Abstract
The suprachiasmatic nucleus is synchronized by the light:dark cycle and is the master biologic clock that serves as a pacemaker to regulate circadian rhythms. We explored the hypothesis that spectral transmittance (tint) of light through caging alters circadian rhythms of endocrine and metabolic plasma constituents in nonpigmented Sprague–Dawley rats. Rats (Crl:SD; n = 12 per group) were housed in a 12:12-h light:dark environment (300 lx; 123.0 μW/cm2; lights on, 0600) in either clear-, amber-, blue-, or red-tinted rodent cages. Blood was collected at 0400, 0800, 1200, 1600, 2000, and 2400 and measured for melatonin, total fatty acids, pH, glucose, lactic acid, corticosterone, insulin, and leptin. As expected, plasma melatonin levels were low during the light phase but higher during the dark phase in all groups; however, when compared with the clear-cage group, rats in amber-, blue-, and red-tinted cages had 29%, 74%, and 48%, respectively, greater total daily melatonin levels due to an increased duration and, in some cases, amplitude of the nocturnal melatonin signal. No differences were found in dietary and water intake, body growth rates, total fatty acids, pH, or glucose among groups. Disruptions in circadian rhythms, manifesting as alterations in phase timing, amplitude, or duration, occurred in the melatonin, lactic acid, corticosterone, insulin, and leptin levels of rats in tinted compared with clear cages. Therefore, the use of variously tinted animal cages significantly alters circadian rhythms in plasma measures of metabolism and physiology in laboratory rats, thus potentially altering the outcomes of scientific investigations.
Abbreviations: ipRGC, intrinsically photosensitive retinal ganglion cells; SCN, suprachiasmatic nucleus
Light prompts photobiological changes by way of the photopigment melanopsin in intrinsically photosensitive retinal ganglion cells (ipRGC),19 with some modulation by the visual rod and cone photoreceptors.2,17 This stimulus leads to a cascade of molecular events sending signals via the neural pathway of the retinohypothalmic tract to the suprachiasmatic nucleus (SCN), the master biologic clock5 which is located in the brain. The SCN synchronizes numerous circadian rhythms and contributes to regulation of homeostasis and circadian physiology via several circadian outputs including the pineal gland's production of melatonin. Studies have shown that changes in lighting intensity, duration, wavelength, and timing can disturb these circadian rhythms.7-9 Not all light is equivalent. The responses to different wavelengths produced by visible and nonvisual electromagnetic radiation in an organism form what is known as an action spectrum. These wavelengths are perceived as a color or tint by the visual system of the primary optic tract. In mammals, the range generally between 450 and 550 nm (that is, blue light) has the strongest influence on neuroendocrine and circadian responses; however, longer wavelength (that is, red) light at high intensities can have this effect also.7,18,29
Melanopsin is a photosensitive pigment in the plasma membrane of ipRGC, which are directly activated by light to drive photoentrainment of the circadian system.14,28 The ipRGC reside in the ganglion cell layer, which is the first retinal layer to receive incoming light. Melanin, however, is a distinctly different pigment from the chromaphore melanopsin. Commonly, animals are considered to be pigmented due to the presence of melanin, a derivative of tyrosine. Such is the case of the nude rats used in our previous studies.11 An animal with a deficiency or absence of melanin in the retinal epithelium, hair, or skin is considered to be nonpigmented. Animals commonly used in biomedical research include albino (nonpigmented) Sprague–Dawley rats that lack melanin in the eyes, hair, and skin. Studies have found that the lack of melanin results in the abnormal development of the retinal pigmented epithelium and central retina and rods, as well as abnormal neural connections between the eye and brain.21,26 Previous studies conducted with tinted laboratory cages demonstrated significant disruptions of metabolic and endocrine parameters in pigmented female nude rats.11 To our knowledge, testing of a nonpigmented rat has not been attempted.
As the field of laboratory animal science grows and advances, changes are being made in industry standards for laboratory animal housing and care for various reasons. These changes include the use of materials more suitable for high-temperature cleaning and of cage designs that accommodate viewing. In response to these shifting needs, alterations in cage design, material, or coloration that have been implemented may have unintended consequences for animal physiology. Unfortunately, most of these changes have little to no scientific basis supporting their use. For example, researchers and animal care personnel commonly expose animals to red light for extended periods of time for continual dark-phase observation or procedures when, in fact, such exposure may be detrimental to animal physiology, metabolism, and behavior depending on exposure duration.18 In addition, the eighth edition of the Guide22 suggests the use of red-tinted windows for rat and mouse holding rooms for animal observation and blocking hallway light from entering the room.
In this regard, our previous research investigated the effect of spectral transmittance (for example, cage tint) through standard laboratory caging in female pigmented athymic nude rats and showed that animals developed chronobiologic disruptions in various endocrine and metabolic constituents in plasma.11 Although the circadian rhythm of total fatty acids in plasma remained unchanged, all other metabolic and physiologic rhythms measured were significantly altered when rats were housed in either amber- or blue-tinted cages as compared with clear cages. These disruptions in plasma analyte levels encompassed changes in rhythm duration, phasing (for example, timing), amplitude, or some combination of these elements. Clear cages allowed the full spectrum of animal room polychromatic fluorescent light to pass through the cage, whereas tinted cages limited the transmittance of specific wavelengths.
In the present study, we expand our previous findings in pigmented rats by exploring the effect of changes in spectral transmittance during the light period in male albino Sprague–Dawley rats. These animals were selected not only because they are nonpigmented but also because they are among the most widely used rat strains in scientific research. We hypothesized that changes in the tint (for example, spectral transmittance) of laboratory cages disrupts circadian rhythmicity and therefore the production of various endocrine and metabolic plasma constituents in Sprague–Dawley rats throughout a 24-h light:dark cycle.
Materials and Methods
Reagents.
HPLC-grade chloroform, ethyl ether, methanol, glacial acetic acid, heptane, and hexane were purchased from Fisher Scientific (Pittsburg, PA). Free fatty acid, rapeseed oil methyl ester standards, boron–trifluoride–methanol, potassium chloride, sodium chloride, and perchloric and trichloroacetic acids were purchased from Sigma Scientific (St Louis, MO). Ultrapure water (catalog no. 400000) was purchased from Cayman Chemical (Ann Arbor, MI).
Animals, housing conditions, and diet.
The male Sprague–Dawley rats (age, 3 to 4 wk) used in this study were purchased from Charles River (Crl:CD(SD)), Kingston, NY) and were certified by the vendor to be free for all known rodent bacterial, viral, and parasitic pathogens. Rats were maintained in the vivarium of Tulane University School of Medicine, which is an AAALAC-accredited facility. All animal use and accompanying procedures were in accordance with The Guide for the Care and Use of Laboratory Animals22 and approved by the IACUC.
Rats were maintained in cages on hardwood maple bedding (no. 7090, Sanichips, Harlan Teklad, Madison, WI; 2 or 3 bedding changes weekly). Serum samples from sentinel animals on combined soiled bedding derived from cages that housed project animals were tested quarterly via Multiplex Fluorescent Immunoassay 2 (Idexx Research Animal Diagnostic Laboratory, Colombia, MO) for rat coronavirus, Sendai virus, pneumonia virus of mice, sialodacryoadenitis virus, Kilham rat virus, Toolan H1 virus, reovirus type 3, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, mouse adenovirus 1 and 2, Hantaan virus, Encephalitozoon cuniculi, cilia-associated respiratory bacillus, parvovirus NS1, rat parvoviruses, rat minute virus, and rat theilovirus in addition to external and internal parasites to ensure animals remained infection-free; all test results were negative during the course of the experiment. Rats were given free access to Purina 5053 Irradiated Laboratory Rodent Diet (Richmond, IN) and acidified water. Quadruplicate determinations of this diet contained 3.9 g total fatty acid per 100 g of diet composed of 1.06% myristic (C14:0), 15.94% palmitic (C16:0), 1.47% palmitoleic (C16:1n7), 3.97% stearic (C18:0), 22.22% oleic (C18:1n9), 54.54% linoleic (C18:2n6), and 0.26% arachidonic (C20:4n6) acids. Minor amounts of other fatty acids comprised 0.54%. Food and water intake was measured 2 or 3 times weekly, and body weights were measured weekly. This experiment was repeated once to verify results.
Caging, lighting regimens, and spectral transmittance measurements.
On arrival, rats were assigned randomly to standard laboratory rat caging (3 rats per cage; 10.5 in. × 19 in. × 8 in.; wall thickness, 0.1 in.) for a 1-wk acclimation period. After this time, animal groups were kept together and randomly transferred into 1 of 4 experimental cages with clear, amber, blue, or red hues (n = 6 per group) for an exposure period of 2 wk. Rodent cages were purchased from Ancare (polycarbonate translucent clear, catalog no. R20PC; polysulfone translucent amber, catalog no. R20PLF; Bellmore, NY) or Lab Products (polycarbonate translucent blue, catalog no. 80778CC; polycarbonate translucent red, catalog no. 18780M; Seaford, DE). All cages had identical stainless steel lids (catalog no. 10SS, Ancare) for cradling food and the water bottle and were covered with polysulfone translucent microfilter tops (catalog no. N10MBT, Ancare). The rats were maintained in climate-controlled rooms (25 °C; 50% to 55% humidity) with diurnal lighting set for 12:12-h light:dark (lights on, 0600). Animal rooms were lighted as described previously11 with overhead white fluorescent lamps and were completely devoid of light contamination during the dark phase. Daily during the course of this experiment, the animal room lighting intensity was measured at 1m above the floor in the center of the room and outside, within, and at the front of the animal cages by using a radiometer–photometer and radiance detector with filter and diffuser, as previously reported;11 these instruments were calibrated regularly. Each day at the same time (0800), light measurements were taken. All cages were placed on the room shelf in premeasured, specific positions to ensure that there were no significant differences between cage type in lighting intensity inside the cages (at rodent eye level). Cage-cleaning procedures, as previously reported,11 did not cause any variations in light intensity measurements throughout the course of the study, as evidenced by lighting measurements.
Light measures of cages used were performed at Thomas Jefferson University in a 17.5 ft × 13.5 ft windowless room that was illuminated by three 2 ft × 4 ft lighting fixtures each containing four 32-W, 48-in. T8, 3500-K color temperature fluorescent lamps (Osram Sylvania, Westfield, IN) behind clear, prismatic acrylic diffusers. Correlated color temperature was measured by using a chromameter (model CL-200A, Konica-Minolta, Tokyo, Japan). Spectral transmittances were quantified by using a handheld spectroradiometer (FieldSpec, ASD, Boulder, CO). The 4 cage types (clear, amber, blue, and red) were held in place, upside-down, covering the cosine receptor foreoptic attachment used for irradiance measurements. The optical sensor on the meter was centered inside each cage and oriented in each of 4 horizontal directions of the room while spectral power measurements were recorded. In addition, the sensor was directed upward, directly toward the overhead fluorescent lamps lighting the room, with a distance of 1.4 m to the light source. Spectral power distribution was recorded when the meter was pointing directly at the overhead fluorescent lighting source. This measure was the most stable among the 5 measurements taken for each cage and, therefore, was used for a comparison between cages.
Blood collection.
After exposure of the rats for 2 wk in the respective cages and lighting conditions, blood was collected via cardiocentesis under gas anesthesia in a series of 6 (time points 0400, 0800, 1200, 1600, 2000, 2400) small-volume collections 3 to 5 d apart, as previously reported.6,11-13 Briefly, 2 L/min of 100% CO2 was passed into the bottom of a acrylic gas anesthesia chamber (Braintree; 10 in. × 8 in. × 8 in.), displacing air at a rate of 20% volume per minute. On loss of righting reflex, each animal was removed and allowed to breathe room air while blood (1 mL; less than 5% total blood volume) was withdrawn by using a 3 mL leur-lock syringe (Tyco Healthcare, Mansfield, MA) and 25 gauge, 3/8-in. needle (Tyco) moistened with sodium heparin (5000 USP U/mL, Sargent Pharmaceuticals, Schaumburg, IL). CO2 and O2 were measured (model no. 8762, serial no. 01120587, IAQ-Calc Indoor Air Quality Meter, TSI Instruments, Shoreview, MN, and Eagle Series portable multigas detector, type 401, serial no. E065052, RKI Instruments, Union City, CA).
Dark-phase sampling was performed in less than 1 min under 1 or 2 safelight red lamps (120 V, 15 W; type 1A, model B, catalog no. 152 1517; Kodak, Rochester, NY) to preserve the normal nocturnal melatonin surge. Exposure at rodent eye level was 45 to 179.5 µW/cm2, depending on the use of 1 or 2 lights and the position of the light(s) during sampling.
Glucose, lactate, and pH measurements.
After each collection, a portion of the whole-blood sample was used to measure pH, glucose, and lactate (iSTAT1 Analyzer with CG4+ and CG8+ cartridges, Abbott Laboratories, East Windsor, NJ). Minimal levels of detection for pH, glucose, and lactate were 0.01, 0.2 mg/dL, and 0.01 mmol/L, respectively, with a sensitivity range of 1% to 5%. The remaining blood sample was centrifuged at 12,000 × g for 10 min at 4 °C (accuSpin Micro17R centrifuge, Fisher Scientific, Germany). Plasma was transferred to a new microtube (Sigma-Aldrich) and stored at −20 °C until assayed for melatonin, total fatty acids, corticosterone, insulin, and leptin.
Melatonin analysis.
Plasma melatonin levels were measured by using a melatonin 125I-radioimmunoassay kit (Bühlmann Laboratories, Schönenbuch, Switzerland) and analyzed on an automated gamma counter (Cobra 5005, Packard, Palo Alto, CA) as previously described.11 Briefly, C18 reverse-phase extraction columns included in the kit were used to extract the melatonin from the samples by using 0.125 mL of plasma for 2400 and 0400 time points. For the 0800, 1200, 1600, and 2000 time points, 0.25 mL of plasma from 2 animals within the same group were pooled to make a total of 0.5 mL, due to the low levels of melatonin in these samples. The functional sensitivity for the assay was 0.9 pg/mL. The intraassay precision of column extraction and radioimmunoassay combined was 8.2%.
Fatty acid extraction and analysis.
Plasma free fatty acids were extracted as previously described11 from 0.05 mL samples in duplicate by using an internal standard consisting of heptadecanoic acid (100 µg) dissolved in chloroform. A gas chromatograph fitted with a flame ionization detector, auto injector (both adjusted to 220 °C), and integrator (model 58990A, Hewlett Packard, Palo Alto, CA) was used to analyze the methyl esters of fatty acids according to their retention time compared with known standards. A 0.25 mm × 30 cm capillary column using helium as the carrier gas was used for separations. The minimum detection level for the assay was 0.05 µg/mL.
ELISA analysis of corticosterone, insulin, and leptin.
Plasma samples were prepared in duplicate for measurement of corticosterone (catalog no. 55-CORMS-E01), insulin (catalog no. 80-INSRTH-E01; rat high-range), and leptin (catalog no. 22-LEPMS-E01; mouse and rat) levels by using chemiluminescent ELISA diagnostic kits (ALPCO, Salem, NH) and measured on a microplate reader (450 nM; VersaMax, Molecular Devices, Sunnyvale, CA) as previously described.11 Detection sensitivity for corticosterone, insulin, and leptin were 4.5 ng/mL, 0.124 ng/mL, and 10 pg/mL, respectively. Lower limits of detection for corticosterone, inulin, and leptin were 15 ng/mL, 0.15 ng/mL, and 10 pg/mL, respectively; and the coefficient of variation of all assays was less than 4.0%.
Statistical analysis.
All data are presented as the mean ± SE and were compared by using one-way ANOVA followed by the Bonferroni multiple-comparison test or 2-tailed t test (Prism, GraphPad, La Jolla, CA). Due to assay errors or protocol sample requirements (noted earlier for melatonin samples), the number of samples varies between assays but is greater than 6, unless otherwise noted. Cosinor analysis was used to determine rhythmicity for plasma total fatty acid and corticosterone. All data for both replicate experiments are combined. Differences among the group means were considered statistically significant at a P value of less than 0.05.
Pearson correlations were performed to determine similarities and differences of the spectral power distributions between cages. Based on irradiance measures, the correlation coefficient of the spectral power distributions from 380 to 760 nm was determined. As a more detailed method of analysis, the spectral power distribution was divided into 100-nm bins (that is, 400 to 500 nm), and Pearson correlations were determined between cage conditions. For the red and clear cages, photon flux was calculated in 50-nm bins between 400 to 700 nm.
Results
Animal room illumination and caging spectral transmittance measurements.
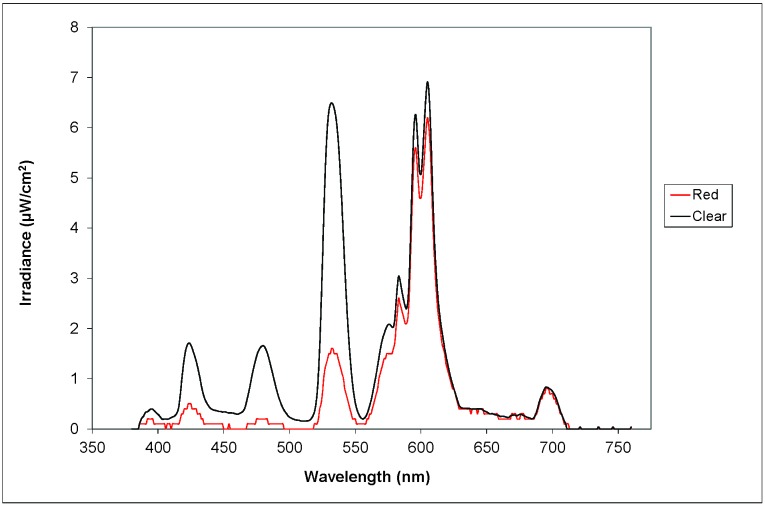
Mean daytime animal room illumination at the center of the room 1 m above the floor with the detector facing the ceiling had relatively small variance and measured 152.6 ± 8.4 µW/cm2. Laboratory rodent cages used in this experiment are shown from left to right and include translucent clear, amber, blue, and red cages (Figure 1). Measurements of radiometric irradiance from inside the front of each cage at rodent eye level were made daily and showed little intercage variability and are reported here as the mean measurement for all cages (32.7 ± 0.1 µW/cm2). Spectral power distributions of light measured through the wall of each cage type is illustrated in Figure 2. Radiometric and photometric values are shown in Table 1. There were marked differences in the correlated color temperatures, irradiance, and photon density between the clear and red cages. Table 2 demonstrates irradiance values and comparisons of peak wavelength differences. Red cages had the lowest irradiances in all wavelength segments. At the shorter wavelengths (400 to 550 nm), blue cages contained the highest peak amplitude (at 424 nm: 5.9% higher than clear, 27% higher than amber, and 74% higher than red). At the longer wavelengths (550 to 700 nm; specifically at 605 nm), the irradiance for clear cages was 8.4%, 3.4%, and 9.8% greater than that for the blue, amber, and red cages, respectively.
Figure 1.

Translucent clear and amber-, blue-, and red-tinted rat cages (from left to right). All cages were of equal dimensions (19 in. × 10.5 in. × 8 in.; wall thickness, 0.1 in.).
Figure 2.
Measurements of spectral transmittance through translucent clear and amber-, blue-, and red-tinted cages. Values are plotted as light irradiance (µW/cm2) and wavelength (nm).
Table 1.
Radiometric and photometric values inside cages
| Clear | Amber | Blue | Red | |
| Irradiance (µW/cm2) | 84 | 80 | 74 | 63 |
| Photon density (× 1015) | 1.20 | 1.12 | 1.17 | 0.72 |
| Illuminance (lx) | 238 | 238 | 216 | 162 |
| CCT (K) | 3250 | 3142 | 3484 | 2300 |
CCT, correlated color temperature.
Table 2.
Irradiance values and comparisons of peak wavelength differences
| Peak wavelength (nm) | ||||
| Measured irradiance (µW/cm2) | 424 | 480 | 532 | 605 |
| Clear cage | 1.71 | 1.66 | 6.49 | 6.91 |
| Amber cage | 1.31 | 1.45 | 6.05 | 6.67 |
| Blue cage | 1.81 | 1.77 | 6.69 | 6.33 |
| Red cage | 0.47 | 0.19 | 1.57 | 6.23 |
| Percentage (%) difference | ||||
| Clear versus blue | −5.9 | −6.7 | −3.0 | 8.4 |
| Clear versus amber | 23.0 | 12.6 | 6.9 | 3.4 |
| Clear versus red | 72.5 | 88.6 | 75.8 | 9.8 |
| Blue versus amber | 27.3 | 18.1 | 9.6 | −5.4 |
Dietary and water intake and body growth rates.
There were no significant differences in daily dietary and water intake or weekly body growth rates among caging groups and are reported as follows as the mean for all groups (diet: 8.7 ± 1.9 g/100 g body weight; water: 15.6 ±1.9 mL/100 g body weight; growth rate: 45 ± 15.3 g weekly).
Anesthesia chamber measurements.
Atmospheric CO2 inside the anesthesia chamber was greater than 6000 ppm within 1 min, and atmospheric O2 measured 24.1%. After the top was opened and closed, simulating the removal of one subject and replacement of the next, measurements for CO2 and O2 were greater than 6000 ppm and 31.9% respectively. A third measurement, taken after opening and closing the lid, revealed greater than 6000 ppm CO2 and 37.2% O2, demonstrating that the atmospheric conditions of the chamber were constant for each cardiocentesis sampling.
Plasma melatonin.
Circadian rhythms of plasma melatonin concentrations are shown for each group in Figure 3. Melatonin concentrations were low (less than 25 pg/mL) from 0800 to 2000 for all groups. A significant rhythm was found in all rats maintained in the 4 groups. Melatonin concentrations for rats in amber, blue, or red cages were not significantly different from those of rats in clear cages at the 0800, 1200, 1600, and 2400 time points. At 2000, there was a significant difference between the red (9.5 ± 1.5 pg/mL) and clear (21.1 ± 2.9 pg/mL) cage groups only. Peak levels of melatonin occurred at 2400 h for the amber, red, and clear groups (209 ± 13 pg/mL, 230 ± 17 pg/mL, 210 ± 15 pg/mL, respectively), whereas the peak for the blue group was phase-delayed by 4 h to occur at 0400. For melatonin and other parameters, phase delays were determined by comparing the timing of peak values, also known as the acrophase, of experimental groups (amber, blue, and red) with that of the control clear peak. A phase delay was defined as a shift in a group peak level to a later time point, whereas a phase advance was a shift in a group peak level to an earlier time point. Hormone levels were significantly (P < 0.05) higher at the 0400 time point in the amber, blue, and red groups (181 ± 24 pg/mL, 289 ± 27 pg/mL, 223 ± 27 pg/mL, respectively) as compared with the clear group (79 ± 93 pg/mL). Areas under the curve for amber, blue, clear, and red cages were 436 pg/mL, 587 pg/mL, 337 pg/mL, and 499 pg/mL, respectively.
Figure 3.
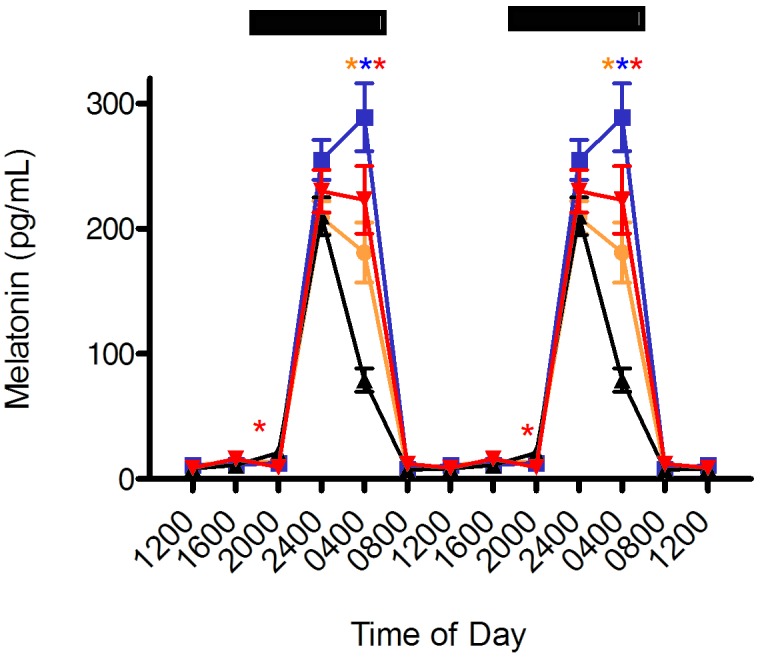
Diurnal plasma melatonin levels (pg/mL; mean ± SEM) of male Sprague–Dawley rats maintained for 6 wk (12:12 light:dark; lights on, 0600) in either translucent clear (black triangles), amber (amber circles), blue (blue squares), or red (red triangles) rodent cages and on free-choice water and standard rodent chow. Data are plotted twice. The dark phase (1800 to 0600) is designated by dark bars. Asterisks indicate concentrations that are significantly (P < 0.05) different from those of rats in clear cages.
Plasma total fatty acids.
All plasma total fatty acid values displayed a significant rhythm, which was almost identical between groups (Figure 4) for Sprague–Dawley rats with free access to food. In all groups, the nadir occurred at 1600 (1348 ± 76 µg/mL), with a peak at 0400 (5173 ± 230 µg/mL); there were no significant differences between groups. The daily combined mean of total fatty acids was 2872 ± 1279 µg/mL plasma.
Figure 4.
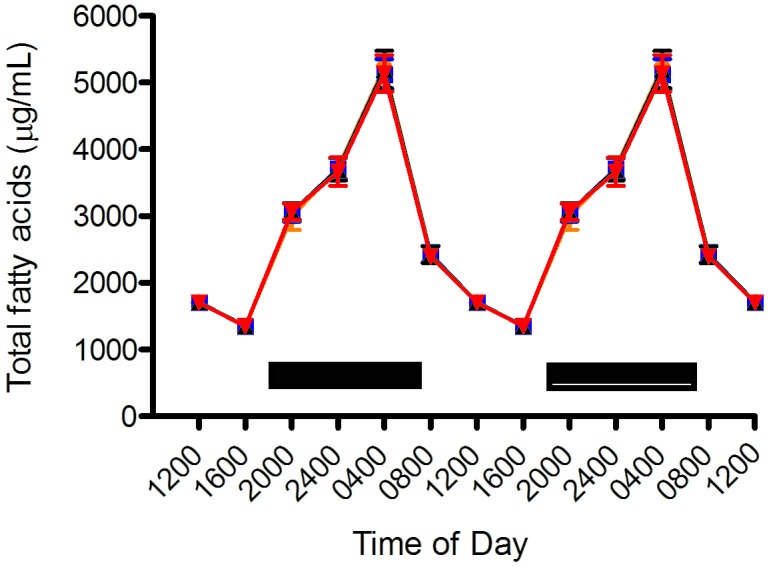
Diurnal plasma total fatty acid concentrations (µg/mL; mean ± SEM) of adult male Sprague–Dawley rats maintained for 6 wk (12:12 light:dark; lights on, 0600) in either translucent clear (black triangles), amber (closed amber circles), blue (blue squares), or red (red triangles) rodent cages and on free-choice water and standard rodent chow. Total fatty acid values were the sums of myristic, palmitic, palmitoleic, stearic, oleic, linoleic, and arachidonic acids. Data are plotted twice. The dark phase (1800 to 0600) is designated by dark bars. No significant differences between rats in any tinted compared with clear cages were found at any time point.
Blood pH.
No significant rhythm was found in any group at any time points for blood pH (7.17 ± 0.01); therefore data were averaged together.
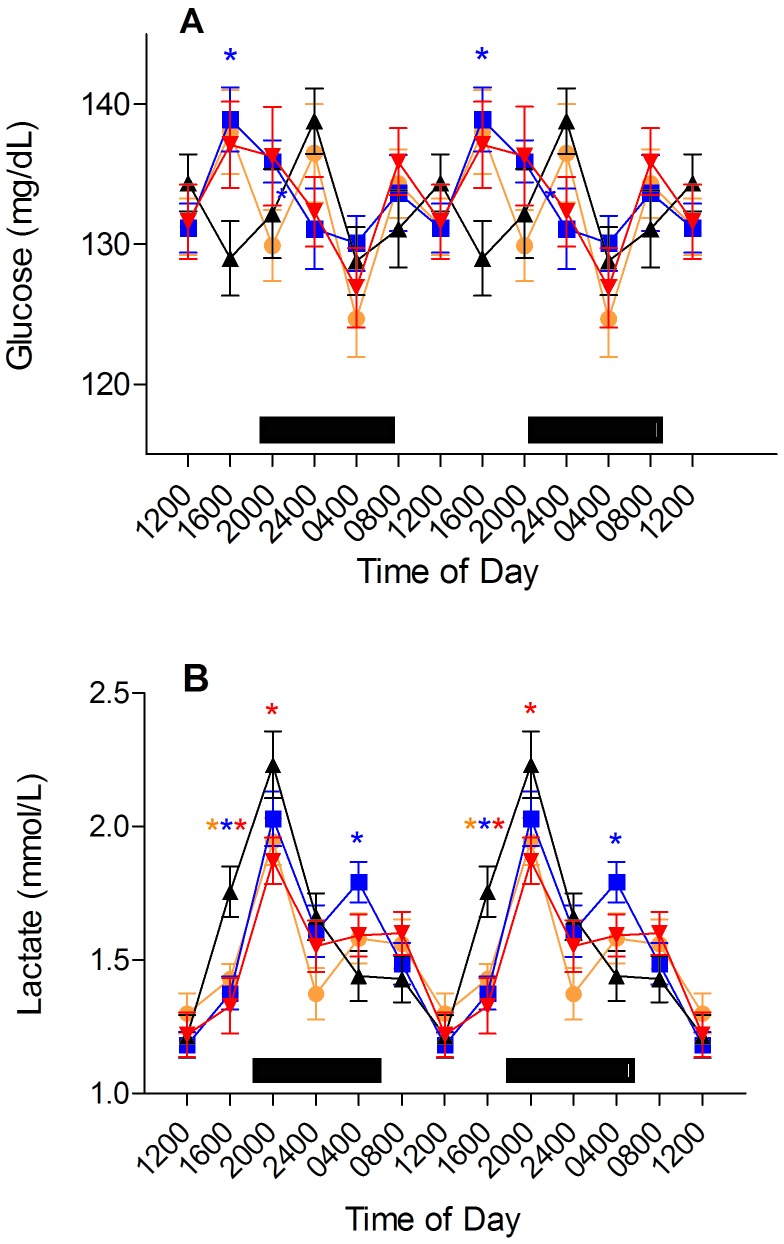
Blood glucose.
Figure 5 A depicts daily rhythms in levels of glucose. Significant circadian rhythms were found for all groups. Blood glucose in the clear group peaked at 2400 with a smaller second peak at 1200. The peak in glucose in the amber, blue, and red groups occurred at 1600, which is phase-advanced by 8 h when compared with the clear group. A smaller, second peak transpired at 2400 in the amber group. The red group's second peak occurred at 0800. Nadirs for all groups took place at 0400. Glucose in the blue group was significantly (P < 0.05) higher than that of the clear group at 1600. No significant differences were found in peak amplitude or nadirs among groups at 0400, 0800, 1200, 2000, or 2400.
Figure 5.
Diurnal changes in (A) plasma glucose and (B) lactic acid levels (mg/dL and mmol/L, respectively; mean ± SEM) of male Sprague–Dawley rats maintained for 6 wk (12:12 light:dark; lights on, 0600) in either translucent clear (black triangles), amber (amber circles), blue (blue squares), or red (red triangles) rodent cages and on free-choice water and standard rodent chow. Data are plotted twice. The dark phase (1800 to 0600) is designated by dark bars. Asterisks indicate concentrations that are significantly (P < 0.05) different from those of rats in clear cages.
Blood lactate.
Circadian rhythms were found in lactate concentrations for Sprague–Dawley rats from all groups and were similar (Figure 5 B). Nadirs and peaks were consistent at 1200 and 2000, respectively. The peak blood lactate level for the red group (1.9 ± 0.1 mmol/L) was significantly (P < 0.05) lower than that of the clear group (2.2 ± 0.1 mmol/L). Concentrations rose continuously from the nadir and peaked shortly after the onset of darkness. At 1600, blood lactate levels for all experimental groups were significantly (P < 0.05) lower than that of the clear group. There was a second peak in the values for the amber and blue groups at 0400 and for the red group at 0800. However, no distinct second peak was identifiable in the clear group. No significant differences were found at either the 0800, 1200, or 2400 time points among all groups.
Plasma corticosterone.
Values for plasma corticosterone in rats of all groups demonstrated clear circadian rhythms and increased continuously from the nadir, which occurred between 0800 and 1200 (Figure 6). The plasma corticosterone rhythm of rats in the clear cages showed 2 peaks: the first, higher-amplitude peak at 2000 and a second, lower amplitude peak at 0400. In comparison, the amber group's peak corticosterone level was phase-delayed 8 h and did not have a second peak. The blue group's peak was phase-delayed 4 h and did not have a second peak. The red group's peak coincided with the clear group's first peak at 2000 but did not have a second peak. The corticosterone peak in the red group was significantly (P < 0.05) lower than that of the clear group at 0400.
Figure 6.
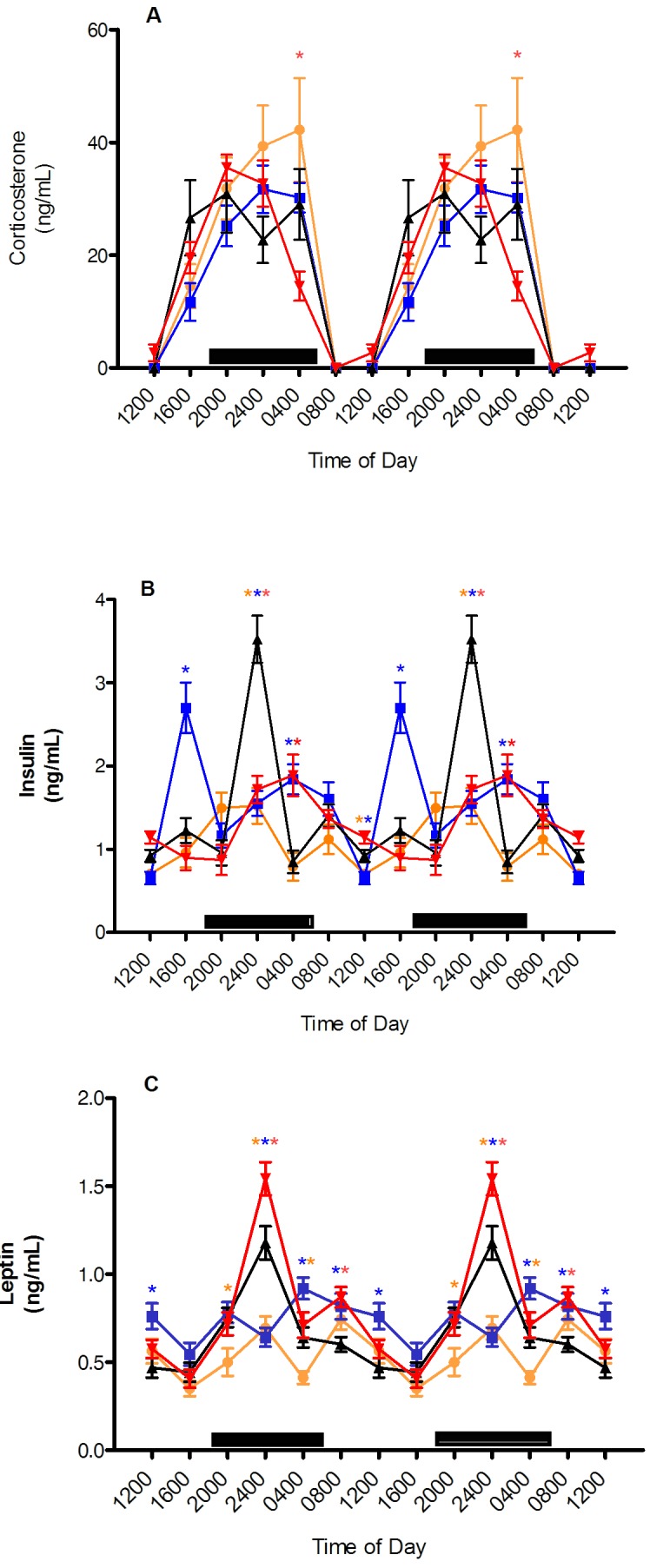
Diurnal changes in plasma (A) corticosterone, (B) insulin, and (C) leptin levels (ng/mL; mean ± SEM) of male Sprague–Dawley rats maintained for 6 wk (12:12 light:dark; lights on, 0600) in either translucent clear (black triangles), amber (amber circles), blue (blue squares), or red (red triangles) tinted cages and on free-choice water and standard rodent chow. Data are plotted twice. The dark phase (1800 to 0600) is designated by dark bars. Asterisks indicate concentrations that are significantly (P < 0.05) different from those of rats in clear cages.
Plasma insulin.
Figure 6 also reveals distinct circadian rhythmicity in the insulin levels of all groups. The plasma insulin rhythm of the clear-cage (control) group was the only one with 3 peaks, which occurred at 1600, 2400 (the highest peak), and 0800. The first (and higher) peak of the amber group (1.5 ± 0.2 ng/mL) was coincident with but significantly (P < 0. 05) lower than that of the clear group at 2400 (3.5 ± 0.3 ng/mL), whereas the second insulin peak of the amber group occurred at 0800 and was parallel with the clear group's third peak. The blue group's first (and higher) peak (2.7 ± 0.4 ng/mL) was at 1600, corresponding to but significantly (P < 0.05) higher than the clear group's first peak (1.2 ± 0.2 ng/mL). The second insulin peak of the blue-caged rats was phase-delayed 4 h (0400) and was significantly (P < 0.05) different from that of the clear group. The red group had only one peak (at 0400), which was phase-delayed 4 h and significantly (P < 0.05) higher than that of the clear group. At 1200, plasma insulin concentrations differed significantly (P < 0.05) between the amber and blue groups, and at 2400, all groups in tinted cages had significantly (P < 0.05) lower plasma insulin than did the clear group. No differences were found between any groups at the 0800 or 2000 time points.
Plasma leptin.
Circadian rhythms in plasma leptin concentration were present (Figure 6) and increased prior to the onset of the dark phase in rats of all groups. The peak leptin concentration in rats in red cages (1.5 ± 0.1 ng/mL) occurred at 2400 and was significantly (P < 0.05) higher than that of the clear group (1.2 ± 0.1 ng/mL). The blue group was significantly different from the clear group at 1200 and 2400; however, the rhythm peak for this group was phase-delayed by 4 h (0400; (P < 0.05) compared with clear group). The mean leptin concentration of the blue-caged rats at 0800 was significantly (P < 0.05) higher than that of the clear group. A second, but lower-amplitude, peak occurred in rats in the amber (nonsignificant) and red (P < 0.05) cages at 0800. The nadir occurred at 1600 in all groups and did not differ between them. The clear groups did not have a distinct second peak. In addition, only the leptin value of the amber group was significantly (P < 0.05) lower than that of controls at 2000, whereas all tinted-caging groups differed from the clear-cage group at the 2400 time point.
Discussion
Circadian rhythms occur in hormones released from the pineal gland, pituitary gland, and peripheral endocrine glands and tissues.7,11 These hormones are under control of the SCN, which is entrained to the light:dark cycle mainly by way of the photopigment melanopsin located in ipRGC and the secretion of melatonin by the pineal gland at night. The regulatory effects of light on SCN activity are dependent on exposure parameters including intensity, wavelength, duration, and timing. Disruptions in SCN function to entrainment cause irregular circadian rhythms of markers of cellular physiology and metabolism and may contribute to disease.6,13 Therefore, knowledge of the potential effects of tinted laboratory cages on experimental research results from commonly used laboratory rat strains is essential. We postulated that changing the tint of animal caging would disrupt normal hormonal and metabolic circadian rhythms in nonpigmented male Sprague–Dawley rats. We compared rats exposed to different spectral transmittances of light passing through tinted cages with animals housed in clear cages and therefore exposed to the full spectrum of polychromatic fluorescent animal-room lighting.
The radiometric and photometric data that we obtained illustrate that the photic environments were different from each other inside of the 4 tinted cage types (clear, amber, blue, or red). Prior research has illustrated that environmental factors such as light spectrum, irradiance, and illuminance can influence circadian, neuroendocrine, and neurobehavioral responses in different species of rodents housed in plastic cages.7-9,11-13,30,31 This literature was incorporated into earlier recommendations regarding housing in typical animal quarters and in experimental animal facilities for spaceflight.20 Several of the photometric and radiometric values varied concurrently (Figure 2, Tables 1 and 2), thus demonstrating the rationale for our placement of the cages in specific locations on the rack. This placement allowed us to eliminate light irradiance as a possible variable, given that there were no significant differences between groups in these measurements, which were obtained at the front inside of each cage at rodent eye level. Further studies are needed to discern if combined factors (for example, spectral transmittance and photon density) are required to evoke the biologic changes found in this study.
The circadian rhythm of plasma total fatty acids was not affected by cage-color exposure in rats with free access to food and water. The rhythm in the current study was identical to that of previous experiments in male Sprague–Dawley rats and similar in peak timing (0400) and intensity.12 This finding confirms that this circadian rhythm remains entrained independently from other SCN-generated rhythms.
Similarly, none of our groups of rats demonstrated significant rhythms in blood pH. The phasing, amplitude, and duration in the circadian rhythmicity in blood glucose concentration were nearly the same among groups in amber-, blue-, or red-tinted cages; all of these peaks were phase-advanced 8 h when compared with that of the clear-cage group. The mean blood glucose concentration of the blue group were higher than that of the clear group at 1600, but all samples were within standard ranges reported for Sprague–Dawley rats.15,27 The overall 24-h integrated levels of glucose were nearly identical among all groups, but blood lactate levels exhibited significant circadian rhythms in all groups. Peak lactate in the rats housed in red-tinted cages was lower than that of the clear group. In addition, second peaks in lactate concentration were present in all groups but the clear group, but only that of the blue group differed (higher) from that of the clear group. When compared with previous studies in Sprague–Dawley rats, the lactate rhythms in our current study are similar, in that the rats showed 2 peaks during the dark phase and a nadir at 1200.12 Lactate is no longer considered to be a waste product only; lactate has proven to be important in wound repair and regeneration and as an intermediary in metabolism and may have key roles in muscle fatigue, anoxia, and dysoxia.16
The clear caged group exhibited a long-duration square wave elevation in corticosterone levels that revealed a peak at the end of the light phase and continued throughout the dark phase. In all of the tinted cage groups, the peak amplitudes were delayed by 4 to 8 h, and the highest-amplitude rhythm occurred in the amber group during the second half of the dark phase. Corticosterone is an important circadian output signal and a highly sensitive hormonal marker of stress. Therefore, time of day and experimental design should be considered carefully when using these measurements for the evaluation of stress. Simple procedures, such as animal handling and placement in an unfamiliar environment, as well as some anesthetics, can increase levels of corticosterone.1,3 For example, the present study used brief CO2 anesthesia for support during blood collections, which has been reported to induce behavioral signs of stress.10 According to the present data, however, and in comparison to similar studies using isoflurane anesthesia, brief CO2 anesthesia did not illicit a physiologic response in these rats, irrespective of cage tint and spectral transmittance. Therefore, normal circadian rhythms of plasma corticosterone were evident at levels not indicative of a stress response to cardiocentesis under brief exposure to CO2 anesthesia.
Insulin plays an important role in energy homeostasis by increasing the uptake and utilization of glucose by peripheral tissues, such as skeletal muscle, liver, and adipose tissue.25 As noted in a previous study,27 a single sharp peak in insulin concentration occurred during the middark phase in the clear controls. In the colored cage groups, however, the nocturnal insulin peaks were broader and markedly blunted and were either phase-advanced or -delayed relative to that in the clear group. In addition, the blue cage group evinced a secondary sharp peak at the end of the light phase.
Secreted by adipocytes, leptin increases energy expenditure and decreases food intake. Leptin circulates in proportion to body fat content, is generally higher in females than males, and is under control of the SCN.4,23 Our results of plasma leptin in male Sprague–Dawley rats showed a distinct peak in both the clear and red groups during middark phase but a lack of a distinct peak in the blue and amber groups. Comparing our data from the current study with those in female nude rats11 reveals that our rats in clear cages have a lower peak amplitude but a similar rhythm of lesser amplitude. This lower amplitude could be the result of small amounts of body fat found in the nude strain and, correspondingly, the large amount of body fat in male Sprague–Dawley rats.
A link exists between leptin and insulin; often they are both increased under conditions such as insulinomas.23,24 Because insulin stimulates glucose transport, these 3 metabolic parameters exhibit a close relationship. Leptin has been shown to directly inhibit insulin-controlled glucose transport by binding to adipocyte receptors.24 The importance of these complex relationships is not completely understood at this time.
As one of the most commonly used animals in biomedical research, albino (nonpigmented) Sprague–Dawley rats lack melanin in the eyes, hair, and skin. The lack of melanin in these rats results in abnormal development of the retinal pigmented epithelium, central retina, and rods, in addition to abnormal neural connectivity between the eyes and brain.21,26 If ipRGC development or melanopsin levels or function were affected in albino rats, we speculate that some of the differences we found in the current study when compared with previous studies with athymic nude rats could be due to these changes in the pigmentation of the retinal epithelium and therefore result from strain-associated differences. Additional studies are warranted to explore this theory.
Additional investigation is warranted to evaluate the differences we found between the current and previous studies in nude rats. The peak spectral sensitivity of ipRGC is in the blue wavelengths (480 nm). More blue is expected to cause more suppression of melatonin. However, surprisingly, in a previous study,11 nude rats demonstrated 7-fold increases in peak melatonin levels in blue- compared with clear-caged rats, whereas our male Sprague–Dawley rats in blue cages showed insignificant increases. Insulin peak levels in male Sprague–Dawley rats of both the blue and clear groups were higher than those of all groups of female nude rats.11 These differences, in addition to other changes found in metabolic and physiologic parameters, suggest the presence of strain-associated variability in responses to caging of various tints.
The eighth edition of The Guide22 recommends the use of red-tinted windows to filter incoming light for mouse and rat observation. Investigators and institutions that commonly use red filtered light at night should also be cautioned. We found that rats in red-tinted cages had higher melatonin levels at 0400, which were sustained from peak levels at 2400. In addition, lactate and insulin peak concentrations in the red group were lower than those of rats in clear cages, whereas the leptin peak was significantly higher. These are just some of the changes resulting from red-tinted light-phase exposure on circadian rhythms; therefore, we recommend that this exposure should not be considered a harmless alternative.
Knowing that cage tint causes significant circadian disruptions, we are currently extending this research to evaluate whether colored enrichment devices commonly used for enrichment in laboratory rodent cages affect circadian rhythms of metabolism and physiology.
Acknowledgments
This work was supported by NIH grant 1 R25 RR032028 (MAW, TGO, and RPB), Tulane University School of Medicine and Louisiana Cancer Research Consortium Startup grant 631455 (DEB), and an AALAS Grant for Laboratory Animal Science (GLAS) Award (RTD). Additional support for the Jefferson coinvestigators (JPH, BW, MJ, and GCB) was from The Institute for Integrative Health (Baltimore). We thank Michael Webb for his outstanding care of the animals, Lynell Dupepe for her facility management knowledge, and Erin Dauchy for her technical training and statistical expertise.
References
- 1.Abelson KSP, Adem B, Royo F, Carlsson HE, Hau J. 2005. High plasma corticosterone levels persist during frequent automatic blood sampling in rats. In Vivo 19:815–819 [PubMed] [Google Scholar]
- 2.Altimus CM, Güler AD, Villa KL, McNeill DS, LeGates TA, Hattar S. 2008. Rods–cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci USA 105:19998–20003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold M, Langhans W. 2010. Effects of anesthesia and blood sampling techniques on plasma metabolites and corticosterone in the rat. Physiol Behav 99:592–598 [DOI] [PubMed] [Google Scholar]
- 4.Bagnasco M, Kalra PS, Kalra SP. 2002. Plasma leptin levels are pulsatile in adult rats: effects of gonadectomy. Neuroendocrinology 75:257–263 [DOI] [PubMed] [Google Scholar]
- 5.Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295:1070–1073 [DOI] [PubMed] [Google Scholar]
- 6.Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, Sauer LA, Rivera-Bermudez MA, Dubocovich ML, Jasser SA, Lynch DR, Rollag MD, Zalatan F. 2005. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res 65:111174–111184 [DOI] [PubMed] [Google Scholar]
- 7.Brainard GC, Hanifin JP. editors. 2005. Photons, clocks, and consciousness. J Biol Rhythms 20:314–325 [DOI] [PubMed] [Google Scholar]
- 8.Brainard GC, Richardson BA, King TS, Reiter RJ. 1984. The influence of different light spectra on the suppression of pineal melatonin content in the Syrian hamster. Brain Res 294:333–339 [DOI] [PubMed] [Google Scholar]
- 9.Brainard GC, Vaughan MK, Reiter RJ. 1986. Effect of light irradiance and wavelength on the Syrian hamster reproductive system. Endocrinology 119:648–654 [DOI] [PubMed] [Google Scholar]
- 10.Conlee KM, Stephens ML, Rowan AN, King LA. 2005. Carbon dioxide for euthanasia: concerns regarding pain and distress, with special reference to mice and rats. Lab Anim 39:137–161 [DOI] [PubMed] [Google Scholar]
- 11.Dauchy RT, Dauchy EM, Hanifin JP, Gauthreaux SL, Mao L, Belancio VP, Ooms TG, Dupepe LM, Jablonski MR, Warfield B, Wren MA, Brainard GC, Hill SM, Blask DE. 2013. Effects of spectral transmittance through standard laboratory cages on circadian metabolism and physiology in nude rats. J Am Assoc Lab Anim Sci 52:146–156 [PMC free article] [PubMed] [Google Scholar]
- 12.Dauchy RT, Dauchy EM, Tirrell RP, Hill CR, Davidson LK, Greene MW, Tirrell PC, Wu J, Sauer LA, Blask DE. 2010. Dark-phase light contamination disrupts circadian rhythms in plasma measures of physiology and metabolism. Comp Med 60:348–356 [PMC free article] [PubMed] [Google Scholar]
- 13.Dauchy RT, Sauer LA, Blask DE, Vaughan GM. 1997. Light contamination during the dark phase in ‘photoperiodically controlled’ animal rooms: effect on tumor growth and metabolism in rats. Lab Anim Sci 47:511–518 [PubMed] [Google Scholar]
- 14.Galindo-Romero C, Jimenez-Lopez M, Garcia-Ayuso D, Salinas-Navarro M, Nadal-Nicolas FM, Agudo-Barriuso M, Villegas-Perez MP, Aviles-Trigueros M, Vidal-Sanz M. 2013. Number and spatial distribution of intrinsically photosensitive retinal ganglion cells in the adult albino rat. Exp Eye Res 108:84–93 [DOI] [PubMed] [Google Scholar]
- 15.Giknis MLA, Clifford CB. 2006. Clinical laboratory parameters for Crl:CD(SD) rats. Wilmington (MA): Charles River Laboratories. [Google Scholar]
- 16.Gladden LB. 2004. Lactate metabolism: a new paradigm for the 3rd millennium. J Physiol 558:5–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gooley JJ, Ho Mien I, St Hilaire MA, Yeo SC, Chua EC, van Reen E, Hanley CJ, Hull JT, Czeisler CA, Lockley SW. 2012. Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J Neurosci 32:14242–14253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanifin JP, Stewart KT, Smith P, Tanner R, Rollag M, Brainard GC. 2006. High-intensity red light suppresses melatonin. Chronobiol Int 23:251–268 [DOI] [PubMed] [Google Scholar]
- 19.Hattar S, Liao H-W, Takao M, Berson DM, Yau K-W. 2002. Melanopsin-containing retinal ganglion cells: architecture, projections and intrinsic photosensitivity. Science 295:1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holley DC, Winget CM, Leon HA; Ames Research Center 1988. Lighting requirements in microgravity: rodents and nonhuman primates, vol 101077, NASA Technical Memorandum. Moffett Field (CA): Ames Research Center [Google Scholar]
- 21.Ilia M, Jeffery G. 2000. Retinal cell addition and rod production depend on early stages of ocular melanin synthesis. J Comp Neurol 420:437–444 [PubMed] [Google Scholar]
- 22.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 23.Kalsbeek A, Fliers E, Romijn JA, LaFleur SE, Wortel J, Bakker O, Endert E, Buijs RM. 2001. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology 142:2677–2685 [DOI] [PubMed] [Google Scholar]
- 24.Lynch HJ, Rivest RW, Ronsheim PM, Wurtman RJ. 1981. Light intensity and the control of melatonin secretion in rats. Neuroendocrinology 33:181–185 [DOI] [PubMed] [Google Scholar]
- 25.Margetic S, Gazzola C, Pegg GG, Hill RA. 2002. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord 26:1407–1433 [DOI] [PubMed] [Google Scholar]
- 26.Mecklenburg L, Schraermeyer U. 2007. An overview on the toxic morphological changes in the retinal pigment epithelium after systemic compound administration. Toxicol Pathol 35:252–267 [DOI] [PubMed] [Google Scholar]
- 27.Merino B, Somoza B, Ruiz-Gayo M, Cano V. 2008. Circadian rhythm drives the responsiveness of leptin-mediated hypothalamic pathway of cholecystokinin 8. Neurosci Lett 442: 165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Provencio I, Warthen DM. 2012. Melanopsin, the photopigment of intrinsically photosensitive retinal ganglion cells. Wiley Interdiscip Rev Membr Transp Signal 1:228–237 [Google Scholar]
- 29.Rahman SA, Kollara A, Brown TJ, Casper RF. 2008. Selectively filtering short wavelengths attenuates the disruptive effects of nocturnal light on endocrine and molecular circadian phase markers in rats. Endocrinology 149:6125–6135 [DOI] [PubMed] [Google Scholar]
- 30.Sun JH, Yaga K, Reiter RJ, Garza M, Manchester LC, Tan DX, Poeggeler B. 1993. Reduction in pineal N-acetyltransferase activity and pineal and serum melatonin levels in rats after their exposure to red light at night. Neurosci Lett 149:56–58 [DOI] [PubMed] [Google Scholar]
- 31.Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. 1984. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature 308:186–188 [DOI] [PubMed] [Google Scholar]