Abstract
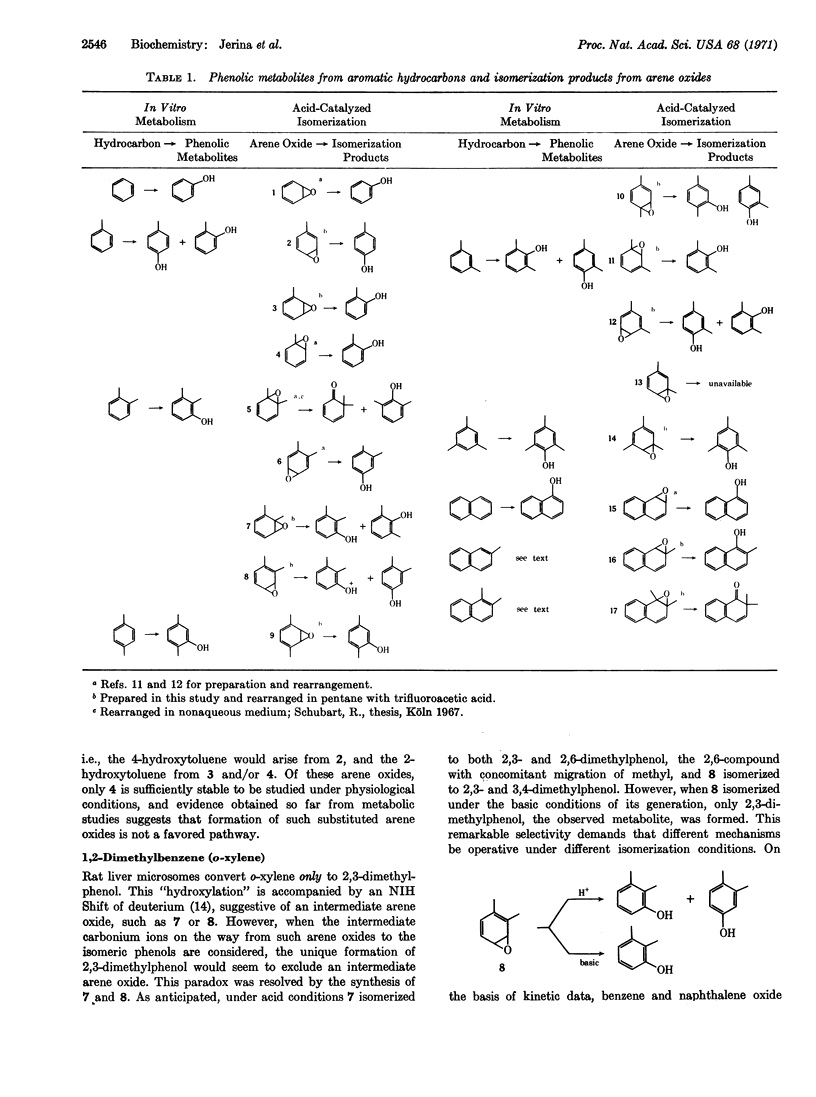
Phenolic products obtained on isomerization of the three arene oxides of toluene, the nine arene oxides of ortho-, meta-, and para-xylene, the arene oxide of mesitylene, and 2-methyl and 1,2-dimethylnaphthalene-1,2-oxides are compared to the phenolic products obtained by hepatic metabolism of the parent aromatic hydrocarbons. The results are compatible with the intermediacy of certain of these arene oxides in the metabolic pathway from hydrocarbons to phenols. Migrations of methyl groups as well as a remarkable apparent migration of oxygen are observed. The isomerization of 1,4-dimethylbenzene oxide to 2,4-dimethylphenol is analogous to the methyl migration observed in the enzymatic conversion of 4-methylphenylalanine to 3-methyltyrosine with phenylalanine hydroxylase. There are multiple pathways of isomerization of alkylarene oxides to phenols and they are determined by environmental and structural factors. The proportion and nature of products vary greatly with pH, which suggests the presence of different mechanisms for acid-catalyzed or spontaneous isomerizations of arene oxides.
Keywords: oxidation mechanisms, microsomal hydroxylations, multiple rearrangements, carcinogencsis
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodie B. B., Reid W. D., Cho A. K., Sipes G., Krishna G., Gillette J. R. Possible mechanism of liver necrosis caused by aromatic organic compounds. Proc Natl Acad Sci U S A. 1971 Jan;68(1):160–164. doi: 10.1073/pnas.68.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J., Guroff G. Production of m-methyltyrosine and p-hydroxymethylphenylalanine from p-methylphenylalanine by phenylalanine hydroxylase. Arch Biochem Biophys. 1968 Apr;125(1):136–141. doi: 10.1016/0003-9861(68)90647-4. [DOI] [PubMed] [Google Scholar]
- Daly J., Jerina D. Migration of deuterium during aryl hydroxylation. 3. Effect of ortho- and meta- substituents. Arch Biochem Biophys. 1969 Oct;134(1):266–268. doi: 10.1016/0003-9861(69)90280-x. [DOI] [PubMed] [Google Scholar]
- Daly J., Jerina D., Witkop B. Migration of deuterium during hydroxylation of aromatic substrates by liver microsomes. I. Influence of ring substitutents. Arch Biochem Biophys. 1968 Nov;128(2):517–527. doi: 10.1016/0003-9861(68)90059-3. [DOI] [PubMed] [Google Scholar]
- Grover P. L., Sims P., Huberman E., Marquardt H., Kuroki T., Heidelberger C. In vitro transformation of rodent cells by K-region derivatives of polycyclic hydrocarbons. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1098–1101. doi: 10.1073/pnas.68.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guroff G., Daly J. W., Jerina D. M., Renson J., Witkop B., Udenfriend S. Hydroxylation-induced migration: the NIH shift. Recent experiments reveal an unexpected and general result of enzymatic hydroxylation of aromatic compounds. Science. 1967 Sep 29;157(3796):1524–1530. doi: 10.1126/science.157.3796.1524. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Witkop B. The role of arene oxide-oxepin systems in the metabolism of aromatic substrates. II. Synthesis of 3,4-toluene-4-2H oxide and subsequent "NIH shift" to 4-hydroxytoluene-3-2H. J Am Chem Soc. 1968 Nov 6;90(23):6523–6525. doi: 10.1021/ja01025a057. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Witkop B., Zaltzman-Nirenberg P., Udenfriend S. 1,2-naphthalene oxide as an intermediate in the microsomal hydroxylation of naphthalene. Biochemistry. 1970 Jan 6;9(1):147–156. doi: 10.1021/bi00803a019. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Witkop B., Zaltzman-Nirenberg P., Udenfriend S. The role of arene oxide-oxepin systems in the metabolism of aromatic substrates. 3. Formation of 1,2-naphthalene oxide from naphthalene by liver microsomes. J Am Chem Soc. 1968 Nov 6;90(23):6525–6527. doi: 10.1021/ja01025a058. [DOI] [PubMed] [Google Scholar]
- Selkirk J. K., Huberman E., Heidelberger C. An epoxide is an intermediate in the microsomal metabolism of the chemical carcinogen, dibenz(a,h)anthracene. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1010–1016. doi: 10.1016/0006-291x(71)90562-6. [DOI] [PubMed] [Google Scholar]



