Abstract
Our study evaluated and compared the false-negative rates (FNR) of a wide array of fur-mite diagnostic tests, including 2 postmortem tests (pelt exam and sticky paper) and 3 antemortem tests (adhesive tape, fur pluck, and PCR). Past publications examining fur-mite diagnostic techniques primarily used paired comparisons, evaluating tests by their level of agreement with only one other test. However, different combinations or pairs of diagnostics are used in the different studies, making the results of these comparisons difficult to interpret across all available diagnostics. In the current study, mice from a conventionally maintained colony endemic for Myobia musculi were identified as positive based on at least one positive diagnostic test. From this pool of positive animals, the FNR of all tests were quantified. The PCR assay and the pelt exam performed the best, with 0% and 2% FNR respectively, whereas tape, fur-pluck, and sticky-paper tests showed 24%, 26%, and 36% FNR, respectively. Our study shows that for mice in a colony naturally infested with Myobia musculi, PCR testing can be used for reliable antemortem detection, and pelt exam performed by experienced examiners is reliable for postmortem detection.
Abbreviation: FNR, false-negative rate
Fur mites are a common pathogen in laboratory rodents, and infestations usually are caused by Myobia musculi, Myocoptes musculinis, or Radfordia affinis.1,2,12,13,16 Infestation can result in mite-associated ulcerative dermatitis,10,34 prolonged inflammatory responses,18 systemic immune effects,19-21,26 and decreased reproductive indices.34 Numerous treatment modalities have been reported,2-4,9,14,16,25,27,28,31,37 but their success at eradicating outbreaks varies. A survey of institutions conducted in 20085 revealed that 30% and 40% of respondents reported infestations of Myobia musculi and Myocoptes musculinis, respectively, whereas recent prevalence data suggests an overall incidence of 12% in North America.30 Many institutions with barrier facilities exclude fur mites,1,16,18,34 and policies to reject imports from facilities with endemic or sporadic infestations disrupts collaborations between investigators.18
Low prevalence in barrier housing coupled with poor diagnostic methods makes detection challenging.7 Despite the multiple testing methodologies available, successful identification of mite-infested mice remains problematic.4,32 Soiled-bedding sentinels, the current standard in colony health surveillance, has conflicting reports of reliability in the detection of fur-mite infestations.23,32 Low cage densities and incorrect sampling site strategies may further decrease detection.24
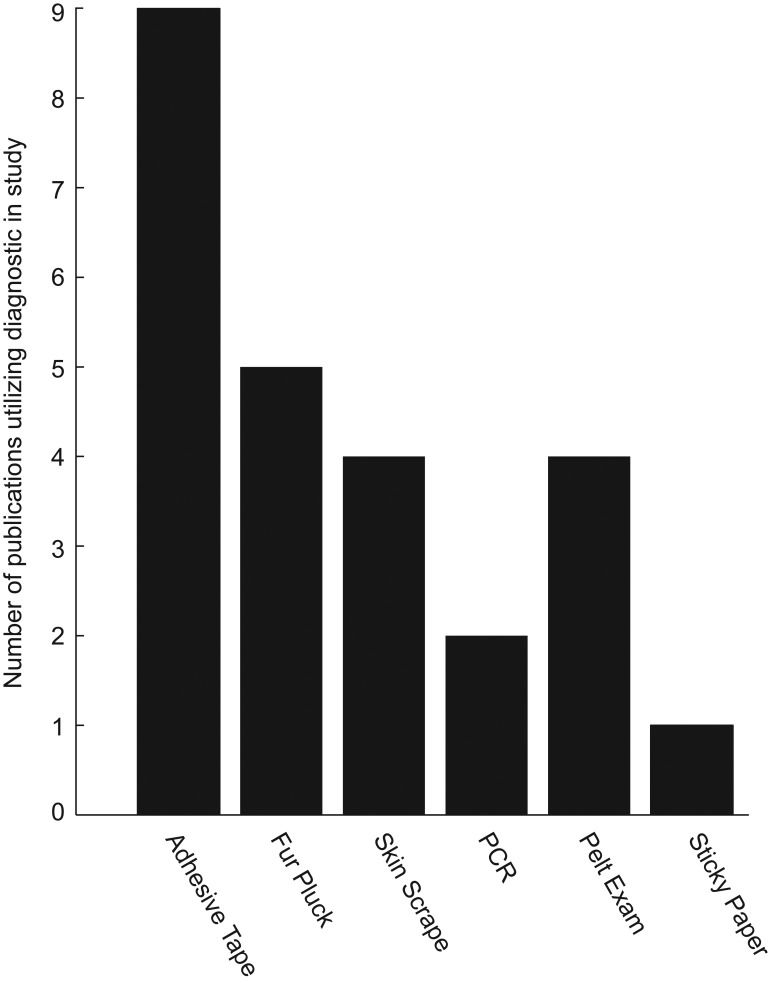
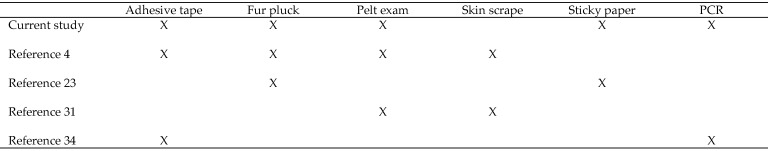
Active infestations in live mice are often diagnosed via transparent adhesive-tape tests,4,14,25 but they can also be identified by skin scrape, fur pluck, sticky paper, pelt examinations,2,12 and PCR tests.3,6,35 A survey of 16 studies3,4,9,14,17,18,23-25,29,31-36 involving the diagnosis and treatment of fur mites revealed that the transparent-tape test is the most commonly performed diagnostic test, followed by fur pluck (Figure 1). One group of authors4 reported the skin scrape test to be the most reliable method in their experience and gauged other tests by the level of agreement to the skin scrape. Their study4 concluded that skin scrape was the most reliable test, followed by pelt examination, transparent adhesive-tape test, fur pluck, and observation. Recent studies suggest direct or environmental sampling PCR can be a sensitive means for detection of mites17 and that PCR prior to treatment is sensitive for fur mites.35 Other studies24,35 compared results between 2 selected tests (fur pluck compared with sticky paper;24 transparent adhesive tape compared with PCR35), making it difficult to infer how the evaluated diagnostics compare with other methods not included in the study. When the 5 available comparative fur-mite studies are assessed, it becomes clear that there is little overlap in the diagnostic tests assessed (Figure 2). In the current study, we evaluated 4 commonly used diagnostic methods to enable a side-by-side comparison. Two of those tests were repeated on a second cohort of mice, which also were tested by PCR. This process allowed us to rank the 5 tests used in the study. A false-negative rate (FNR), defined by the occurrence of negative test results in subjects known to be infested, was calculated, instead of agreement between pairs of diagnostics. Here we evaluated the FNR of a wide array of diagnostics, including 2 postmortem and 3 antemortem tests, in the same study.
Figure 1.
Number of publications using various fur-mite diagnostic techniques in studies. Results were compiled by reviewing 16 studies evaluating fur-mite diagnosis or treatment, many of which used multiple diagnostic tests.
Figure 2.
Overview of comparative fur-mite diagnostic studies.
Materials and Methods
Mice.
Experimentally naïve male and female mice (n = 100; age, 16 wk to 15 mo) on a mixed BALB/c background from a colony naturally infected with Myobia spp. were donated for the current study. The conventionally maintained colony is endemic for mouse hepatitis virus, mouse norovirus, mouse parvovirus, minute virus of mice, polyoma virus, Aspicularis tetraptera, Myobia musculi, Entamoeba muris, and Syphacia obvelata. Mice were housed in groups of 3 to 5 per cage. The facility is tested semiannually by using soiled-bedding sentinels, one per rack side, and is negative for cilia-associated respiratory bacillus, ectromelia virus, Encephalitozoon cuniculi, epizootic diarrhea of infant mice virus, hantavirus, K virus, lymphocytic choriomeningitis virus, mouse cytomegalovirus, mouse adenovirus, mouse thymic virus, pneumonia virus of mice, reovirus type 3, Sendai virus, Theiler mouse encephalomyelitis virus, Mycoplasma spp. and Salmonella spp. Any mouse with pruritus or dermatitic lesions was excluded from the study.
Facilities.
The Division of Veterinary Resources at the NIH (Bethesda, MD) is an AAALAC-accredited animal care and use program. All animal care was in compliance with the Guide for the Care and Use of Laboratory Animals,15 and experimental procedures were in accordance with federal policies and guidelines governing the use of animals and approved by the Office of Research Services Animal Care and Use Committee. Mice were housed on hardwood and cedar shavings, with continuous access to rodent diet (NIH31, Zeigler Brothers, Gardner, PA) and acidified water (pH 2.7 to 3.0). Mice were housed in polycarbonate shoebox-type cages with open wire-bar lids, which were kept on wall-mounted static racks and changed once weekly. Environmental enrichment included shredded paper bedding (EnviroDri, Shepherd Specialty Papers, Richland, MI) and a weekly rotation of paper tubes and plastic enrichment devices. The housing room was maintained at 69 to 75 °F (20.6 to 23.9 °C), with an average humidity between 30% and 70% and on a 12:12-h light:dark cycle.
Experimental design.
Cohort 1.
To compare the sticky-paper test and postmortem pelt exam with 2 traditional antemortem fur-mite diagnostic tests, 50 mice were tested with transparent adhesive tape, and fur was plucked prior to euthanasia. We then performed euthanasia, pelt exam, and overnight incubation on sticky paper.
Cohort 2.
To compare the FNR of a fur-mite PCR test with the sticky-paper test and postmortem pelt exam, 50 mice were swabbed prior to euthanasia for the PCR assay. We then performed euthanasia, pelt exam, and overnight incubation on sticky paper.
The FNR is defined by the occurrence of negative test results in subjects known to have the disease or infestation. Mite-positive mice were defined as those with a positive result on at least one diagnostic test. A negative result from a mite-positive mouse counted as a false negative.
Diagnostic techniques used.
Fur-mite PCR assay.
Individually wrapped adhesive swabs provided by the commercial laboratory (Charles River Labs, Wilmington, MA) were used to sample the fur from around the ears, base of neck, under the chin, inguinal areas, and base of tail, as directed by the vendor.6 Swabs then were placed in sterile microcentrifuge tubes, and the tips cut off by using scissors. Samples were transported back to the commercial laboratory by using overnight delivery on ice packs.
Adhesive-tape technique.
A 4 × 2-cm piece of clear transparent adhesive tape (3M, St Paul, MN) was adhered to the head, neck, base of tail, and ventral abdomen of each mouse. The tape then was affixed to a glass slide and examined under a light microscope (model S or D Leitz Laborlux, Leica Microsystems, Buffalo Grove, IL) at 16× magnification for the presence of adult mites or mite eggs.
Fur-pluck technique.
Fur was plucked from the head caudal to the ears and eyes, the base of the tail, and ventral abdomen. Samples from these regions were pooled for each mouse and placed in 2 to 3 drops of mineral oil on a glass slide, covered by a glass coverslip, and examined under a light microscope (model S or D, Leitz Laborlux) at 16× magnification for the presence of adult mites or mite eggs.
Pelt exam technique.
Mice were euthanized by carbon dioxide asphyxiation and immediately placed under a dissecting light microscope (model 439168, Wild Heebrugg, Gais, Switzerland) for whole-body examination for the presence of mite eggs or adults. Swiss jeweler's forceps (Miltex, York, PA) were used to part the hair, and the entire pelt was examined beginning at the muzzle and ending at the base of the tail. The mice then were placed in dorsal recumbency for examination of the ventral abdomen and inguinal regions. Each pelt exam lasted approximately 2 min.
Sticky-paper technique.
After pelt examination, mice were placed in lateral recumbency on the center of adhesive covers for 96-well plates (Falcon, Franklin Lakes, NJ) that were trimmed to fit into deep culture dishes with lids (Thermo Scientific, Rochester, NY). After overnight incubation at room temperature (68 to 72 °F) under a biosafety class II cabinet, mice were removed from the sticky paper, and the paper was scored under a dissecting microscope (model 439168, Wild Heebrugg) for the presence or absence of adult mites or eggs.
Scoring method.
Two experienced readers (one with 3.5 y of experience, the other with 5 y of experience) read and recorded results for each sample independently. Readers were blinded to each other's results. When disagreement was identified, the slide, carcass, or sticky paper was reexamined and a definitive result (either positive or negative) agreed on. Positive results meant at least one mite egg or adult was observed. A negative result indicated no mite eggs or adults. Reader disagreement occurred only during the scoring of sticky-paper tests, resulting in repeat sample evaluation by both readers. In each case of disagreement, the sample was definitively scored as positive, meaning one reader initially recorded a false-negative result.
Statistics.
A χ2 test was used to assess whether at least 2 of the 5 tests had significantly different FNR. Then, we analyzed which pair or pairs of diagnostics had significantly different FNR by using the Tukey Honest Significant Difference multiple-comparisons test among proportions.38 A P value of less than 0.05 was used to define statistical significance. All analyses were done in Matlab (Mathworks, Natick, MA).
Results
In contrast with previously reported results,24 we found that of the 2 postmortem tests, pelt exams (FNR, 2%) were more sensitive in detecting Myobia musculi on mice than was the sticky-paper test (FNR, 36%). PCR had a 0% FNR, whereas 2 traditional antemortem tests, fur pluck and transparent adhesive-tape exams, had FNR of 24% and 26%, respectively. In the first cohort of 50 mice, only 2 (5%) of the pelt exams produced false-negative results, whereas 10 (26%) of the sticky-paper tests were falsely negative. Fourteen of 50 known-infested mice were negative across all 4 testing modalities.
In second cohort of 50 mice, we compared the PCR assay to the most effective test for the first cohort (pelt exam). The sticky-paper test was included to confirm the difference in detection efficacy of the 2 postmortem tests in the first cohort. As for the first cohort, mite-positive mice were identified as those with a positive result on at least one diagnostic test. This time, all 50 mice were mite-positive. The pelt exam yielded no false-negative results, and the sticky-paper test again performed worse (22 false-negative results, 44%) than did the pelt exam. The PCR assay obtained no false-negative results and was specific to the identified mite species, indicating the presence of Myobia spp. and not Myocoptes spp.
When the 2 cohorts were combined (100 mice), the sticky-paper test had the highest FNR. A χ2 test confirmed significantly (χ2 = 49.37, P < 10−9) different FNR between at least 2 of the 5 diagnostic tests we evaluated. Using a multiple-comparisons test among proportions, we found that both the PCR assay and pelt examination had significantly (P < 0.01) lower false-negative rates than did the other 3 tests (Table 1). We found no significant difference in sensitivity between the sticky-paper, fur-pluck, and tape tests.
Table 1.
Differences in FNR according to the multiple-comparisons test among proportions
| P | |
| PCR compared with pelt exam | not significant |
| PCR compared with fur pluck | < 0.001 |
| PCR compared with adhesive tape | < 0.001 |
| PCR compared with sticky paper | < 0.001 |
| Pelt exam compared with fur pluck | < 0.005 |
| Pelt exam compared with adhesive tape | < 0.01 |
| Pelt Exam compared with sticky paper | < 0.001 |
| Fur pluck compared with adhesive tape | not significant |
| Fur pluck compared with sticky paper | not significant |
| Adhesive tape compared with sticky paper | not significant |
Because different sets of diagnostics were combined in each cohort, differences in overall detection rates may have occurred. To ensure that diagnostic rankings derived from the pairwise FNR comparisons were not erroneous due to different testing criteria, we recalculated FNR by using the pelt exam as a ‘gold standard.’ By using this detection method, the criteria for the 2 cohorts was identical, and the PCR assay continued to have significantly lower FNR than did the sticky-paper, tape, and fur-pluck tests (P values for pairwise comparisons were less than 0.001).
Several mice that tested negative by sticky paper but positive by pelt exam were reexamined under dissecting microscope just after incubation and removal from the sticky paper. We found that Myobia and eggs were still present, with live mites clinging to the end of individual hairs. Readers noted that mites adhering to the sticky paper were most frequently found on the direct contact surface on which the mouse was laid. Very few mites jumped off the carcass and onto the sticky paper, thus resulting in a positive test.
Discussion
In our study, the PCR assay for fur-mite detection in mice had a 0% FNR, proving to be the most sensitive of all antemortem tests we used. The pelt exam had a 2% FNR, demonstrating the highest sensitivity of all postmortem tests examined. The traditional antemortem fur-mite diagnostic assays (transparent adhesive-tape and fur-pluck tests) had similar, lower sensitivities than that of pelt exam.
Our result differs from a recent study, which found that the FNR of PCR can be quite high when mice that have been treated for mites are tested.35 Indeed, in that study,35 the PCR test had a much lower FNR when applied to untreated animals. Six weeks after mice were treated for infestation, the FNR of PCR increased dramatically from 0% to 29%.35 Another potential reason for the apparent discrepancy between the current and previous results is that different commercial laboratories were used for PCR testing in the 2 studies. Although there is no published data to explain the difference in sensitivity, it is possible that the lower mite burden that resulted from treatment may have contributed to the false-negative results. Perhaps the discrepancy between commercial laboratories can be attributed to sampling locations, in which we sampled around the ears, base of neck, under chin, inguinal areas, and base of tail. By comparison, the laboratory used in the previous study35 recommends swabbing the head, base of tail, and inguinal area of each mouse. Another possibility is that the type of swab used for collection may affect false-negative results, and several types of swabs are commercially available. No data are currently available that addresses differences in performance between sticky adhesive, flocked, or polyester swabs or the effects of mite load on diagnostic sensitivity.
Differences in primer sets and amplification conditions8 have previously been proposed to explain differences in testing results between commercial laboratories, as well as human error, reagent failure, or equipment malfunction. The labs used in the previous35 and current studies both run a generic PCR test for the presence of mite DNA, followed by a secondary PCR test to confirm the exact species detected. However, the primer sets and amplification conditions used remain proprietary information.
The debate regarding whether to switch to PCR testing from traditional methods is not a new one.8,11 PCR can directly detect infectious agents, and its high sensitivity allows for the pooling of samples and perhaps cost savings. Another advantage is that PCR can be used for quarantined and on-study mice, where the survival of tested animals is necessary. Due to the decreased need for handling when taking the sample, PCR may be less stressful, compared with fur-pluck and adhesive-tape tests, with PCR showing increased gains in sensitivity prior to treatment for fur mites.35 A recent study demonstrated a high probability of detecting fur mites by using environmental sampling, exhaust plenums in particular.17 However, the PCR assay's sensitivity can also be a disadvantage in situations of cross contamination, resulting in false-positive results. Mice treated for infestation may no longer have active infestations, but the presence of materials such as empty egg casings or mite parts may persist, given that it takes longer than 6 mo for a mouse to shed its entire coat.32 One limitation of the current study is that we were unable to evaluate the rate of false-positive results from PCR tests.
Our study also shows that pelt exams are more sensitive in detecting Myobia than was previously reported for mixed Myobia and Myocoptes infestations.4 Myobia mites concentrate at the head and face, whereas Myocoptes mites spread out on the ventral abdomen and inguinal regions.22 Perhaps our pelt exams were more sensitive because of a single, rather than mixed infestation, given that mixed infestations increase the number of sites requiring examination, resulting in evaluator fatigue. One study4 reported high sensitivity by using pelt exams, in contrast to another report32 of low sensitivity, but neither study reported the amount of experience of the laboratory technician doing the pelt exam. Perhaps the pelt exam's sensitivity is linked to technician's experience. In our study, the 2 technicians had more than 8 y combined experience reading pelts. Our institute performs whole-body pelt exams without incubation, which is another difference from other studies in which pelts were removed and placed in dishes for incubation.32,33 Because Myobia tend to concentrate around the eyes and muzzle, removal of the pelt from the carcass may lower the sensitivity of the exam. It should be noted that the evaluators were aware that they were taking part in a fur-mite diagnostic study and of the health status of the mice they were examining, thus perhaps resulting in greater numbers of positive diagnoses across all traditional testing modalities.
Our experience with the sticky-paper test was dramatically different than that of other colleagues,24 who saw very high sensitivity in the diagnosis of Myocoptes postmortem. We suspect that differences in natural biology of the 2 mite species explain the difference in the detection of Myocoptes compared with Myobia species. Myobia musculi and Myocoptes musculinis complete their entire life cycles on or above the host's skin surface,22 but after the death of the host, Myobia spp. are not as active in their effort to leave carcasses as are Myocoptes spp.22 Myocoptes spp. are more sensitive to environmental conditions, leaving the skin surface 30 to 45 min after the death of the host.22 However, in the case of Myobia spp., large numbers of mites will remain in feeding positions and subsequently die there,22 as we observed in our study. More recent data attest to the difference in mobility between the 2 species, in which Myocoptes spp. effectively transferred from soiled bedding to result in active infestation, whereas Myobia spp. proved relatively inefficient.33 In our experience, the sticky-paper test involved high labor with no gains in sensitivity. One advantage over the transparent adhesive-tape test may be, due to carcass positioning, sticky paper's higher surface area sampling for the ventral abdomen, where Myocoptes spp. tend to concentrate. However, sticky paper did not perform significantly better than did the transparent-tape test in our study.
Another limitation of our study was that we did not evaluate the skin-scrape test, although it has been deemed reliable in past studies.4,32 Although skin scrapes are performed as often as are pelt exams (Figure 1), we were limited by the total number of sampling procedures performed on each study subject. Because fur mites tend to concentrate in specific anatomic regions, repeated sampling in the same location could result in false-negative results for the tests performed last in order, especially in cases of low mite burden. In our institute, the skin scrape is not commonly used for the detection of fur mites. Skin scraping tends to be labor-intensive and may cause trauma to mice, in the case of inexperienced sample collectors.
In our study, 14 of 100 mice tested negative across 4 traditional diagnostic methods. Negative results, however, did not prove that the mice were not infested with fur mites, and the possibility of those being false-negative results remains. False-negative results, regardless of diagnostic method, have potentially devastating consequences; releasing an animal from quarantine or accepting imports on the basis of false-negative results may lead to a facility outbreak of fur mites. Because no test is 100% sensitive or specific. it is important to perform confirmatory testing on positive results. In the case of PCR-based pinworm screening, multiple methods of diagnosis is recommended to increase diagnostic efficacy.11 Additional studies should be performed to assess the effect on sensitivity of pooled compared with individual samples, environmental compared with direct animal testing, various levels of mite burden, and mixed compared with single infestations.
According to our results, PCR testing for fur mites is highly sensitive in detecting active infestation with Myobia spp. Our analysis showed there was no statistical difference in the FNR of PCR and pelt exam, so facilities should continue to use pelt exams in health surveillance where Myobia infestations are suspected. The analysis also revealed that there was no significant difference between the FNR of fur-pluck, transparent-tape, and sticky-paper tests for detection of Myobia infestations. Given the results of a previous study,24 perhaps a combination of pelt exam and sticky-paper tests should be used in situations of mixed or unknown infestations. Due to its high sensitivity, PCR is useful for screening, but we recommend follow-up testing to confirm an active infestation, especially in treated mice. PCR assays are becoming an integral part of laboratory animal health surveillance. However, until more data become available regarding its sensitivity and specificity in various treatment and sampling situations, we recommend the use of multiple methods for accurate detection and confirmation of active fur-mite infestations of mice.
Acknowledgments
We thank the laboratory of Wendy DuBois for the donation of mite-infested mice; Sharon Miller, Katie Sadnavitch, Dariyen Carter, and Tamikia Lamb for technical assistance; and Mattias P Karlsson for statistical analysis.
This study was supported by the Division of Veterinary Resources, Office of Research Services, and NIH.
References
- 1.Baker DG. 2007. Arthropods p 565–579 In: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL. The mouse in biomedical research, 2nd ed. San Diego (CA): Academic Press [Google Scholar]
- 2.Baker DG. 2007. Parasites of rats and mice, p 359–363 In: Baker DG. Flynn's parasites of laboratory animals. Ames (IA): Blackwell Publishing [Google Scholar]
- 3.Bornstein DA, Scola J, Rath A, Warren HB. 2006. Multimodal approach to treatment for control of fur mites. J Am Assoc Lab Anim Sci 45:29–32 [PubMed] [Google Scholar]
- 4.Burdett EC, Heckmann RA, Ochoa R. 1997. Evaluation of 5 treatment regimens and 5 diagnostic methods for murine mites (Myocoptes musculinus and Myobia musculi). Contemp Top Lab Anim Sci 36:73–76 [PubMed] [Google Scholar]
- 5.Carty AJ. 2008. Opportunistic infections of mice and rats: Jacoby and Lindsey revisited. ILAR J 49:272–276 [DOI] [PubMed] [Google Scholar]
- 6.Charles River Laboratories. [Internet] 2011. Fur-mite PCR now available from Charles River. [Cited 18 October 2012]. Available at: http://www.criver.com/en-US/NewsEvents/WhatsNew/Pages/Fur_Mite_PCR.aspx
- 7.Clifford CB, Watson J. 2008. Old enemies still with us after all these years. ILAR J 49:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compton SR, Riley LK. 2001. Detection of infectious agents in laboratory rodents: traditional and molecular techniques. Comp Med 51:113–119 [PubMed] [Google Scholar]
- 9.Conole J, Wilkinson MJ, McKellar QA. 2003. Some observations on the pharmacological properties of ivermectin during treatment of a mite infestation in mice. Contemp Top Lab Anim Sci 42:42–45 [PubMed] [Google Scholar]
- 10.Dawson DV, Whitmore SP, Bresnahan JF. 1986. Genetic control of susceptibility to mite-associated ulcerative dermatitis. Lab Anim Sci 36:262–267 [PubMed] [Google Scholar]
- 11.Dole VS, Zaias J, Kyricopoulos-Cleasby DM, Banu LA, Waterman LL, Sanders K, Henderson KS. 2011. Comparison of traditional and PCR methods during screening for and confirmation of Aspiculuris tetraptera in a mouse facility. J Am Assoc Lab Anim Sci 50:904–909 [PMC free article] [PubMed] [Google Scholar]
- 12.Fox JG, Anderson LC, Loew FM, Quimby FW . 2002. Laboratory animal medicine, 2nd ed. San Diego (CA): Academic Press [Google Scholar]
- 13.Friedman S, Weisbroth SH. 1977. The parasitic ecology of the rodent mite, Myobia musculi. IV. Life cycle. Lab Anim Sci 27:34–37 [PubMed] [Google Scholar]
- 14.Huerkamp MJ, Zitzow LA, Webb S, Pullium JK. 2005. Cross-fostering in combination with ivermectin therapy: a method to eradicate murine fur mites. Contemp Top Lab Anim Sci 44:12–16 [PubMed] [Google Scholar]
- 15.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 16.Jacoby RO, Fox JG, Davisson M. 2002. Biology and diseases of mice, p 35–120 In: Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine, 2nd ed. San Diego (CA): Academic Press [Google Scholar]
- 17.Jensen ES, Allen KP, Henderson KS, Szabo A, Thulin JD. 2013. PCR testing of a ventilated caging system to detect murine fur mites. J Am Assoc Lab Anim Sci 52:28–33 [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston NA, Trammell RA, Ball-Kell S, Verhulst S, Toth LA. 2009. Assessment of immune activation in mice before and after eradication of mite infestation. J Am Assoc Lab Anim Sci 48:371–377 [PMC free article] [PubMed] [Google Scholar]
- 19.Jungmann P, Freitas A, Bandeira A, Nobrega A, Coutinho A, Marcos MA, Minoprio P. 1996. Murine acariasis. II. Immunological dysfunction and evidence for chronic activation of Th2 lymphocytes. Scand J Immunol 43:604–612 [DOI] [PubMed] [Google Scholar]
- 20.Jungmann P, Guenet JL, Cazenave PA, Coutinho A, Huerre M. 1996. Murine acariasis. I. Pathological and clinical evidence suggesting cutaneous allergy and wasting syndrome in BALB/c mouse. Res Immunol 147:27–38 [DOI] [PubMed] [Google Scholar]
- 21.Laltoo H, Van Zoost T, Kind LS. 1979. IgE antibody response to mite antigens in mite-infested mice. Immunol Commun 8:1–9 [DOI] [PubMed] [Google Scholar]
- 22.Letscher RM. 1970. Observations concerning the life cycle and biology of Myobia musculi (Schrank) and Myocoptes musculinis (Koch). [MS Thesis]. College Station (TX): Texas A&M University [Google Scholar]
- 23.Lindstrom KE, Carbone LG, Kellar DE, Mayorga MS, Wilkerson JD. 2011. Soiled bedding sentinels for the detection of fur mites in mice. J Am Assoc Lab Anim Sci 50:54–60 [PMC free article] [PubMed] [Google Scholar]
- 24.Metcalf Pate KA, Rice KA, Wrighten R, Watson J. 2011. Effect of sampling strategy on the detection of fur mites within a naturally infested colony of mice (Mus musculus). J Am Assoc Lab Anim Sci 50:337–343 [PMC free article] [PubMed] [Google Scholar]
- 25.Mook DM, Benjamin KA. 2008. Use of selamectin and moxidectin in the treatment of mouse fur mites. J Am Assoc Lab Anim Sci 47:20–24 [PMC free article] [PubMed] [Google Scholar]
- 26.Morita E, Kaneko S, Hiragun T, Shindo H, Tanaka T, Furukawa T, Nobukiyo A, Yamamoto S. 1999. Fur mites induce dermatitis associated with IgE hyperproduction in an inbred strain of mice, NC/Kuj. J Dermatol Sci 19:37–43 [DOI] [PubMed] [Google Scholar]
- 27.Papini R, Marconcini A. 1991. Treatment with ivermectin in drinking water against Myobia musculi and Myocoptes musculinus mange in naturally infected laboratory mice. Angew Parasitol 32: 11–13 [PubMed] [Google Scholar]
- 28.Pence BC, Demick DS, Richard BC, Buddingh F. 1991. The efficacy and safety of chlorpyrifos (Dursban) for control of Myobia musculi infestation in mice. Lab Anim Sci 41:139–142 [PubMed] [Google Scholar]
- 29.Pollicino P, Rossi L, Rambozzi L, Farca AM, Peano A. 2008. Oral administration of moxidectin for treatment of murine acariosis due to Radfordia affinis. Vet Parasitol 151:355–357 [DOI] [PubMed] [Google Scholar]
- 30.Pritchett-Corning KR, Cosentino J, Clifford CB. 2009. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43:165–173 [DOI] [PubMed] [Google Scholar]
- 31.Pullium JK, Brooks WJ, Langley AD, Huerkamp MJ. 2005. A single dose of topical moxidectin as an effective treatment for murine acariasis due to Myocoptes musculinus. Contemp Top Lab Anim Sci 44:26–28 [PubMed] [Google Scholar]
- 32.Ricart Arbona RJ, Lipman NS, Wolf FR. 2010. Treatment and eradication of murine fur mites. II. Diagnostic considerations. J Am Assoc Lab Anim Sci 49:583–587 [PMC free article] [PubMed] [Google Scholar]
- 33.Roble GS, Boteler W, Riedel E, Lipman NS. 2012. Total IgE as a serodiagnostic marker to aid murine fur-mite detection. J Am Assoc Lab Anim Sci 51:199–208 [PMC free article] [PubMed] [Google Scholar]
- 34.Weisbroth SH, Friedman S, Scher S. 1976. The parasitic ecology of the rodent mite, Myobia musculi. III. Lesions in certain host strains. Lab Anim Sci 26:725–735 [PubMed] [Google Scholar]
- 35.Weiss EE, Evans KD, Griffey SM. 2012. Comparison of a fur-mite PCR assay and the tape test for initial and posttreatment diagnosis during a natural infection. J Am Assoc Lab Anim Sci 51:574–578 [PMC free article] [PubMed] [Google Scholar]
- 36.Welter A, Mineo JR, de Oliveira Silva DA, Lourenco EV, Vieira Ferro EA, Roque-Barreira MC, Maria da Silva N. 2007. BALB/c mice resistant to Toxoplasma gondii infection proved to be highly susceptible when previously infected with Myocoptes musculinus fur mites. Int J Exp Pathol 88:325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wing SR, Courtney CH, Young MD. 1985. Effect of ivermectin on murine mites. J Am Vet Med Assoc 187:1191–1192 [PubMed] [Google Scholar]
- 38.Zar J. 1999. Biostatistical analysis. New Jersey (NJ): Prentice Hall [Google Scholar]