Abstract
Objective. The objective of this study was to evaluate the incidence of diabetes among patients with PsA and RA in the general population.
Methods. We conducted a cohort study using an electronic medical records database representative of the UK general population (1986–2010). We estimated hazard ratios (HRs) for incident diabetes in PsA, psoriasis and RA cohorts compared with age- and sex-matched comparison cohorts without the corresponding conditions, adjusting for BMI, smoking, alcohol use, co-morbidities and glucocorticoids at baseline.
Results. Cohorts included 4196 persons with PsA, 59 281 with psoriasis and 11 158 with RA, with mean follow-up times of 5.9, 5.8 and 5.5 years, respectively. Incidence rates for diabetes were 7.3, 6.4 and 6.3 cases per 1000 person-years among individuals with PsA, psoriasis and RA, respectively. Age- and sex-matched HRs for diabetes were 1.72 (95% CI 1.46, 2.02) in PsA, 1.39 (95% CI 1.32, 1.45) in psoriasis and 1.12 (95% CI 1.01, 1.25) in RA. After adjustment for BMI, smoking and alcohol, the HRs were attenuated substantially (1.43, 1.24 and 1.00, respectively). With further adjustment for baseline glucocorticoid use and co-morbidities, the HRs were 1.33 (1.09, 1.61) in PsA, 1.21 (1.15, 1.27) in psoriasis and 0.94 (0.84, 1.06) in RA.
Conclusion. This general population study suggests an increased incidence of diabetes in PsA and RA, which is substantially explained by obesity and lifestyle factors. These findings support the importance of managing such factors in PsA and RA patients.
Keywords: psoriatic arthritis, psoriasis, rheumatoid arthritis, diabetes
Introduction
PsA and RA are both chronic, progressive and destructive joint diseases that are associated with systemic inflammation and often lead to significant complications. Diabetes risk may be elevated due to inflammatory arthritis, although studies of diabetes in RA patients have had inconsistent results [1, 2] and diabetes risk, specifically in PsA, has not been previously reported. As psoriatic skin disease (PsO) is associated with diabetes, independent of obesity and other traditional diabetes risk factors [3, 4], diabetes risk in PsA may be elevated due to the systemic inflammatory burden from both skin and joint disease. Furthermore, the prevalence of obesity in PsA patients is greater than in PsO or RA patients, conferring diabetes risk [5]. Therefore, due to underlying systemic inflammation and obesity, we suspected diabetes risk in PsA may be greater than that seen in inflammatory skin disease or joint disease alone. To address these issues, we evaluated the risk of incident diabetes among persons with PsA and RA in a large UK cohort in which data on obesity and lifestyle variables have been collected. To demonstrate the previously shown association with PsO (as a positive control) [4, 6], we also analysed diabetes risk among individuals with PsO.
Methods
Data source
The Health Improvement Network (THIN) is a database of electronic medical records in which general practitioners (GPs) in the UK have systematically recorded data on approximately 7.3 million patients. Because >92% of the British population is registered with a GP [7, 8], THIN is a population-based cohort representative of the UK general population. THIN includes demographics, details from GP visits, specialist referrals and hospital admissions, results of laboratory tests and additional health information. The Read code classification is used to identify specific clinical diagnoses, and a drug dictionary based on the Multilex classification is used to code medications [9–11]. Health information is recorded with quality control procedures to maintain high data completion rates and accuracy. Thus THIN data represent routine medical practice in a population-based setting.
Study design and cohort definitions
We conducted a cohort analysis of incident diabetes among adults with incident PsA (PsA cohort) compared with age-, sex- and entry year-matched individuals without PsA (comparison cohort) using data from THIN. We conducted equivalent cohort analyses among individuals with incident PsO and RA. Participants were required to be continuously enrolled in the database for 12 months prior to inclusion in the cohort, and those with diabetes prior to study entry were excluded.
PsA and PsO were defined using the earliest diagnostic code for the respective condition using the Read classification [10,11], definitions previously found to have positive predictive values (PPVs) of 85% and 90%, respectively, in THIN [12,13]. Secondary definitions of PsA and PsO included at least two diagnostic codes for the respective conditions.
Our primary definition for RA required one RA diagnostic Read code followed by at least one prescription for a DMARD using THIN drug codes based on the Multilex classification [9]. This RA definition has been found to have a specificity of 96% (vs the 1987 ACR criteria) [14]. Secondary definitions of RA included (i) a single diagnostic record for RA and (ii) at least two visits with RA diagnoses [2].
For the comparison cohorts corresponding to each exposure cohort, we matched up to 10 individuals without the corresponding exposure condition to each exposed subject based on age, sex and year of study entry. Our study period was 1 January 1986 through 31 May 2010. Participants entered the cohort when all inclusion criteria were met or the matched date for subjects in the comparison cohorts. Participants were followed until they developed diabetes, died or the follow-up ended, whichever came first.
Diabetes mellitus outcome assessment
Our outcome of interest was development of diabetes requiring medication treatment. Diabetes was defined by a prescription for a medication used for the treatment of diabetes, including all insulin preparations and oral agents, as done previously [2].
Assessment of covariates
Data on demographics, BMI, smoking and alcohol use were collected from the database using the most recent value before study entry. We calculated the Charlson co-morbidity index using Read diagnostic codes and assessed glucocorticoid use during the 12 months prior to diagnosis of the respective exposure condition (RA, PsA or PsO), or the matched date for subjects in the comparison cohorts.
Statistical analysis
We compared the baseline characteristics between the exposure cohorts (i.e. PsA, PsO and RA cohorts) and corresponding comparison cohorts. We identified incident cases of diabetes during follow-up and calculated incidence rates (IRs) for diabetes. We estimated the cumulative incidence of diabetes in each cohort accounting for the competing risk of death [15]. Examination of log-log survival curves for each of the exposure variables (RA, PsA and PsO) in our model demonstrated that assumptions of proportional hazards were met. We employed Cox proportional hazard regression models to assess the multivariable-adjusted hazard ratios (HRs) after stratifying by matched clusters (age, sex and calendar year of study entry). We imputed missing values for BMI and smoking using a sequential regression method based on a set of covariates as predictors (IVEware for SAS, version 9.2; SAS Institute, Cary, NC, USA) [16]. To minimize random error, we imputed five datasets and then combined estimates from these datasets. The imputed datasets were used for all primary analyses. Our multivariable analyses were further adjusted for baseline assessments of BMI (in kg/m2, using the World Health Organization classification), smoking (non-smoker, current smoker and past smoker), use of oral and topical glucocorticoids, and Charlson comorbidity index. For all HRs, we calculated 95% CIs. All P-values were two-sided. This study was approved by the UK National Health Service Research Ethics Committee (Protocol 12-007) and was judged exempt from review by the Boston Medical Center Institutional Review Board (Protocol H-31390).
Results
Our primary analyses included 4196 individuals with PsA (50% female, mean age 49 years, mean BMI 27.6), 59 281 persons with PsO (51% female, mean age 49 years, mean BMI 26.7), 11 158 individuals with RA (68% female, mean age 58 years, mean BMI 26.8) and their matched comparison cohorts (Table 1). Relative to the comparison cohorts, the exposed cohorts had a higher baseline prevalence of overweight or obese status, past or current smoking, oral and topical glucocorticoids use and mean comorbidity indices. Patients with PsO and PsA had higher frequencies of current alcohol use than their respective comparison cohorts.
Table 1.
Baseline characteristics of the study populations
| PsA (n = 4196) | No PsA (n = 41 794) | P-value | PsO (n = 59 281) | No PsO (n = 581 248) | P-value | RA (n = 11 158) | No RA (n = 110 375) | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Age, mean (s.d.), years | 48.7 (13.9) | 48.7 (13.8) | 0.92 | 49.2 (17.1) | 48.9 (17) | <0.001 | 58.4 (14.2) | 58.2 (14.1) | 0.33 |
| Women, n (%) | 2104 (50.1) | 20 965 (50.2) | 0.98 | 30 330 (51.2) | 297 675 (51.2) | 0.82 | 7582 (68) | 75 093 (68) | 0.86 |
| BMI, mean (s.d.), kg/m2 | 27.6 (5.6) | 26.2 (5.1) | <0.001 | 26.7 (5.4) | 25.9 (5.0) | <0.001 | 26.8 (5.2) | 26.3 (5.0) | <0.001 |
| <18.5, n (%) | 33 (0.8) | 580 (1.4) | <0.001 | 893 (1.5) | 9115 (1.6) | <0.001 | 167 (1.5) | 1546 (1.4) | <0.001 |
| 18.5–24.9, n (%) | 1170 (27.9) | 13 698 (32.8) | 18 227 (30.7) | 185 857 (32) | 3522 (31.6) | 35 626 (32.3) | |||
| 25.0–29.9, n (%) | 1152 (27.5) | 10 580 (25.3) | 15 772 (26.6) | 140 238 (24.1) | 3238 (29.0) | 29 777 (27.0) | |||
| ≥30.0, n (%) | 948 (22.6) | 5653 (13.5) | 9859 (16.6) | 71 193 (12.2) | 2017 (18.1) | 15 919 (14.4) | |||
| Unknown, n (%) | 893 (21.3) | 11 283 (27) | 14 530 (24.5) | 174 845 (30.1) | 2214 (19.8%) | 27 507 (24.9) | |||
| Smoking, n (%) | |||||||||
| Never | 1967 (46.9) | 19 730 (47.2) | <0.001 | 23 828 (40.2) | 267 871 (46.1) | <0.001 | 4730 (42.4) | 54 696 (49.6) | <0.001 |
| Past | 807 (19.2) | 5811 (13.9) | 10 846 (18.3) | 78 779 (13.6) | 2351 (21.1) | 17 516 (15.9) | |||
| Current | 929 (22.1) | 9285 (22.2) | 16 770 (28.3) | 122 223 (21) | 2798 (25.1) | 20 521 (18.6) | |||
| Unknown | 493 (11.7) | 6968 (16.7) | 7837 (13.2) | 112 375 (19.3) | 1279 (11.5) | 17 642 (16) | |||
| Alcohol, n (%) | |||||||||
| Never | 521 (12.4) | 4670 (11.2) | <0.001 | 7041 (11.9) | 67 642 (11.6) | <0.001 | 1870 (16.8) | 15 363 (13.9) | <0.001 |
| Past | 49 (1.2) | 398 (1.0) | 726 (1.2) | 5376 (0.9) | 175 (1.6) | 1182 (1.1) | |||
| Current | 2756 (65.7) | 25 806 (61.7) | 37 147 (62.7) | 335 973 (57.8) | 6770 (60.7) | 66 374 (60.1) | |||
| Unknown | 870 (20.7) | 10 920 (26.1) | 14 367 (24.2) | 172 257 (29.6) | 2343 (21.0) | 27 456 (24.9) | |||
| Oral glucocorticoid use, n (%) | 343 (8.2) | 1096 (2.6) | <0.001 | 2525 (4.3) | 15 354 (2.6) | <0.001 | 2470 (22.1) | 3707 (3.4) | <0.001 |
| Topical glucocorticoid use, n (%) | 1475 (35.2) | 1808 (4.3) | <0.001 | 14 844 (25) | 23 956 (4.1) | <0.001 | 900 (8.1) | 5493 (5) | <0.001 |
| Charlson comorbidity index, mean (s.d.) | 0.13 (0.38) | 0.07 (0.34) | <0.001 | 0.1 (0.39) | 0.08 (0.37) | <0.001 | 0.21 (0.51) | 0.1 (0.41) | <0.001 |
All characteristics were assessed prior to study entry. Unknown refers to subjects with missing values for the variable indicated. P-values were calculated by t-test or Wilcoxon rank-sum test for continuous variables or chi-square test for indicator or categorical variables.
The cumulative incidence of diabetes in each cohort is depicted in Fig. 1. New diagnoses of diabetes occurred among 179 persons during 24 625 person-years (PY) in the PsA cohort, among 2202 persons during 345 507 PY in the PsO cohort and among 383 persons during 61 087 PY in the RA cohort, corresponding to incidence rates of 7.3, 6.4 and 6.3 cases per 1000 PY, respectively (Table 2). As shown in Fig. 1, the risk of diabetes was higher among PsA, PsO and RA subjects than their counterparts, respectively. Relative to the comparison cohorts, the age-, sex- and entry time-matched HRs for diabetes were 1.72 (95% CI 1.46, 2.02) for PsA, 1.39 (95% CI 1.32, 1.45) for PsO and 1.12 (95% CI 1.01, 1.25) for RA. After further adjustment for baseline BMI, smoking and alcohol use, these HRs were attenuated but remained significant in the PsA and PsO cohorts (HRs 1.43 and 1.24, respectively), but not in the RA cohort (HR 1.00). With further adjustment for baseline glucocorticoid use and co-morbidities, the HRs became 1.33 (95% CI 1.09, 1.61) in PsA, 1.21 (95% CI 1.15, 1.27) in PsO and 0.94 (95% CI 0.84, 1.06) in RA.
Fig. 1.
Cumulative incidence of diabetes in the PsA, psoriasis and RA cohorts and age-, sex- and cohort entry-matched comparison cohorts.
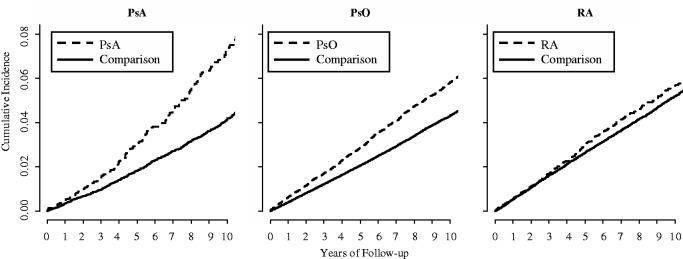 Estimates accounted for the competing risk of death. HRs corresponding to these figures were 1.72 (95% CI 1.46, 2.02) in PsA, 1.39 (95% CI 1.32, 1.45) in psoriasis and 1.12 (95% CI 1.01, 1.25) in RA. However, after adjustment for covariates, the HRs were attenuated to 1.33 (1.09, 1.61) in PsA, 1.21 (1.15, 1.27) in psoriasis and 0.94 (0.84, 1.06) in RA.
Estimates accounted for the competing risk of death. HRs corresponding to these figures were 1.72 (95% CI 1.46, 2.02) in PsA, 1.39 (95% CI 1.32, 1.45) in psoriasis and 1.12 (95% CI 1.01, 1.25) in RA. However, after adjustment for covariates, the HRs were attenuated to 1.33 (1.09, 1.61) in PsA, 1.21 (1.15, 1.27) in psoriasis and 0.94 (0.84, 1.06) in RA.
Table 2.
Incidence rates and HRs for diabetes among the PsA, PsO, RA and comparison cohorts
| PsA | No PsA | PsO | No PsO | RA | No RA | |
|---|---|---|---|---|---|---|
| Diabetes cases, n | 179 | 1029 | 2202 | 15 359 | 383 | 3477 |
| Total follow-up time, PY | 24 625 | 236 780 | 345 507 | 3 294 591 | 61 087 | 605 507 |
| Mean follow-up time, years | 5.9 | 5.7 | 5.8 | 5.7 | 5.5 | 5.5 |
| Incidence rate, per 1000 PY | 7.3 | 4.3 | 6.4 | 4.7 | 6.3 | 5.7 |
| Age-, sex- and entry time-adjusted HR (95% CI) | 1.72 (1.46, 2.02) | 1.00 (referent) | 1.39 (1.32, 1.45) | 1.00 (referent) | 1.12 (1.01, 1.25) | 1.00 (referent) |
| + BMI-, smoking- and alcohol-adjusted HR (95% CI) | 1.43 (1.2, 1.7) | 1.00 (referent) | 1.24 (1.18, 1.31) | 1.00 (referent) | 1.00 (0.89, 1.12) | 1.00 (referent) |
| + Oral and topical glucocorticoids and Charlson index-adjusted HR (95% CI) | 1.33 (1.09, 1.61) | 1.00 (referent) | 1.21 (1.15, 1.27) | 1.00 (referent) | 0.94 (0.84, 1.06) | 1.00 (referent) |
Sensitivity analyses did not materially change the results, including (i) the use of two Read code definitions for PsA (multivariable HR 1.50, 95% CI 1.10, 2.06), PsO (multivariable HR 1.27, 95% CI 1.17, 1.39) and RA (multivariable HR 0.97, 95% CI 0.85, 1.11) (see supplementary Table S1, available at Rheumatology Online); (ii) a single Read code definition for RA (multivariable HR 1.02, 95% CI 0.93, 1.11) (supplementary Table S1, available at Rheumatology Online); (iii) restriction to only those subjects without missing values for covariates (supplementary Table S2, available at Rheumatology Online); (iv) exclusion of subjects who were ever prescribed oral steroids (supplementary Table S3, available at Rheumatology Online); (v) exclusion of subjects ever prescribed oral or topical steroids (supplementary Table S4, available at Rheumatology Online) and (vi) restriction of RA cases to those after 1995, to evaluate diabetes risk in a more recent context (multivariable HR 0.96, 95% CI 0.84, 1.00; data not shown).
Discussion
In this large general practice cohort representative of the UK population, we found that diabetes risk was 72% higher among individuals with PsA than age- and sex-matched individuals without PsA. When we took baseline BMI, smoking, alcohol use and other covariates into account, the association was attenuated, but remained significant (33% increase over the comparison cohort). In contrast, the overall risk of diabetes among individuals with RA was only 12% higher than age- and sex-matched individuals without RA, and this increased risk was nullified after adjustment for lifestyle risk factors. This finding was replicated using alternate definitions of RA and other sensitivity analyses. Taken together, our findings provide the first general population evidence of increased diabetes risk specifically in PsA and the lack of an increased risk in RA after adjustment for obesity and lifestyle factors.
These findings suggest that obesity and lifestyle factors partially explain the increased risk of diabetes in PsA and PsO patients. Indeed, in the general population up to 91% of type 2 diabetes is attributable to obesity, smoking and alcohol [17]. However, these three factors have also been associated with the risk of incident PsO, and obesity has been associated with the risk of PsA, which leads to the potential for confounding and the need to adjust for such factors in estimating diabetes risk [18]. These risk estimates were similar to those from a large prospective cohort study (Nurses’ Health Study) in which the risk of diabetes among individuals with PsO was attenuated after adjustment for obesity and lifestyle factors (from 2.08 to 1.63) [4].
Similar confounding likely exists in assessing the impact of RA on diabetes risk, because development of RA is more common among smokers and obese individuals [19–21]. Although our unadjusted HR (1.12) of diabetes in RA patients was directionally consistent with the previous Canadian study (crude HR 1.5) [2], risk in our cohort disappeared after adjustment for obesity and lifestyle factors (HR 1.01), whereas the increased risk in the Canadian study did not change after adjustment for available covariates in the insurance claim database (BMI and lifestyle variables unavailable). The HR for diabetes for RA patients in our study was lower than in the Canadian study due to the lower diabetes incidence rate in our RA population (6.3 vs 8.6 cases per 1000 PY), not due to different incidence rates in the comparison populations (5.7 vs 5.8 cases per 1000 PY). Further supporting the absence of any increased risk, the Olmsted County RA cohort reported a crude diabetes incidence rate ratio of 0.78 [1]. Finally, our consistent results from multiple sensitivity analyses provide no support for an independent impact of RA on the risk of diabetes.
The differential risks of diabetes in RA and PsA, despite both being progressive arthritic conditions associated with systemic inflammation, may indicate different mechanisms for the development of diabetes. Although obesity is common in both populations, PsA patients tend to have higher BMIs than RA patients (29.6 vs 27.9), and differences in body composition (central obesity or fat mass) may potentially be associated with differential effects of adipocytokines [5, 22, 23]. Notably, systemic inflammation has also been associated with insulin resistance and diabetes, suggesting an inflammatory basis for diabetes [24]. The arthritic component of PsA may further add to the inflammatory mechanisms due to PsO. However, it remains unclear which, if any, inflammatory factors specific to psoriatic conditions underlie the increased diabetes risk. Therefore, in addition to obesity-associated metabolic factors, immune-mediated inflammatory processes specific to psoriatic conditions could provide the potential links between PsA and diabetes.
The strengths and limitations of our study deserve comment. This study was performed using a large UK general practice database and therefore findings are likely to be generalizable to the general population. Our database is derived from the electronic medical records used for patient care, thus the overall diagnostic accuracy is expected to be high [13, 14, 25], but remains a potential concern. For example, the PPV estimate for PsA based on a relatively small sample size was 85% [12]. However, any non-differential misclassification would have biased the study results toward the null, and our results persisted in sensitivity analyses that used alternate definitions of PsA, PsO and RA. We attempted to define populations with incident PsA, PsO and RA. However, it is possible that some subjects did not truly have incident disease, in which case our diabetes risk estimate would reflect diabetes risk among those with new-onset and long-standing PsA, PsO or RA. While there were missing values for covariates in some subjects, it was reassuring that our primary results with imputation closely agreed with those restricted to only those subjects without missing values. Finally, as our study assessed the main links between PsA, PsO, RA and the risk of diabetes, future studies may identify more specific risk factors for diabetes related to these rheumatic conditions.
In conclusion, this large general population study suggests that the overall risk of diabetes is increased in PsA, which is partially explained by obesity and lifestyle factors. Nevertheless, PsA (and PsO) remained associated with the risk of diabetes even after taking these factors into account. In contrast, we found that the risk of diabetes among patients with RA was entirely due to obesity and smoking. Overall, these findings suggest that diabetes risk should not be ascribed only to the presence of inflammatory disease. Future diabetes prevention efforts may be directed at weight loss and smoking cessation, in addition to treatment of the underlying disease, in PsA and PsO patients.
Rheumatology key messages.
Diabetes risk in RA patients was elevated due to known risk factors.
Diabetes risk in PsA was due to known risk factors and the underlying disease.
Supplementary Material
Acknowledgements
Thanks to Christine Peloquin for statistical assistance.
Funding: This work was supported in part by grants from the NIAMS (P60AR047785), National Heart, Blood and Lung Institute (R01 HL089744 to J.M.G.), NIH (K24-AR064310) and Boston University School of Medicine. The funding sources had no role in the design, conduct or reporting of the study or in the decision to submit the manuscript for publication.
Disclosure statement: J.G. serves as a consultant to Amgen, Abbott, Centocor, Celgene, Novartis, Merck and Pfizer and has received honoraria. J.G. has received grants from Amgen, Abbott, Pfizer, Novartis and Genentech. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Gonzalez A, Maradit Kremers H, Crowson CS, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 2008;67:64–9. doi: 10.1136/ard.2006.059980. [DOI] [PubMed] [Google Scholar]
- 2.Solomon DH, Love TJ, Canning C, et al. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69:2114–7. doi: 10.1136/ard.2009.125476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azfar RS, Seminara NM, Shin DB, et al. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. 2012;148:995–1000. doi: 10.1001/archdermatol.2012.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379–82. doi: 10.1001/archdermatol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhole VM, Choi H, Burns L, et al. Differences in body mass index among individuals with PsA, psoriasis, RA and the general population. Rheumatology. 2012;51:522–6. doi: 10.1093/rheumatology/ker349. [DOI] [PubMed] [Google Scholar]
- 6.Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 7.Attribution Dataset GP Registered Populations Scaled to ONS Population Estimates, 2011. 2012. http://www.ic.nhs.uk/statistics-and-data-collections/population-and-geography/gp-registered-populations/attribution-dataset-gp-registered-populations-scaled-to-ons-population-estimates-2011 (20 November 2012, date last accessed)
- 8.Population Estimates for England and Wales, Mid-2011 (2011 Census-based) 2012. http://www.ons.gov.uk/ons/rel/pop-estimate/population-estimates-for-england-and-wales/mid-2011–2011-census-based-/index.html (16 November 2012, date last accessed)
- 9.Multilex Drug Database. http://www.fdbhealth.com/multilex-overview/ (29 April 2012, date last accessed)
- 10.Chishom J. The Read clinical classification. BMJ. 1990;300:1092. doi: 10.1136/bmj.300.6732.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuart-Buttle CD, Read JD, Sanderson HF, et al. A language of health in action: Read codes, classifications and groupings. Proc AMIA Annu Fall Symp. 1996:75–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the United Kingdom. Rheumatology. 2013;52:568–75. doi: 10.1093/rheumatology/kes324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seminara NM, Abuabara K, Shin DB, et al. Validity of the Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol. 2011;164:602–9. doi: 10.1111/j.1365-2133.2010.10134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas SL, Edwards CJ, Smeeth L, et al. How accurate are diagnoses for rheumatoid arthritis and juvenile idiopathic arthritis in the general practice research database? Arthritis Rheum. 2008;59:1314–21. doi: 10.1002/art.24015. [DOI] [PubMed] [Google Scholar]
- 15.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;30:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.Raghunathan TE, Lepkowski JM, Van Hoewyk J, et al. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85–95. [Google Scholar]
- 17.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 18.Love TJ, Zhu Y, Zhang Y, et al. Obesity and the risk of psoriatic arthritis: a population-based study. Ann Rheum Dis. 2012;71:1273–7. doi: 10.1136/annrheumdis-2012-201299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowson CS, Matteson EL, Davis JM, III, et al. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Care Res. 2013;65:71–7. doi: 10.1002/acr.21660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen M, Jacobsen S, Klarlund M, et al. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res Ther. 2006;8:R133. doi: 10.1186/ar2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Symmons DP, Bankhead CR, Harrison BJ, et al. Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis: results from a primary care-based incident case-control study in Norfolk, England. Arthritis Rheum. 1997;40:1955–61. doi: 10.1002/art.1780401106. [DOI] [PubMed] [Google Scholar]
- 22.Eder L, Jayakar J, Pollock R, et al. Serum adipokines in patients with psoriatic arthritis and psoriasis alone and their correlation with disease activity. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202325. Advance Access published 14 December 2012. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y, Shin H, Vassy JL, et al. Comparison of regional body composition and its relation with cardiometabolic risk between BMI-matched young and old subjects. Atherosclerosis. 2012;224:258–65. doi: 10.1016/j.atherosclerosis.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Svenson KL, Pollare T, Lithell H, et al. Impaired glucose handling in active rheumatoid arthritis: relationship to peripheral insulin resistance. Metabolism. 1988;37:125–30. doi: 10.1016/s0026-0495(98)90005-1. [DOI] [PubMed] [Google Scholar]
- 25.de Burgos-Lunar C, Salinero-Fort MA, Cárdenas-Valladolid J, et al. Validation of diabetes mellitus and hypertension diagnosis in computerized medical records in primary health care. BMC Med Res Methodol. 2011;28:146. doi: 10.1186/1471-2288-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


