recordings. In the current study, the observers were directly supervised, and results were verified by 2 experienced veterinary technicians who had assisted in developing the behavior categories and had participated in previous pilot studies. The only training the observers received before the start of the project was instruction in the categories of behavior patterns to be measured. This process was followed to avoid giving observers preconceived notions of the behaviors that might be observed. The use of naïve observers has been used by other workers in animal behavior research to provide participants with the freedom to make intuitive judgments when observing and expressing behavioral characteristics; free-choice profiling is such an example.31 Despite the blind nature of the study, as the study progressed, it became clear to the observers which mice belonged to the intact group by their appearance (Figure 6). This knowledge potentially could have compromised the blinding of the study..
Figure 6.
Mice at the end of the project. Left, intact male mouse; right,castrated male mouse.
Our current study has demonstrated that castration performed postpubertally eliminates aggression in group-housed male Swiss mice. This refinement strategy may have application in teaching organizations that need to house large number of male mice and in laboratory animal health-screening programs. Some colleagues have recommended housing intact male mice in groups of 3 to maintain stable dominance hierarchies and minimize aggression.28,29 Using our strategy, we have been able to group-house as many as 10 castrated mice, in a large rat cage with enrichment materials, in harmonious social conditions. This practice enables institutions to house male mice in a cost-effective yet ethical manner. The castration technique is simple to perform and generally void of postoperative complications. In our opinion, the welfare benefits that result from castration outweigh any negative wellbeing and human resources costs associated with the performance of surgery. In conclusion, castration is a simple and effective method to reduce male mouse aggression as part of a colony-management strategy.
Acknowledgments
We thank the students of the Diploma of Animal Technology program and the staff of the Veterinary and Applied Science Centre. Additional thanks are extended to Dr A Livingston for editorial support. The study was funded by TAFESA.
References
- 1.Bartolomucci A. 2007. Social stress, immune functions, and disease in rodents. Front Neuroendocrinol 28:28–49 [DOI] [PubMed] [Google Scholar]
- 2.Castro JE. 1975. Immunological effects of orchidectomy. Br J Urol 47:89–95 [DOI] [PubMed] [Google Scholar]
- 3.Chrétien M. 1977. Action of testosterone on the differentiation and secretory activity of a target organ: the submaxillary gland of the mouse. Int Rev Cytol 50:333–396 [DOI] [PubMed] [Google Scholar]
- 4.Committee on Recognition and Alleviation of Distress in Laboratory Animals 2008. Recognition and alleviation of distress in laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 5.Evans CM, Brain PF. 1978. Effects of age at castration on testosterone-induced aggression-promoting cues in groups of male mice. Physiol Behav 21:19–23 [DOI] [PubMed] [Google Scholar]
- 6.Floryk D, Kurosaka S, Tanimoto R, Yang G, Goltsov A, Park S, Thompson TC. 2011. Castration-induced changes in mouse epididymal white adipose tissue. Mol Cell Endocrinol 345:58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant EC, Mackintosh JH. 1963. A comparison of the social postures of some common laboratory rodents. Behaviour 21:246–259 [Google Scholar]
- 8.Haemisch A, Gartner K. 1994. The cage design affects intermale aggression in small groups of male laboratory mice: strain-specific consequences on social organisation and endocrine activations in 2 inbred strains (DBA/2J and CBA/J). J Exp Anim Sci 36:101–116 [PubMed] [Google Scholar]
- 9.Haemisch A, Voss I, Gartner K. 1994. Effects of environmental enrichment on aggressive behaviour, Dominance hierarchies and endocrine status in male DBA/2J mice. Physiol Behav 56:1041–1048 [DOI] [PubMed] [Google Scholar]
- 10.Howerton CL, Garner JP, Mench JA. 2008. Effects of running-wheel–igloo enrichment on aggression, hierarchy linearity, and stereotypy in group-housed male CD1 (ICR) mice. Appl Anim Behav Sci 115:90–103 [Google Scholar]
- 11.Hurst JL, Beynon RJ. 2004. Scent wars: the chemobiology of competitive signalling in mice. Bioessays 26:1288–1298 [DOI] [PubMed] [Google Scholar]
- 12.Inoue T, Zakikhani M, David S, Algire C, Blouin MJ, Pollak M. 2010. Effects of castration on insulin levels and glucose tolerance in the mouse differ from those in man. Prostate 70:1628–1635 [DOI] [PubMed] [Google Scholar]
- 13.Laboratory Animal Science Association. [Internet] 1998. The production and disposition of laboratory rodents surplus to the requirements for scientific procedures. Report of a LASA Task Force Meeting, 12 June 1998. [Cited 23 July 2013]. Available at www.lasa.co.uk/PDF/Surplus.pdf [Google Scholar]
- 14.Lofgren JL, Erdman SE, Hewes C, Wong C, King R, Chavarria TE, Discua AR, Fox JG, Maurer KJ. 2012. Castration eliminates conspecific aggression in group- housed CD1 male surveillance mice (Mus musculus). J Am Assoc Lab Anim Sci 51:594–599 [PMC free article] [PubMed] [Google Scholar]
- 15.Mellor DJ. 1999. What determines minimum farm animal welfare standards—animals’ actual needs, animals’ perceived needs, or economics? Proceedings of 16th Annual Seminar of the Society of Dairy Cattle Veterinarians of the NZVA 192:143–152 [Google Scholar]
- 16.Mellor DJ. 2012. Animal emotions, behavior, and the promotion of positive welfare states. N Z Vet J 60:1–8 [DOI] [PubMed] [Google Scholar]
- 17.Mellor DJ, Reid CSW. 1994. Concepts of animal wellbeing and predicting the impact of procedures on experimental animals, p 3–18. In: Baker RM, Jenkin G, Mellor DJ.Improving the well-being of animals in the research environment. Adelaide (Australia): Australian and New Zealand Council for the Care of Animals in Research and Teaching, Glen Osmond, South Australia [Google Scholar]
- 18.Mellor DJ, Stafford KJ. 2001. Integrating practical, regulatory, and ethical strategies for enhancing farm animal welfare. Aust Vet J 79:726–728 [DOI] [PubMed] [Google Scholar]
- 19.Motelica-Heino I, Edwards DA, Roffi J. 1993. Intermale aggression in mice: does hour of castration after birth influence adult behavior? Physiol Behav 53:1017–1019 [DOI] [PubMed] [Google Scholar]
- 20.Nagy A, Gertsenstein M, Vintersten K, Behringer RR. 2003. Manipulating the mouse embryo. New York (NY): Cold Spring Harbor Laboratory Press [Google Scholar]
- 21.National Health and Medical Research Council . 2011. Australian code of practice for the care and use of animals for scientific purposes (draft),3.7 Conduct of projects, 3.6.6 (i) [Google Scholar]
- 22.National Health and Medical Research Council 2004. Australian code of practice for the care and use of animals for scientific purposes. Canberra: Australian Government [Google Scholar]
- 23.Peters PJ, Bronson FH, Whitsett JM. 1972. Neonatal castration and intermale aggression in mice. Physiol Behav 8:265–268 [DOI] [PubMed] [Google Scholar]
- 24.Phillips C. 2009. Welfare of animals: the silent majority. New York (NY): Springer–Verlag [Google Scholar]
- 25.Poole TB, Morgan HDR. 1973. Differences in aggressive behaviour between male mice (Mus musculus) in colonies of different sizes. Anim Behav 21:788–795 [DOI] [PubMed] [Google Scholar]
- 26.Silver LM.[Internet] 1995. Mouse genetics: concepts and applications. Oxford University Press. [Cited 4 April 2013]. Available at: http://www.informatics.jax.org/silver/
- 27.Speaking of Research . [Internet]. US statistics. Available at: http://speakingofresearch.com/facts/statistics/ [Google Scholar]
- 28.Van Loo PLP, Mol JA, Koolhaas JM, Van Zutphen BFM, Baumans V. 2001. Modulation of aggression in male mice: influence of group size and cage size. Physiol Behav 72:675–683 [DOI] [PubMed] [Google Scholar]
- 29.Van Loo PLP, Van Zutphen LFM, Baumans V. 2003. Male management: coping with aggression problems in male laboratory mice. Lab Anim 37:300–313 [DOI] [PubMed] [Google Scholar]
- 30.Webster J. 2005. Animal welfare: limping towards Eden. Chicester (UK): Wiley-Blackwell [Google Scholar]
- 31.Wemelsfelder F, Hunter TEA, Mendl MT, Lawrence AB. 2001. Assessing the ‘whole animal’: a free-choice profiling approach. Anim Behav 62:209–220 [Google Scholar]
- 32.Whittaker AL, Howarth GS, Hickman DL. 2012. Effects of space allocation and housing density on measures of wellbeing in laboratory mice: a review. Lab Anim 46:3–13 [DOI] [PubMed] [Google Scholar]