Abstract
Aims: Peroxiredoxin 6 (Prdx6), a 1-cys Prdx has both peroxidase and phospholipase A2 activities, protecting against oxidative stress and regulating pulmonary surfactant phospholipid metabolism. This study determined the mechanism by which keratinocyte growth factor (KGF) and the glucocorticoid analogue, dexamethasone (Dex), induce increased Prdx6 expression. Results: Transcriptional activation by KGF in both A549 lung adenocarcinoma cells and rat lung alveolar epithelial type II (ATII) cells utilizes an antioxidant response element (ARE), located between 357 and 349 nucleotides before the PRDX6 translational start, that is also necessary for upregulation of the human PRDX6 promoter in response to oxidative stress. Activation is mediated by binding of the transcription factor, Nrf2, to the ARE as shown by experiments using siRNA against Nrf2 and by transfecting ATII cells isolated from lungs of Nrf2 null mice. KGF triggers the migration of Nrf2 from cytoplasm to nucleus where it binds to the PRDX6 promoter as shown by chromatin immunoprecipitation assays. Activation of transcription by Dex occurs through a glucocorticoid response element located about 750 nucleotides upstream of the PRDX6 translational start. Innovation: This study demonstrates that KGF can activate an ARE in a promoter without reactive oxygen species involvement and that KGF and Dex can synergistically activate the PRDX6 promoter and protect cells from oxidative stress. Conclusion: These two different activators work through different DNA elements. Their combined effect on transcription of the reporter gene is synergistic; however, at the protein level, the combined effect is additive and protects cells from oxidative damage. Antioxid. Redox Signal. 20, 391–402.
Introduction
Peroxiredoxins (Prdxs) are nonseleno-peroxidases that catalyze the reduction of a broad spectrum of peroxides using the thiol groups of their cysteines (Cys) as catalytic centers (37). Prdx 1–5 contain two conserved catalytic Cys and utilize thioredoxin as a reductant. In contrast, Prdx6 contains a single conserved cysteine (18) and utilizes glutathione (GSH) to catalyze the reduction of H2O2 and other organic peroxides including phospholipid hydroperoxides (PLOOH) (12). Although Prdx6 is not a lung-specific protein, lungs exhibit high expression levels compared to other organs (21, 30). Overexpression of Prdx6 in a cell line inhibited membrane phospholipid peroxidation and apoptosis caused by Cu2+/ascorbate treatment (27), while overexpression in transgenic mice increased resistance to oxygen toxicity in the lungs of intact animals (47) and to peroxide stress in isolated alveolar type II (ATII) pneumocytes (44). Conversely, antisense treatment of L2 cells (32) or PRDX6 gene inactivation in mice resulted in decreased resistance to oxidative stress with increased lipid peroxidation in lungs (43, 45, 46) and ATII cells (44) from the Prdx6 null animals.
Innovation.
Peroxiredoxin 6 (Prdx6) is an inducible glutathione peroxidase protecting cells against oxidative stress. This study is the first to elucidate the mechanism of PRDX6 transcriptional induction by keratinocyte growth factor (KGF) and dexamethasone (Dex). Surprisingly, induction by KGF involves the Nrf2 transcription factor binding to an antioxidant response element as previously shown for induction by oxidants. Dex induction requires a previously unknown variant of the glucocorticoid response element. The combination of KGF and Dex results in a synergistic effect on PRDX6 transcription and on protection of cells against oxidative stress. These findings will facilitate modulation of Prdx6 expression, increase antioxidant defenses, and could help protect patients from damage due to oxygen toxicity.
Prdx6 also possesses a phospholipase A2 activity that uses a serine-histidine-aspartate catalytic triad (5) This activity of Prdx6 and its location in the lysosome/lamellar body (in addition to the cytoplasm) suggest that Prdx6 also functions in the regulation of lung phospholipid metabolism. Prdx6 null mice have decreased phospholipase A2 activity, decreased phosphatidylcholine synthesis by the remodeling (reacylation) pathway, and increased phospholipid accumulation in their lungs (11), while overexpressor mice exhibit the inverse effects (13).
We have previously reported that Prdx6 can be induced by H2O2 or paraquat in the L2 cell line, and by hyperoxia in rat lungs (19) and that oxidant stress activates the human PRDX6 promoter through the Nrf2 transcription factor binding to a functional upstream antioxidant response element (6), an ARE (34), required for the transcriptional response to oxidant stress (6); also called an electrophile-response element (51). Nrf2 has previously been shown to bind to AREs in other genes and to be involved in their transcriptional regulation (31).
Keratinocyte growth factor (KGF), also known as fibroblast growth factor 7, is an epithelial-specific growth factor that protects cells of the alveoli from various forms of oxidative stress (2, 33, 35, 39, 50). KGF stimulates ATII cell proliferation (10), increases alveolar epithelial fluid transport (28), decreases apoptosis (1, 26), promotes DNA repair (40), and reduces intracellular reactive oxygen species (ROS) generation in response to ultraviolet B radiation (22). KGF treatment increases Nrf2 mRNA levels 2–3-fold in the HaCaT keratinocyte cell line within 3 h after its addition, suggesting transcriptional regulation (4). KGF had previously been demonstrated to induce Prdx6 in keratinocytes (14) and in a mouse liver cell line upon serum starvation (15), but the molecular mechanism is unclear.
Induction of Prdx6 has also been shown to take place in development. In rats, there was a three-fold increase in mRNA and protein levels after birth (20). Induction of Prdx6 by dexamethasone (Dex) treatment of primary ATII cells from rat lungs and the rat L2 lung epithelial cell line suggested that glucocorticoids are involved in the developmental induction of Prdx6 (20).
The goals of the present study were to identify and contrast the mechanisms for activation of the human PRDX6 promoter by KGF and Dex. In addition, if, as expected, the mechanisms of activation of the human PRDX6 promoter by KGF and by Dex were found to be different, the possibility of obtaining a synergistic increase in Prdx6 expression through treatment with both agonists would be tested and such combined treatment could be more protective of cells than treatment with just one of the agonists alone.
Results
Promoter activity of PRDX6 is increased by KGF in a dose-dependent manner
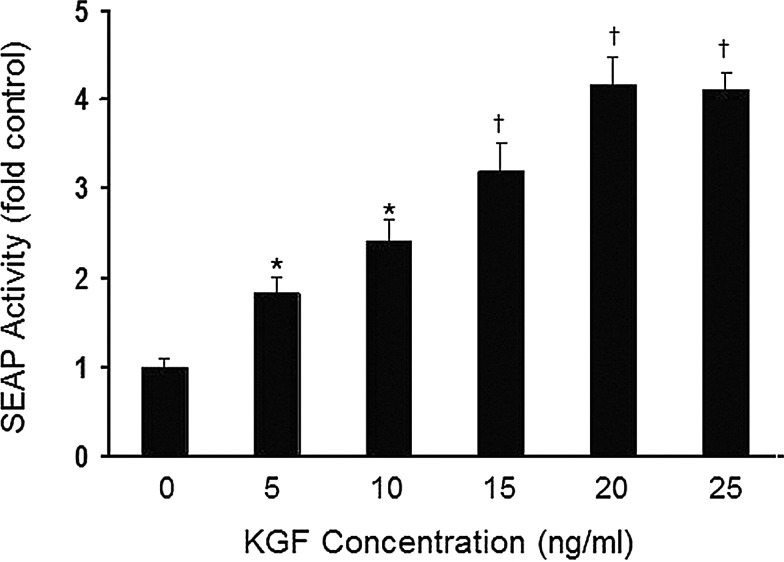
Reporter gene activity promoted by the 1.5 kb region of the human PRDX6 gene directly upstream from the translational start was evaluated 24 h after exposure to various concentrations of KGF in transiently transfected A549 cells. The A549 cell line, derived from a malignancy originating from human bronchoalveolar epithelial cells (25), has been shown to express all six known mammalian Prdxs (24). Activity increased in a dose-dependent manner with increasing amounts of KGF, reaching a plateau at 20 ng/ml of KGF treatment (Fig. 1). This concentration was used for subsequent experiments.
FIG. 1.
PRDX6 promoter activity in A549 cells responds to keratinocyte growth factor (KGF) in a dose-dependent manner. A549 cells, transiently transfected with pSEAP2 reporter plasmid containing the 1.5 kb peroxiredoxin 6 (PRDX6) upstream region, were cultured for 24 h, then treated with a range of KGF concentrations (5 ng/ml-25 ng/ml). Secreted alkaline phosphatase (SEAP) activity was determined 24 h later. The values shown are the mean±SE (n=3). *p<0.05, †p<0.01, compared with the control (untreated) cells.
KGF stimulation of the PRDX6 promoter requires the −410 to −338 promoter region
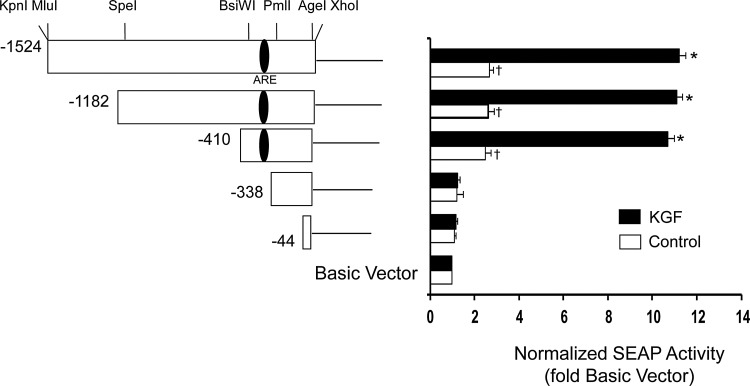
The full-length (1.5 kb) promoter construct pSEAP2-wt and a series of PRDX6 promoter deletions were transiently transfected into A549 cells and secreted alkaline phosphatase (SEAP) activity was determined in the presence and absence of KGF (Fig. 2). The full-length construct produced a two-fold increase in basal SEAP activity compared with the pSEAP2-Basic construct; this was further increased by almost four-fold in response to KGF treatment. The constructs containing 1182 bp and 410 bp before the start of translation (−1182 and −410) exhibited the same ability to direct transcription as the full-length construct in the presence and absence of KGF. However, SEAP activity of cells transfected with the −338 construct was the same as that detected in cells transfected with the pSEAP2-Basic vector control and did not respond to KGF, indicating that the region from −410 to −338 contains regulatory elements controlling KGF-induced expression of Prdx6 in A549 cells, and basal expression (6).
FIG. 2.
Basal and KGF-inducible activity of PRDX6 promoter deletions. Left panel, a series of constructs were generated by restriction enzyme digestion of the 1.5 kb DNA fragment derived from the 5′-flanking region of PRDX6 in the promoterless pSEAP2-Basic vector. Numbers marking the 5′ ends of the deletions are relative to the translational start. The approximate location of the putative antioxidant response element (ARE) is shown within the promoter. Right panel, A549 cells were transiently transfected with reporter vectors and then incubated for 24 h, followed by 20 ng/ml of KGF treatment (or no treatment in control samples). SEAP activity was determined 24 h later. Mean±SE (n=3) are shown. *p<0.01 compared with vehicle control, †p<0.05 compared with pSEAP2-basic vector (no insert).
Role of the ARE
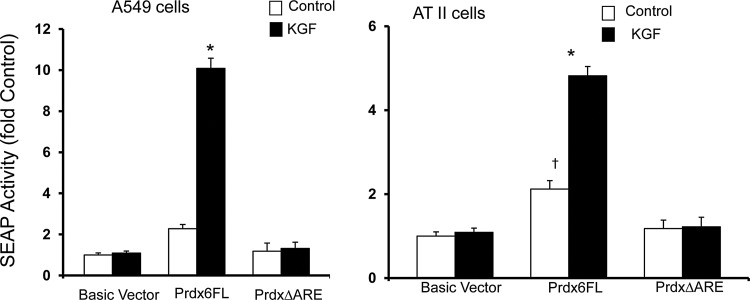
The results with the deletion constructs focused attention on the sequence between −410 and −338. This region contained the ARE consensus sequence (−357 to −349) that we identified as being required for induction of transcription by oxidative stress (6). To examine whether this ARE was also involved in KGF-mediated activation, a 1.5 kb promoter/reporter gene construct in which the ARE had been deleted by site-directed mutagenesis (ΔARE) (6) was transfected into A549 cells and rat ATII cells (Fig. 3) and compared with a wild-type construct. The ΔARE-transfected cells showed markedly diminished basal and KGF-mediated SEAP activity, confirming that the ARE is indispensable for both basal PRDX6 promoter activity, as shown previously (6), and activation by KGF in A549 cells (Fig. 3, left panel) and in rat ATII cells (Fig. 3, right panel). The results in both cell types were similar, indicating that A549 cells are a reasonable model for ATII cells in these PRDX6 promoter studies.
FIG. 3.
Role of the ARE in PRDX6 gene expression. Deletion of the ARE was generated by site-directed mutagenesis of the full-length PRDX6 promoter in the pSEAP2-Basic vector. Cells were transiently transfected with reporter vectors; after 24 h, cells were incubated with 20 ng/ml of KGF. SEAP activity was determined 24 h after KGF treatment. Cells were then harvested, and reporter gene assays performed. Prdx6FL, full-length PRDX6 promoter (1.5 kb); Prdx6ΔARE, full-length PRDX6 promoter with ARE deletion. Transfection and treatment of A549 cells (left panel) or rat primary alveolar type II (ATII) cells (right panel) with 20 ng/ml KGF for 24 h. Values shown are mean±SE (n=3) with untreated pSEAP2-basic vector set to 1. †p<0.05 *p<0.01 compared with untreated control.
Nrf2 knockdown prevents KGF induction of Prdx6 expression in A549 cells
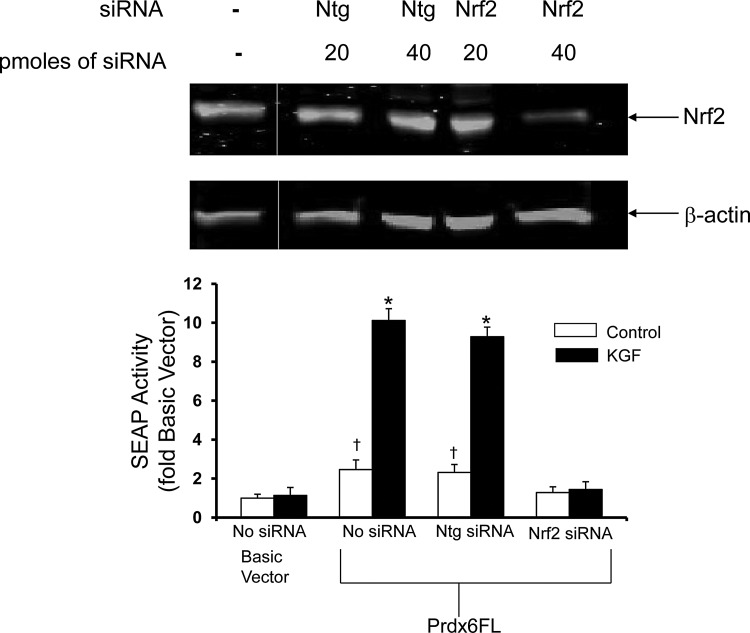
If the Nrf2 transcription factor, which binds to the PRDX6 ARE and activates transcription in response to oxidative stress (6), also regulates the KGF-mediated increase of transcription, then reducing Nrf2 levels would block the effect of KGF. The effect of Nrf2 siRNA on Nrf2 expression was evaluated on a western blot, 72 h after transfection (Fig. 4, upper panel). Treatment with 20 pmol of siRNA had no significant effect: however, 40 pmol of siRNA decreased the level of Nrf2, normalized to β-actin, by 92% compared with non-targeting (Ntg) siRNA and also resulted in an ∼10-fold reduction in SEAP activity from the PRDX6 promoter in cells treated with KGF (Fig. 4, lower panel). A lesser but significant reduction by Nrf2 siRNA on basal levels of reporter gene expression was seen in untreated control cells when compared with cells transfected with nontargeting siRNA (Fig. 4, lower panel).
FIG. 4.
siRNA knockdown of Nrf2 in A549 cells blocks induction of Prdx6 expression by KGF. Upper panel, targeted siRNA knockdown reduces Nrf2 in A549 cells. A549 cells were transfected with 20 or 40 pmoles of non-targeting siRNA (Ntg) or siRNA directed against Nrf2 (Nrf2) and cultured for 72 h. β-actin was used as a loading control. The gel shown was edited to excise irrelevant lanes. The vertical gray line marks the location of the excised portion. Lower panel, A549 cells were transfected with the pSEAP2-Basic plasmid (Basic vector) or the same plasmid with the full-length PRDX6 promoter (Prdx6FL) and, where indicated, siRNA; Ntg as a negative control or siRNA targeted against Nrf2. Assays were performed 24 h after treatment. Control; cells not treated with KGF. KGF; cells treated with 20 ng/ml KGF. Values shown are mean±SE (n=3) with untreated pSEAP2-Basic vector set to 1. *p<0.05 compared with vehicle control, †p<0.05 compared with pSEAP2-Basic vector.
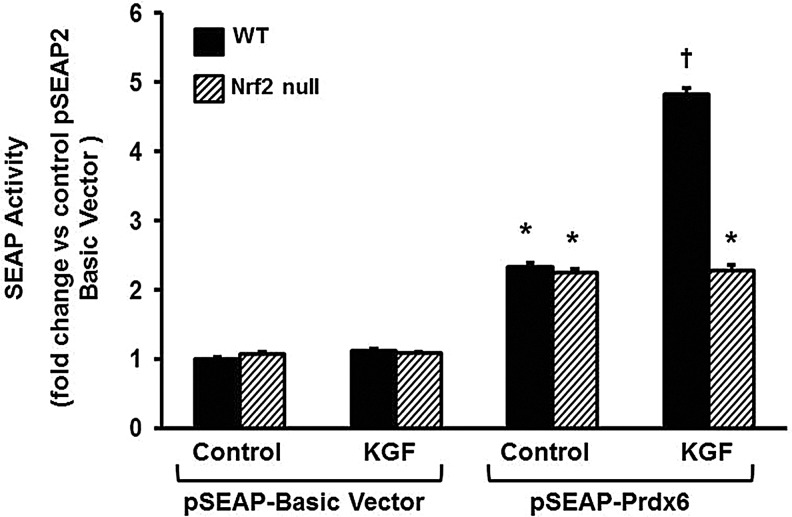
Nrf2 null mice do not exhibit stimulation of the PRDX6 promoter activity in response to KGF
To confirm that KGF-induced expression of Nrf2 regulates PRDX6 gene expression in another model, we compared transfected ATII cells isolated from Nrf2−/− mice or wild-type mice for the ability of KGF to induce PRDX6 promoter-driven SEAP reporter gene expression. The ability of KGF to induce Prdx6 expression was essentially abolished in the Nrf2−/− cells (Fig. 5).
FIG. 5.
KGF treatment does not stimulate promoter activity in Nrf2 knockout mice. Cells were transiently transfected with the indicated reporter vectors; after 24 h, cells were incubated with 20 ng/ml of KGF. SEAP activity was determined 24 h after treatment. Cells were then harvested, reporter gene assays performed and transfection efficiency was normalized to cotransfected β-galactosidase. The values are the mean±SE (n=3).*p<0.05, †p<0.01, compared with pSEAP-2-basic vector in the nontreated cells.
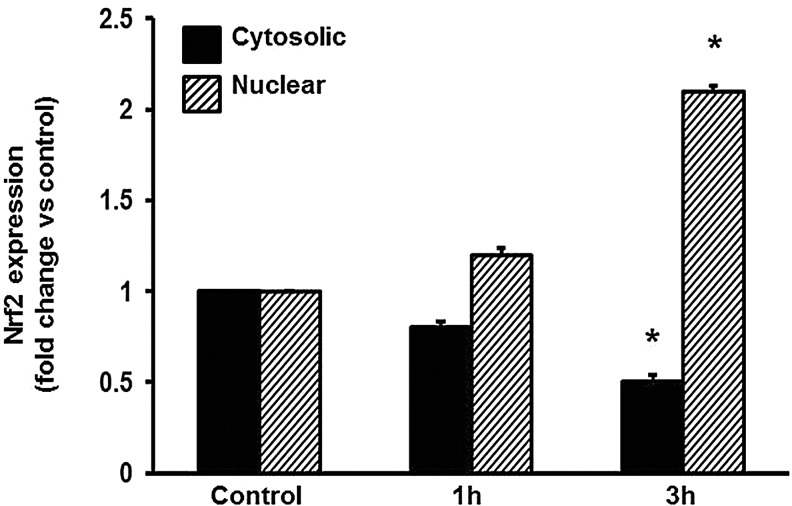
Nuclear localization of Nrf2 in A549 cells
The subcellular localization of Nrf2 is a major determinant of its function. To determine the influence of KGF on Nrf2 localization in A549 cells, we performed subcellular fractionation followed by western blot analyses in cells exposed to KGF. Nrf2 protein content showed a time-dependent decrease in the cytosolic fraction and a concomitant increase in the nuclear fraction (Fig. 6).
FIG. 6.
KGF-induced Nrf2 nuclear translocation in A549 cells. The content of Nrf2 cytosolic and nuclear fractions was determined in the indicated subcellular fraction by western blot analysis. Cells were exposed to 20 ng/ml KGF for the indicated times. Values shown are percent of the values in untreated cells. GAPDH (cytosolic fraction) and PCNA (nuclear fraction) were used as loading controls. Mean±SE (n=3). *p<0.05 compared with control.
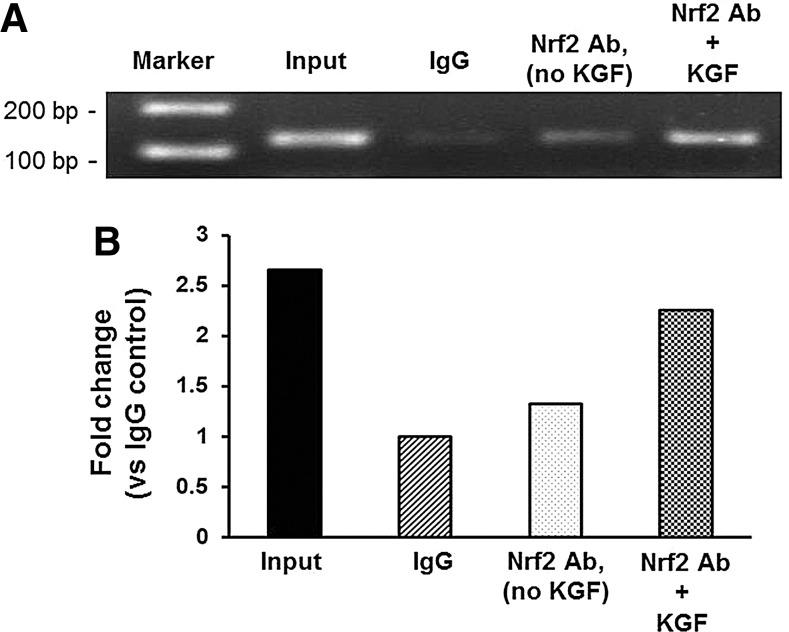
Nrf2 binds to the PRDX6 promoter in vivo
Nrf2 migration to the nucleus could result in its increased binding to the PRDX6 promoter ARE in vivo. To investigate this possibility, we performed chromatin immunoprecipitation (ChIP) assays. The binding of Nrf2 to the PRDX6 ARE under control conditions was substantially increased by KGF treatment (Fig. 7). A minimal signal was detected when IgG was substituted for anti-Nrf2 antibody, indicating specificity of the anti-Nrf2 antibody. The data in Figures 4–7 indicate that Nrf2 is activated and binds to the PRDX6 ARE upon KGF exposure.
FIG. 7.
Chromatin immunoprecipitation (ChIP) assay of the PRDX6 promoter ARE in response to KGF treatment. (A) A549 cells treated with 20 ng/ml KGF for 24 h were processed for ChIP assays using the primer pairs described in Materials and Methods section. Lane 1: Marker; 100 bp ladder: Input; results obtained from DNA that was polymerase chain reaction (PCR) amplified from chromatin extracts before immunoprecipitation, IgG; PCR results obtained after immunoprecipitation with rabbit immunoglobulin G. Nrf2 AB, no KGF; PCR results obtained after immunoprecipitation with Nrf2 antibody; in unstimulated cells: Nrf2 Ab+KGF; PCR results obtained after immunoprecipitation with Nrf2 antibody in cells treated with KGF. Results are representative for n=3. (B) Densitometric quantification of ChIP assay. Quantification of bands in A was performed using the ImageJ built-in measure function. Values are presented as a fold change versus IgG control. Data are shown as fold change versus IgG control.
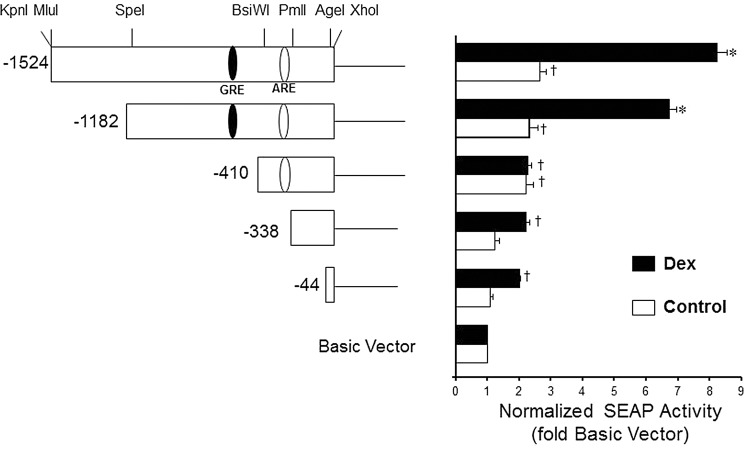
Dex stimulation of the PRDX6 promoter is abolished by deletion of the region between 1182 and 410 nucleotides upstream of the translational start
The same series of promoter deletions that had been used to identify the region that controls response to KGF was also employed to test response to Dex. Previously, we found that maximum induction of PRDX6 mRNA and protein with Dex occurred at a concentration of 1 μM in the L2 cell line (20) and in A549 cells (data not shown). A549 cells were transfected with the reporter gene constructs then treated with 1 μM Dex and SEAP reporter activity was measured. Reporter gene activity increased more than three-fold in response to Dex when the full-length promoter was present (Fig. 8). The region that was identified as necessary for response to Dex was upstream of the ARE, between −1182 and −410 nucleotides and did not appear to have any effect on basal activity. However, its deletion completely abolished the effect of Dex on stimulation of the PRDX6 promoter in A549 cells (Fig. 8).
FIG. 8.
Basal and dexamethasone (Dex)-inducible activity of PRDX6 promoter deletions. Left panel, a series of constructs were generated by restriction enzyme digestion of the 1.5 kb DNA fragment derived from the 5′-flanking region of PRDX6 in the promoterless pSEAP2-Basic vector. Numbers marking the 5′ ends of the deletions are relative to the translational start. The approximate location of the putative glucocorticoid response element (GRE) (and ARE) are shown within the promoter. Right panel, A549 cells were transiently transfected with reporter vectors and then incubated for 24 h, followed by 1 μM Dex treatment (or no treatment in control samples). SEAP activity was determined 24 h later. Mean±SE (n=3) are shown. *p<0.01 compared with vehicle control, †p<0.05 compared with pSEAP2-basic vector (no insert).
Role of the glucocorticoid response element
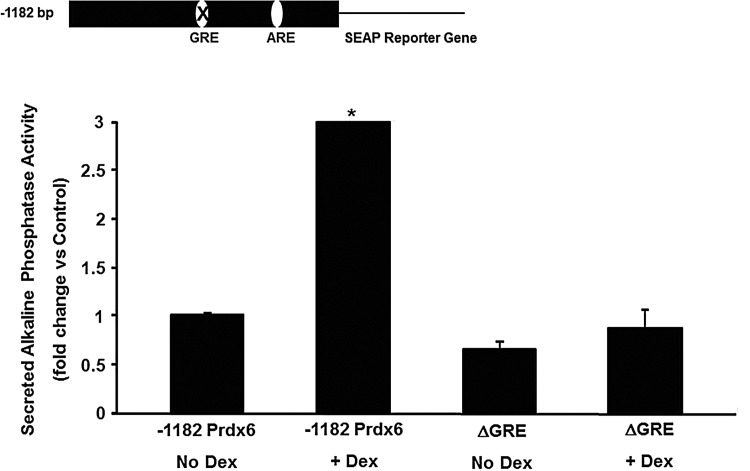
Inspection of the sequence in this region revealed a putative glucocorticoid response element (GRE) located in this region (between −751 and −737). The consensus GRE, as originally described by Evans (9) contains the sequence, GGTACANNNTGTTCT, where N is any nucleotide. The putative GRE reported herein has the sequence GGTACGGGAATTTTT, an identity of 11/15 nucleotides with the consensus GRE as described. The deletion data suggested that this GRE could be responsible for Dex stimulation of the PRDX6 promoter. To test this, a plasmid was constructed in which the putative GRE was specifically deleted by site directed mutagenesis and transfected into A549 cells. This deletion blocked induction by Dex (Fig. 9), indicating that this sequence did indeed control the promoter response to Dex.
FIG. 9.
Role of the GRE in PRDX6 gene expression. Deletion of the GRE was generated by site-directed mutagenesis of the 1.2 kb human PRDX6 promoter in the pSEAP2-Basic vector. Cells were transiently transfected with reporter vectors; after 24 h, cells were incubated with1 μM Dex. SEAP activity was determined 24 h after Dex treatment. Cells were then harvested, and reporter gene assays performed. −1182 Prdx, undeleted 1.2 kb PRDX6 promoter; ΔGRE, 1.2 kb PRDX6 promoter with GRE deletion. Values shown are mean±SE (n=3) with untreated pSEAP2-Basic vector (control) set to 1. *p<0.05 compared with pSEAP2-basic vector.
Effect of KGF and Dex in combination on the PRDX6 promoter and on PRDX6 mRNA and protein in lung epithelial A549 cells
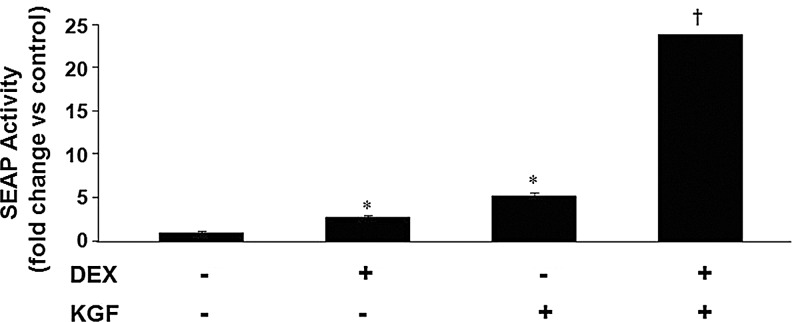
Since KGF and Dex each induced the PRDX6 promoter to increase expression through different DNA binding elements, we evaluated their ability to stimulate increased transcription from the PRDX6 promoter in combination with each other. Although, when acting individually, both KGF and Dex increased reporter gene activity modestly, by two or three fold, the combination of KGF and Dex resulted in an increase of over 20-fold, suggesting a synergistic effect (Fig. 10).
FIG. 10.
Synergistic effect of KGF and Dex on induction of PRDX6 promoter activity. The effect of the combination of KGF and Dex on reporter gene activity in response to the human PRDX6 promoter is shown. The effect of each individual treatment is shown for comparison. The basal level of reporter gene activity is set to 1. Cells were transiently transfected with reporter vectors; after 24 h, cells were incubated with 20 ng/ml of KGF, 1 μM Dex, or both. SEAP activity was determined 24 h after treatment. Cells were then harvested, and reporter gene assays performed. Mean±SE (n=3) are shown. *p<0.05 compared with untreated control, †p<0.01 compared with untreated control.
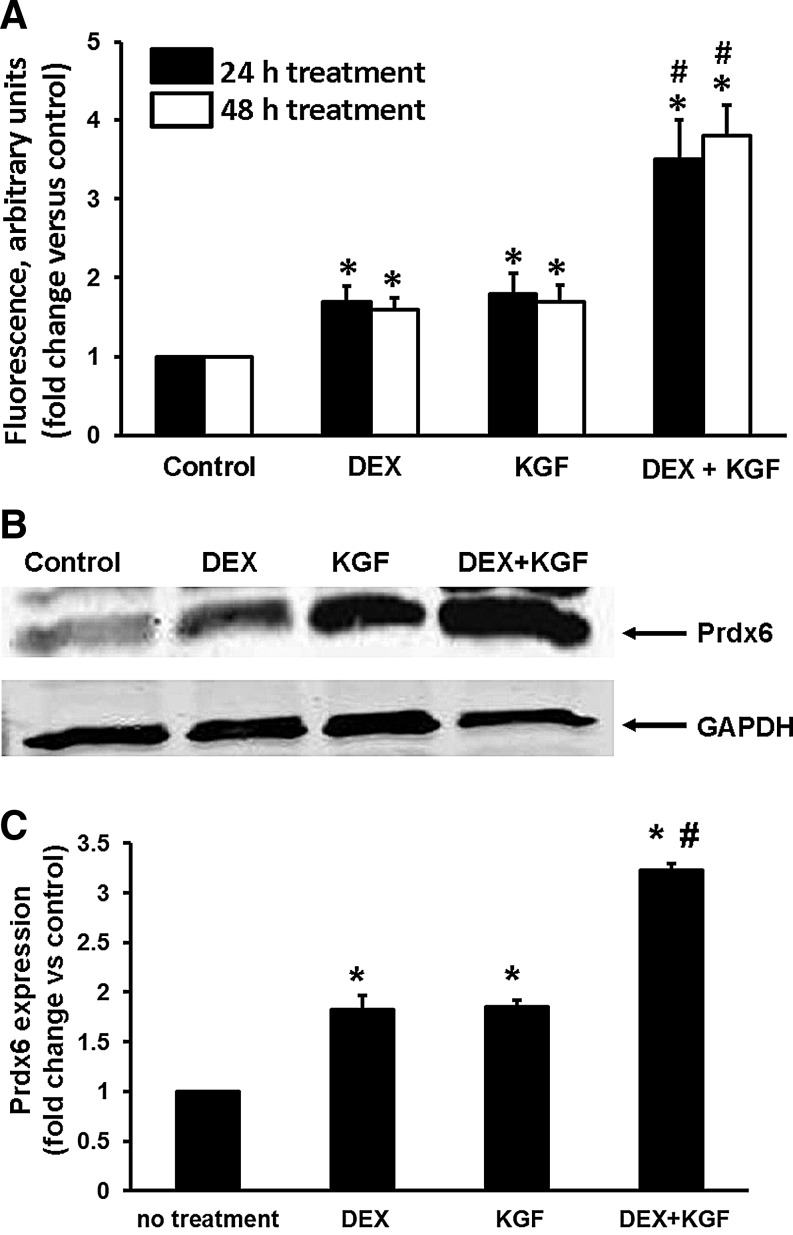
The reporter gene data indicate that both KGF and Dex increase transcription from the human PRDX6 promoter. Since there is not necessarily a correlation between transcriptional activity and protein levels, we next investigated their effect on the levels of Prdx6 protein in A549 cells, quantifying the protein levels by fluorescence activated cell sorting (FACS) analysis. KGF or Dex treatment each increased the level of Prdx6 protein a little less than two-fold. The combination of KGF and Dex also increased Prdx6 protein levels in A549 cells, nearly four-fold over control levels (Fig. 11A). Similar data were obtained by western blotting (Fig. 11B, C).
FIG. 11.
Upregulation of Prdx6 protein expression in A549 cells under the Dex and KGF treatments. (A) Determined by flow cytometry (FCM). Geometric mean fluorescence (arbitrary units) of Prdx6 intracellular content in control (untreated) versus Dex or KGF-treated A549 cells by FCM analysis. Cells underwent KGF (20 ng/ml), Dex (10−6 M), or combined Dex+KGF treatment for 24 or 48 h. Geometric Mean fluorescence of Prdx6 intracellular content was normalized by 50,000 events. Bars demonstrate the fold increase in Prdx6 intracellular concentration in KGF/Dex-treated A549 cells in comparison with basic fluorescence of untreated cells, shown as 1. Mean±SE (n=3) *p<0.05 compared with untreated control; #p<0.001 compared with a single treatment. (B) Western blot analysis of Prdx6 expression in A549 cells after 48 h treatment with 20 ng/ml KGF, 1 μM Dex, or combined treatment. (C) Quantification of western blot analysis of Prdx6 expression in KGF and/or Dex-treated A549 cells. For each sample, Prdx6 expression was normalized for the expression of β-actin or GAPDH. The amount of Prdx6 in non-treated (control) A549 cells is shown as 1. Mean±SE (n=3) *p<0.001 versus control; #p<0.001 versus single treatment.
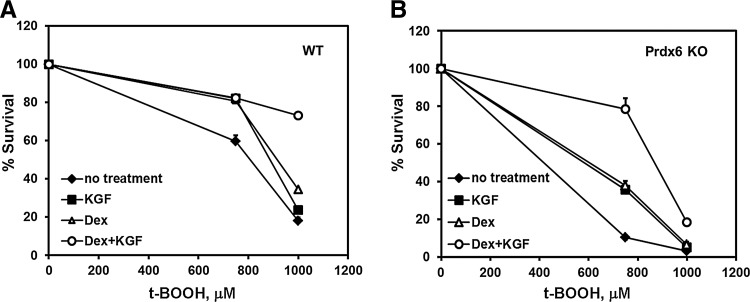
Since KGF and Dex increase Prdx6 protein levels, individually and especially in combination, it was of interest to determine whether their administration would protect against oxidative stress, whether their effect would be synergistic and whether this protection would require Prdx6. To study this, we utilized type II cells derived from wild-type mice and from Prdx6 null mice (30). Cells that had been treated for 24 h with KGF, Dex, both, or untreated controls were treated with tert-butyl hydroperoxide (tBOOH) at the indicated concentrations for 24 h and cell survival was measured. Wild-type cells were relatively resistant to 750 μM tBOOH, but either KGF, Dex, or the combination increased cell survival significantly. At 1 mM tBOOH, <20% of wild-type cells survived and neither KGF nor Dex were very protective. However, the combination of the two increased the survival rate to about 75%. In contrast, the cells from the Prdx6 null mice were much more sensitive to 1 mM tBOOH and only a few survived. Although the combination of KGF and Dex did significantly increase survival at 1 mM tBOOH compared with the other treatments, only about 20% of the cells survived. However, at 750 μM tBOOH, survival of the Prdx6 null cells was dramatically boosted by either KGF or by Dex and the combination increased survival from <10% to about 90% (Fig. 12). Thus, it appears that although KGF and Dex can be protective in the absence of Prdx6 and are more effective in combination, protection by KGF+Dex against higher concentrations of oxidant is much more effective when Prdx6 is present.
FIG. 12.
The effect of KGF and Dex on wild-type (WT) and Prdx6 null mouse ATII cell survival from oxidative stress. Comparison of survival rates for WT (A) or Prdx6 null (B) cells after treatment with indicated concentrations of tBOOH for 24 h were measured by the neutral red assay. Where indicated, cells received a 24 h pretreatment with 20 ng/ml KGF and/or 1 μM Dex. Data shown are mean±SE (n=5). *p<0.0001 compared with other points at the same concentration of tBOOH.
Discussion
KGF ameliorates oxidant-induced lung damage and mortality in animals exposed to hyperoxia, bleomycin, and radiation (2, 33, 35, 39, 40). Its importance in KGF receptor signaling in epithelial repair and cytoprotection is well established (49). Clinical trials have been initiated with KGF for the treatment of wound healing disorders and radiation and chemotherapy-induced mucositis (7). However, the mechanism of action of KGF in cytoprotection is still poorly understood. In this study, we identified the human PRDX6 gene promoter as a target of KGF action and demonstrated that the ability of KGF to upregulate the PRDX6 promoter requires an ARE. It has been previously reported that KGF induces Prdx6 protein and mRNA from the endogenous gene in a mouse liver hepatocyte cell line (15). However, the mechanism was not determined as the induction could not be reproduced using reporter gene constructs. The mouse Prdx6 gene does not contain a consensus ARE in the same position as the human promoter, but does contain one between −475 and −467. However, this potential ARE apparently did not respond to KGF in the experiments described in the prior publication (15).
Nrf2 is normally present in the cytoplasm bound to its repressor, Keap1 (also known as INrf2). Stimuli such as oxidative stress or electrophilic compounds interact with Keap1 and allow release of Nrf2 which, after phosphorylation, travels to the nucleus where it can activate transcription by heterodimerizing with Small Maf Protein (Smaf) and binding to the ARE (48). Several lines of evidence in this report implicate Nrf2 in the induction of PRDX6 transcription by KGF. These include siRNA knockdown resulting in a decrease in PRDX6 promoter activity (Fig. 4), ChIP assay indicating that Nrf2 binds to the PRDX6 promoter in cells (Fig. 7), and loss of KGF-induction of PRDX6 promoter activity in lung ATII cells from Nrf2 null mice (Fig. 5). These results support the concept that Prdx6 expression in response to KGF is regulated by the interaction of Nrf2 with the ARE in the PRDX6 gene promoter region. In addition, we found that Nrf2 translocated into nuclei within 3 h of exposure to KGF (Fig. 6). A previous study (4) indicated that Nrf2 is regulated by KGF at the transcriptional level. Our data do not rule out this possibility.
Our finding that upregulation of Prdx6 expression in response to KGF involves the same ARE and transcription factor (Nrf2) as the response to oxidative stress (6) was unexpected, as oxidative stress can induce Prdx6 expression in the absence of exogenously added KGF. We therefore considered the possibility that KGF may produce ROS through some undefined reaction, which results in upregulation of PRDX6 via Nrf2. However, ROS production was not detected in A549 cells in response to KGF nor did treatment with N-acetylcysteine, a ROS scavenger, block the KGF-mediated increase in reporter gene activity (data not shown). Therefore, it seems likely that KGF activates Nrf2 by an unknown mechanism independent of the generation of oxidants.
Dex, a synthetic glucocorticoid, had been previously shown to induce Prdx6 in cellular models (20). In general, glucocorticoid induction of transcription works through a glucocorticoid receptor that binds the glucocorticoid then translocates to the nucleus where it binds to a GRE in the DNA (9). The GRE that we have identified is not a perfect match for the previously published consensus sequence (9). In fact, it has four mismatches, one in the upstream half-site and three in the downstream half-site. A recent study (41) has shown that many functional GREs do not match the exact consensus described in (9). Our data indicate that the GRE that we have identified in the PRDX6 promoter is functional.
The effect of KGF and Dex on transcription in the reporter gene constructs appears to reflect their effect on the endogenous gene, as Prdx6 protein was substantially increased in A549 cells in response to KGF and to Dex. The ability of KGF to increase Prdx6 protein in keratinocytes has been previously reported (14). Likewise, we have previously reported the ability of Dex to increase Prdx6 in lung epithelial cells (20). The combination of KGF and Dex is more effective at increasing Prdx6 than either one alone. Induction at the reporter gene level is higher than at the protein level, but this is not surprising, as the reporter gene is primarily a reflection of transcription and does not take into account such factors as RNA stability or translational efficiency. Our data suggest that a component of the protective effects of KGF against oxidative stress (35) is due to its effect on Prdx6, Dex is also protective in this context, and that the combination offers more protection than either one alone. Further studies in an animal model will be needed to assess the potential therapeutic use of these findings in patients such as those on oxygen therapy.
In summary, we have shown that KGF stimulates transcription from the PRDX6 promoter through the Nrf2 transcription factor and that the ARE within the PRDX6 promoter is required for the inducibility of the PRDX6 promoter both under oxidative stress (6) and in response to KGF. On the other hand, Dex can also stimulate the PRDX6 promoter through a GRE that is upstream of the ARE. The stimulation by KGF and Dex are independent and can be synergistic. Future studies will be needed to better address the role of KGF in controlling Nrf2 stability, nuclear import and export, and interaction with Keap1 and the role of Prdx6 in mediating the protective effects of KGF and the possible protective role of Dex.
Materials and Methods
Chemicals and reagents
Minimal essential medium (MEM) was from Life Technologies (Grand Island, NY), OptiMEM was from Invitrogen, (Carlsbad, CA), KGF and Dex were from (Sigma-Aldrich, St. Louis, MO). pSEAP2 alkaline phosphatase reporter vectors and an alkaline phosphatase activity assay kit were from BD Bioscience (San Jose, CA). Competent cells, restriction enzymes, and the Beta-Glo assay system were from Promega (Madison, WI). All chemicals were analytical grade or higher. Rabbit polyclonal antibody against GADPH was obtained from Cell Signaling Technology (Denver, MA) and against β-actin was from Sigma-Aldrich.
Animals
Nrf2-disrupted mice on a C57BL/6 background were obtained from RIKEN Genomic Sciences Center, Tsurumi, Yokohama 230-0045, Japan, with the kind approval of Dr. Masayuki Yamamoto, Tohoku University School of Medicine, Sendai, Japan. Mice were produced as described (16) and maintained in our facilities. Genotypes of homozygous wild-type and Nrf2-disrupted mice were confirmed by polymerase chain reaction (PCR) amplification of genomic DNA isolated from tail tips. PCR amplification was performed using three different primers (42), 5′ TGGACGGGACTATTGAAGGCTG-3′ (sense for both genotypes), 5′-GCCGCCTTTTCAGTAGATGGAGG-3′ (antisense for wild-type), and 5′-GCGGATTGACCGTAATGGGATAGG-3′ (antisense for LacZ). All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Rats and mice were housed under high efficiency particulate air (HEPA)-filtered air in facilities maintained by the University of Pennsylvania Laboratory of Animal Resources.
Cell culture and treatments
A549 cells (American Type Culture Collection [ATCC], Manassas, VA) were cultured in MEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified incubator containing 5% CO2 at 37°C. Unless otherwise indicated, cells were exposed to the indicated amount of KGF or Dex (10−6 M) or both for 24–48 h. Cells were rinsed with cold PBS before being harvested.
ATII pneumocytes were isolated from the lungs of anesthetized male Sprague-Dawley rats (8) or wild-type C57Bl/6J mice, Nrf2 null, and Prdx6 null mice (3) according to previously published methods. Rat and mouse pneumocytes were plated on 12-well tissue culture dishes at ∼1.5×106 cells/dish (3.8 cm in diameter). Dishes for mouse pneumocytes were collagen type I coated. Cells were cultured in 10% fetal calf serum (FCS) in Eagle's MEM at 37°C in a humidified incubator with 5% CO2 in air.
Plasmid constructs
Human PRDX6 promoter constructs and truncations of the PRDX6 promoter, −1182, −410, −338, and −44 from the start of translation, produced by digestion of pSEAP2-PRDX6-wt with restriction enzymes have been described (6). [Note: in our previous publication we incorrectly referred to the position of truncations as upstream from the transcriptional start (6)]. Targeted deletions of the ARE and the putative GRE were obtained using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA).
Western blot analysis
Protein concentration was determined using the Coomassie blue assay (Bio-Rad, Hercules, CA). For western blot, 20 μg of total cell protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 12% gel and electrotransferred onto a 0.45 μm pore size nitrocellulose membrane (Invitrogen). Polyclonal antibody against Prdx6 has been described (27). Dilutions of the antibody to human Prdx6 and the fluorescent IRDye TM700 secondary antibody were 1:3000. Anti-Nrf2 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and diluted 1:300 before use. After transfer, images were acquired and analyzed using the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE), following the manufacturer's recommendations. All manipulations of contrast were done for the entire gel.
Transfection procedure and assay of alkaline phosphatase and β-galactosidase activity
Transfection of A549 cells (70%–80% confluence) with plasmids using Lipofectamine 2000 transfection reagent (Invitrogen) and of ATII cells using the Amaxa Nucleofector have been previously described (6). siRNAs targeting Nrf2 (38) were a kind gift of Dr. Shyam Biswal, Johns Hopkins University. About 24 h after transfection, both A549 cells and ATII cells were treated with 20 ng/ml KGF (∼10−9 M) or 10−6 M Dex or both for an additional 24 h. Assays for SEAP and β-galactosidase activities have been described (6). The specific activity of each promoter construct was calculated by normalizing the SEAP activity to the β-galactosidase activity.
Chromatin immunoprecipitation assay
ChIP assay was performed as described previously (6) employing, however, a different commercially available kit (Millipore, Danvers, MA). Briefly, cells were fixed by adding formaldehyde directly into the medium at room temperature for 10 min. After fixation, the cells were collected by scraping and then sonicated. Immunoprecipitation analysis was performed using control rabbit IgG and anti-Nrf2 antibody (Santa Cruz Biotechnology). Immunoprecipitated DNA fragments served as templates for PCR with the PRDX6 promoter primer sequences:
forward, 5′-GCATCCTCAAGCTTCCAGGGGGC-3′;
reverse, 5′-GGACTACCGCGGTCGCTGTTGG-3′. PCR products were analyzed by agarose gel electrophoresis.
Measurement of Nrf2 localization
We used cellular fractionation and western blot analysis to detect the amount of Nrf2 in nuclei and cytoplasm of A549 cells. After 20 ng/ml of KGF treatment, cells were lysed in ice-cold hypotonic buffer (Sigma, St. Louis, MO) containing 10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 10 mM dithiothreitol (DTT), 0.6% NP40, and a cocktail of protease inhibitors (Halt™; Pierce, Rockford, IL). After centrifugation at 4°C at 12,000g for 30 s, the supernatant was collected as the cytosolic faction. The pellet was further extracted using buffer containing 5 mM HEPES, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, 26% glycerol, and 300 mM NaCl at 4°C for 30 min. Soluble nuclear proteins were collected in the supernatant after centrifugation at 12,000g for 10 min and subjected to western blotting as described above.
Flow cytometry analysis
Flow cytometry analysis of Prdx6 expression in A549 cells was performed with a four-color dual-laser FACSCalibur system (Becton Dickinson, San Jose, CA) using CellQuest software as described (17, 23). PRDX6 expression was detected by intracellular single-color staining (29) using a polyclonal primary antibody (27), and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG secondary antibody (Jackson Laboratories, Bar Harbor, ME). Both unstained cells and isotype control IgG-FITC served as negative controls. FACS data were analyzed using CellQuest software (Becton Dickinson, San Jose, CA). Histograms of total fluorescence intensity (arbitrary units) of intracellular Prdx6 content were normalized to 50,000 events.
ATII survival study
Isolated type II cells were plated in MEM (GIBCO, Carlsbad, CA) supplemented with 10% FCS (Sigma), 100 U/ml penicillin, and 100 μg/ml streptomycin (GIBCO) on 24-well collagen type I coated plate and allowed to adhere. The next day, cells underwent a 24 h 20 ng/ml KGF, 1 μM Dex, or combined KGF+Dex treatment, followed by additional 24 h t-BOOH treatment with indicated concentrations of tBOOH. Cell survival rates were measured by the neutral red assay.
Neutral red uptake assay
The neutral red uptake assay with minor modification was used for cell survival studies (36). ATII cells were incubated with neutral red at a final concentration of 50 μg/ml in the culture media at 37°C for 3 h. Cells were then destained with 50% ethanol solution containing 0.5% glacial acetic acid for 10 min. Neutral red concentration in the lysate indicating uptake by live cells was quantified by the absorbance determined at 550 nm. Survival rates were calculated by normalization to the corresponding untreated controls and are expressed as a percentage.
Statistical analysis
Results are expressed as mean±SE. Statistical significance was determined by one-way ANOVA or t-test as appropriate using SigmaStat (Jandell Scientific, San Rafael, CA). p-values of <0.05 were considered significant.
Abbreviations Used
- ARE
antioxidant response element
- ChIP
chromatin immunoprecipitation
- Dex
dexamethasone
- DTT
dithiothreitol
- GSH
glutathione
- GRE
glucocorticoid response element
- KGF
keratinocyte growth factor
- MEM
minimal essential medium
- PCR
polymerase chain reaction
- PRDX6
peroxiredoxin
- ROS
reactive oxygen species
- SEAP
secreted alkaline phosphatase
Acknowledgments
The authors thank Dr. Masayuki Yamamoto for the Nrf2 null mice, Evguenia Arguiri for maintaining the Nrf2 null mouse colonies, and Dr. Shyam Biswal for siRNAs. This work was supported by NIH R01 HL102016 and NIH P01 HL75587 (A.B.F.). Costs associated with the Nrf2 null mouse colony were supported by NIH R01 CA133470 and the American Institute for Cancer Research 03B024 (M.C.S.). I.C. was supported as an NRSA Postdoctoral Fellow by HL T32-07748. This work has been presented in part at the Experimental Biology Meetings of 2009 and 2010.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bao S, Wang Y, Sweeney P, Chaudhuri A, Doseff AI, Marsh CB, and Knoell DL. Keratinocyte growth factor induces Akt kinase activity and inhibits Fas-mediated apoptosis in A549 lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 288: L36–L42, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Barazzone C, Donati YR, Rochat AF, Vesin C, Kan CD, Pache JC, and Piguet PF. Keratinocyte growth factor protects alveolar epithelium and endothelium from oxygen-induced injury in mice. Am J Pathol 154: 1479–1487, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bortnick AE, Favari E, Tao JQ, Francone OL, Reilly M, Zhang Y, Rothblat GH, and Bates SR. Identification and characterization of rodent ABCA1 in isolated type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 285: L869–L878, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, Kan YW, and Werner S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol 22: 5492–5505, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JW, Dodia C, Feinstein SI, Jain MK, and Fisher AB. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J Biol Chem 275: 28421–28427, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury I, Mo Y, Gao L, Kazi A, Fisher AB, and Feinstein SI. Oxidant stress stimulates expression of the human peroxiredoxin 6 gene by a transcriptional mechanism involving an antioxidant response element. Free Radic Biol Med 46: 146–153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danilenko DM. Preclinical and early clinical development of keratinocyte growth factor, an epithelial-specific tissue growth factor. Toxic Pathol 27: 64–71, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Dobbs LG, Gonzalez R, and Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 134: 141–145, 1986 [DOI] [PubMed] [Google Scholar]
- 9.Evans RM. The steroid and thyroid hormone receptor superfamily. Science (New York) 240: 889–895, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehrenbach H, Kasper M, Tschernig T, Pan T, Schuh D, Shannon JM, Muller M, and Mason RJ. Keratinocyte growth factor-induced hyperplasia of rat alveolar type II cells in vivo is resolved by differentiation into type I cells and by apoptosis. Eur Respir J 14: 534–544, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Fisher AB, Dodia C, Feinstein SI, and Ho YS. Altered lung phospholipid metabolism in mice with targeted deletion of lysosomal-type phospholipase A2. J Lipid Res 46: 1248–1256, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Fisher AB, Dodia C, Manevich Y, Chen JW, and Feinstein SI. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J Biol Chem 274: 21326–21334, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Fisher AB, Dodia C, Yu K, Manevich Y, and Feinstein SI. Lung phospholipid metabolism in transgenic mice overexpressing peroxiredoxin 6. Biochim Biophys Acta 1761: 785–792, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Frank S, Munz B, and Werner S. The human homologue of a bovine non-selenium glutathione peroxidase is a novel keratinocyte growth factor-regulated gene. Oncogene 14: 915–921, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Gallagher BM. and Phelan SA. Investigating transcriptional regulation of PRDX6 in mouse liver cells. Free Radic Biol Med 42: 1270–1277, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, and Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313–322, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Jacobberger JW. Increasing the power of cytometry. Nat Methods 3: 343–344, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kang SW, Baines IC, and Rhee SG. Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J Biol Chem 273: 6303–6311, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Manevich Y, Feinstein SI, Pak JH, Ho YS, and Fisher AB. Induction of 1-cys peroxiredoxin expression by oxidative stress in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 285: L363–L369, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Kim HS, Pak JH, Gonzales LW, Feinstein SI, and Fisher AB. Regulation of 1-cys peroxiredoxin expression in lung epithelial cells. Am J Respir Cell Mol Biol 27: 227–233, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Kim TS, Dodia C, Chen X, Hennigan BB, Jain M, Feinstein SI, and Fisher AB. Cloning and expression of rat lung acidic Ca(2+)-independent PLA2 and its organ distribution. Am J Physiol 274: L750–L761, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Kovacs D, Raffa S, Flori E, Aspite N, Briganti S, Cardinali G, Torrisi MR, and Picardo M. Keratinocyte growth factor down-regulates intracellular ROS production induced by UVB. J Dermatol Sci 54: 106–113, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Krutzik PO. and Nolan GP. Fluorescent cell barcoding in flow cytometry allows high-throughput drug screening and signaling profiling. Nat Methods 3: 361–368, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Lehtonen ST, Markkanen PM, Peltoniemi M, Kang SW, and Kinnula VL. Variable overoxidation of peroxiredoxins in human lung cells in severe oxidative stress. Am J Physiol Lung Cell Mol Physiol 288: L997–L1001, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Lieber M, Smith B, Szakal A, Nelson-Rees W, and Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer 17: 62–70, 1976 [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Pan ZZ, Devaux Y, and Ray P. p21-activated protein kinase 4 (PAK4) interacts with the keratinocyte growth factor receptor and participates in keratinocyte growth factor-mediated inhibition of oxidant-induced cell death. J Biol Chem 278: 10374–10380, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Manevich Y, Sweitzer T, Pak JH, Feinstein SI, Muzykantov V, and Fisher AB. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci U S A 99: 11599–11604, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthay MA, Fukuda N, Frank J, Kallet R, Daniel B, and Sakuma T. Alveolar epithelial barrier. Role in lung fluid balance in clinical lung injury. Clin Chest Med 21: 477–490, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Milovanova T, Chatterjee S, Hawkins BJ, Hong N, Sorokina EM, Debolt K, Moore JS, Madesh M, and Fisher AB. Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochim Biophys Acta 1783: 1866–1875, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mo Y, Feinstein SI, Manevich Y, Zhang Q, Lu L, Ho YS, and Fisher AB. 1-Cys peroxiredoxin knock-out mice express mRNA but not protein for a highly related intronless gene. FEBS Lett 555: 192–198, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Nguyen T, Sherratt PJ, and Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Ann Rev Pharmacol Toxicol 43: 233–260, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Pak JH, Manevich Y, Kim HS, Feinstein SI, and Fisher AB. An antisense oligonucleotide to 1-cys peroxiredoxin causes lipid peroxidation and apoptosis in lung epithelial cells. J Biol Chem 277: 49927–49934, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Panos RJ, Bak PM, Simonet WS, Rubin JS, and Smith LJ. Intratracheal instillation of keratinocyte growth factor decreases hyperoxia-induced mortality in rats. J Clin Invest 96: 2026–2033, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papaiahgari S, Kleeberger SR, Cho HY, Kalvakolanu DV, and Reddy SP. NADPH oxidase and ERK signaling regulates hyperoxia-induced Nrf2-ARE transcriptional response in pulmonary epithelial cells. J Biol Chem 279: 42302–42312, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Ray P, Devaux Y, Stolz DB, Yarlagadda M, Watkins SC, Lu Y, Chen L, Yang XF, and Ray A. Inducible expression of keratinocyte growth factor (KGF) in mice inhibits lung epithelial cell death induced by hyperoxia. Proc Natl Acad Sci U S A 100: 6098–6103, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Repetto G, del Peso A, and Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3: 1125–1131, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Rhee SG, Kang SW, Chang TS, Jeong W, and Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life 52: 35–41, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Singh A, Boldin-Adamsky S, Thimmulappa RK, Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F, Watson WH, Gabrielson E, Feinstein E, and Biswal S. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res 68: 7975–7984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugahara K, Iyama K, Kuroda MJ, and Sano K. Double intratracheal instillation of keratinocyte growth factor prevents bleomycin-induced lung fibrosis in rats. J Pathol 186: 90–98, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Takeoka M, Ward WF, Pollack H, Kamp DW, and Panos RJ. KGF facilitates repair of radiation-induced DNA damage in alveolar epithelial cells. Am J Physiol 272: L1174–L1180, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Taniguchi-Yanai K, Koike Y, Hasegawa T, Furuta Y, Serizawa M, Ohshima N, Kato N, and Yanai K. Identification and characterization of glucocorticoid receptor-binding sites in the human genome. J Recept Signal Transduct Res 30: 88–105, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, and Dore S. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med 43: 408–414, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Phelan SA, Forsman-Semb K, Taylor EF, Petros C, Brown A, Lerner CP, and Paigen B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem 278: 25179–25190, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Feinstein SI, and Fisher AB. Peroxiredoxin 6 as an antioxidant enzyme: protection of lung alveolar epithelial type II cells from H2O2-induced oxidative stress. J Cell Biochem 104: 1274–1285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Feinstein SI, Manevich Y, Ho YS, and Fisher AB. Lung injury and mortality with hyperoxia are increased in peroxiredoxin 6 gene-targeted mice. Free Radic Biol Med 37: 1736–1743, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Feinstein SI, Manevich Y, Ho YS, and Fisher AB. Peroxiredoxin 6 gene-targeted mice show increased lung injury with paraquat-induced oxidative stress. Antioxid Redox Signal 8: 229–237, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Phelan SA, Manevich Y, Feinstein SI, and Fisher AB. Transgenic mice overexpressing peroxiredoxin 6 show increased resistance to lung injury in hyperoxia. Am J Respir Cell Mol Biol 34: 481–486, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ware LB. and Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol 282: L924–L940, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine Growth Factor Rev 9: 153–165, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Yi ES, Salgado M, Williams S, Kim SJ, Masliah E, Yin S, and Ulich TR. Keratinocyte growth factor decreases pulmonary edema, transforming growth factor-beta and platelet-derived growth factor-BB expression, and alveolar type II cell loss in bleomycin-induced lung injury. Inflammation 22: 315–325, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Zhang H, Liu H, Dickinson DA, Liu RM, Postlethwait EM, Laperche Y, and Forman HJ. gamma-Glutamyl transpeptidase is induced by 4-hydroxynonenal via EpRE/Nrf2 signaling in rat epithelial type II cells. Free Radic Biol Med 40: 1281–1292, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]