Abstract
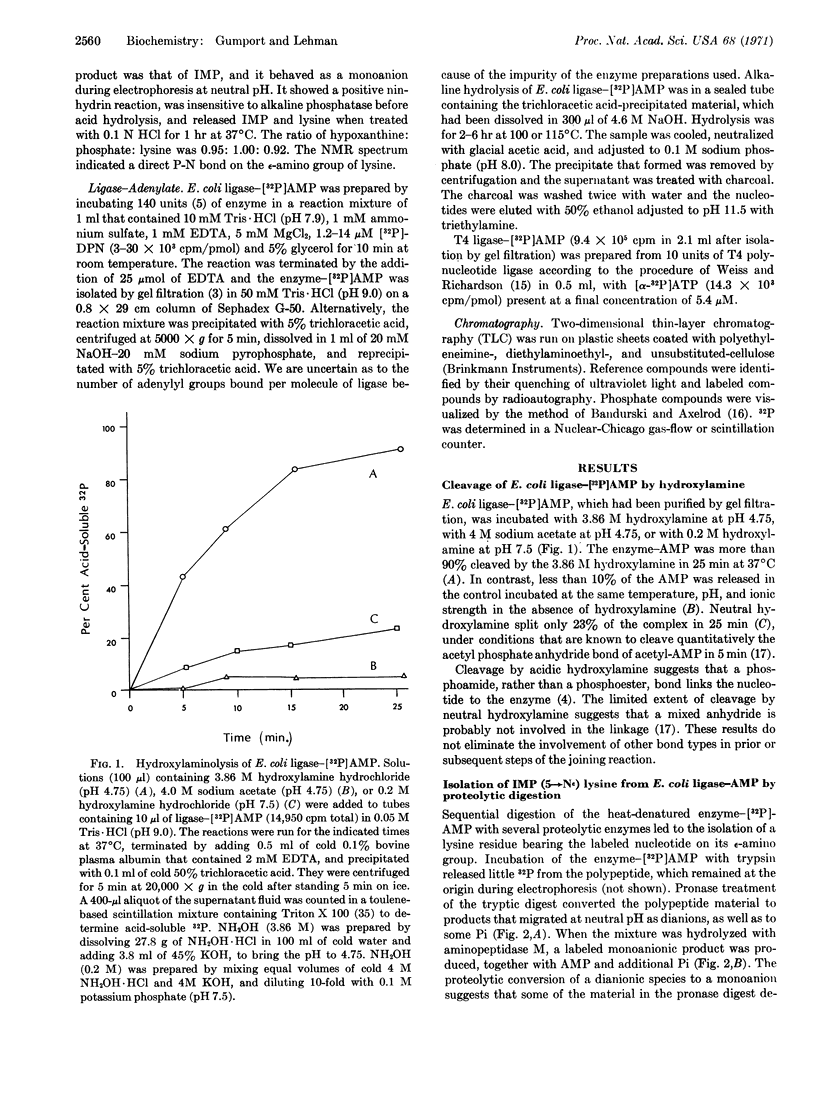
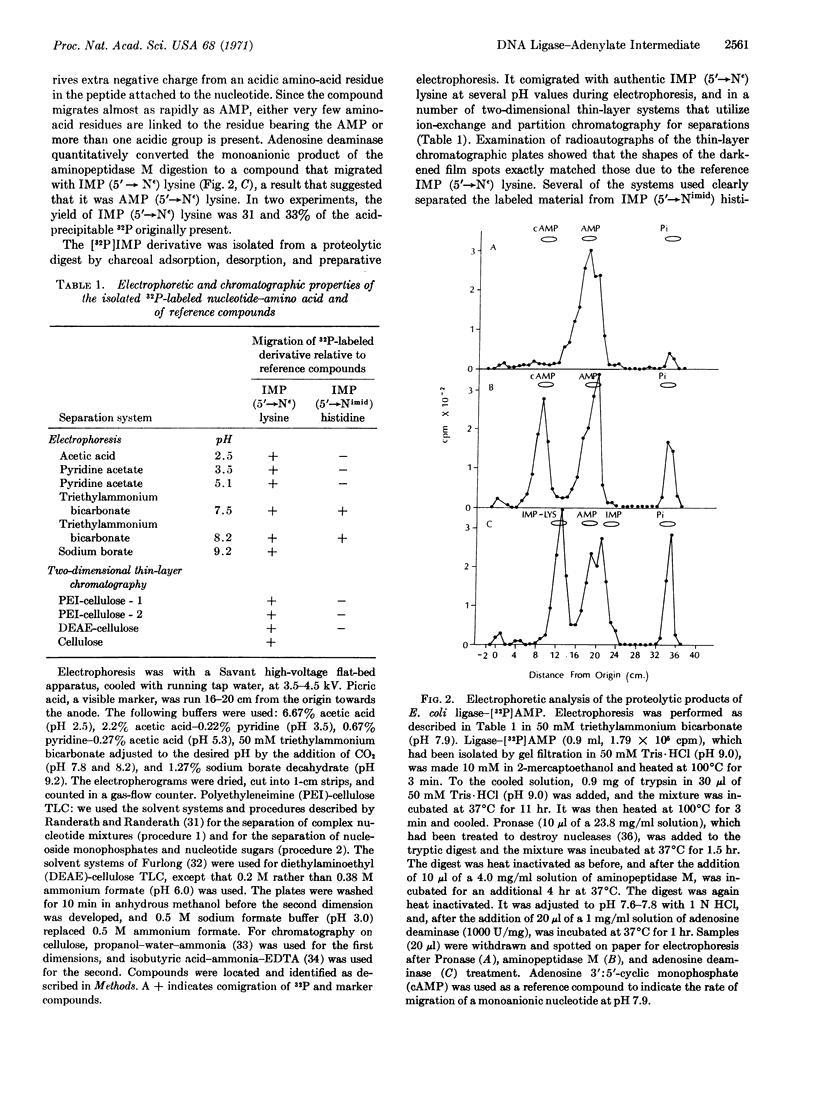
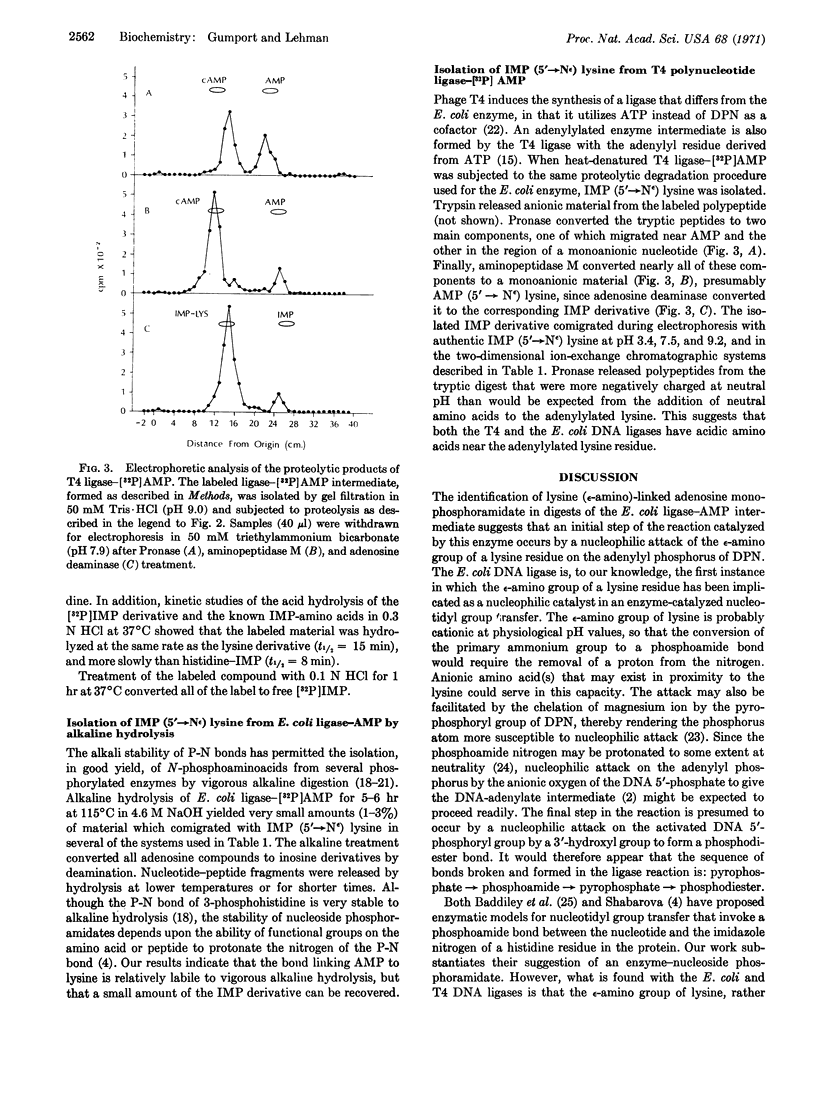
Proteolytic degradation of the Escherichia coli DNA ligase-adenylate intermediate releases adenosine 5′-monophosphate linked to the ε-amino group of lysine by a phosphoamide bond. Measurements of the rate of hydroxylaminolysis of the ligase-adenylate provide further support for a phosphoamide linkage in the native enzyme. Lysine (ε-amino)-linked adenosine monophosphoramidate has also been isolated from the T4 phage-induced ligase-adenylate intermediate. These results indicate that an initial step of the DNA ligase reaction consists of the nucleophilic attack of the ε-amino group of a lysine residue of the enzyme on the adenylyl phosphorus of DPN or ATP that leads to the formation of enzyme-bound lysine (εamino)-linked adenosine monophosphoramidate.
Keywords: nucleotidyl group transfer, covalent catalysis, phosphoamides, T4 phage, E. coli
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- BERG P. Acyl adenylates; the synthesis and properties of adenyl acetate. J Biol Chem. 1956 Oct;222(2):1015–1023. [PubMed] [Google Scholar]
- Chelala C. A., Hirschbein L., Torres H. N. Interconvertible forms of Escherichia coli RNA polymerase. Proc Natl Acad Sci U S A. 1971 Jan;68(1):152–154. doi: 10.1073/pnas.68.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottam G. L., Srere P. A. Nature of the phosphorylated residue in citrate clevage enzyme. Biochem Biophys Res Commun. 1969 Jun 27;35(6):895–900. doi: 10.1016/0006-291x(69)90708-6. [DOI] [PubMed] [Google Scholar]
- DELUCA M., EBNER K. E., HULTQUIST D. E., KREIL G., PETER J. B., MOYER R. W., BOYER P. D. THE ISOLATION AND IDENTIFICATION OF PHOSPHOHISTIDINE FROM MITOCHONDRIAL PROTEIN. Biochem Z. 1963;338:512–525. [PubMed] [Google Scholar]
- Easley C. W. Combinations of specific color reactions useful in the peptide mapping technique. Biochim Biophys Acta. 1965 Sep 13;107(2):386–388. doi: 10.1016/0304-4165(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Englund P. T., Huberman J. A., Jovin T. M., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXX. Binding of triphosphates to deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):3038–3044. [PubMed] [Google Scholar]
- HOTTA Y., BASSEL A. MOLECULAR SIZE AND CIRCULARITY OF DNA IN CELLS OF MAMMALS AND HIGHER PLANTS. Proc Natl Acad Sci U S A. 1965 Feb;53:356–362. doi: 10.1073/pnas.53.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z. W., Lehman I. R. Enzymatic joining of polynucleotides. VI. Activity of a synthetic adenylylated polydeoxynucleotide in the reaction. J Biol Chem. 1969 Jan 10;244(1):43–47. [PubMed] [Google Scholar]
- Harvey C. L., Gabriel T. F., Wilt E. M., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. IX. Synthesis and properties of the deoxyribonucleic acid adenylate in the phage T4 ligase reaction. J Biol Chem. 1971 Jul 25;246(14):4523–4530. [PubMed] [Google Scholar]
- Hultquist D. E., Moyer R. W., Boyer P. D. The preparation and characterization of 1-phosphohistidine and 3-phosphohistidine. Biochemistry. 1966 Jan;5(1):322–331. doi: 10.1021/bi00865a041. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon H. S., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. 8. ATP: glutamine synthetase adenylyltransferase, an enzyme that catalyzes alterations in the regulatory properties of glutamine synthetase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1703–1710. doi: 10.1073/pnas.58.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Zimmerman S. B., Oshinsky C. K., Gellert M. Enzymatic joining of DNA strands, II. An enzyme-adenylate intermediate in the dpn-dependent DNA ligase reaction. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2004–2011. doi: 10.1073/pnas.58.5.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Lehman I. R. Enzymatic joining of polynucleotides. IX. A simple and rapid assay of polynucleotide joining (ligase) activity by measurement of circle formation from linear deoxyadenylate-deoxythymidylate copolymer. J Biol Chem. 1970 Jul 25;245(14):3626–3631. [PubMed] [Google Scholar]
- Olivera B. M., Hall Z. W., Anraku Y., Chien J. R., Lehman I. R. On the mechanism of the polynucleotide joining reaction. Cold Spring Harb Symp Quant Biol. 1968;33:27–34. doi: 10.1101/sqb.1968.033.01.008. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 May;57(5):1426–1433. doi: 10.1073/pnas.57.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Enzymes in DNA metabolism. Annu Rev Biochem. 1969;38:795–840. doi: 10.1146/annurev.bi.38.070169.004051. [DOI] [PubMed] [Google Scholar]
- SHUSTER L., KAPLAN N. O., STOLZENBACH F. E. The reaction of pyridine nucleotides with trifluoroacetic acid anhydride. J Biol Chem. 1955 Jul;215(1):195–209. [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970 Jul 25;245(14):3637–3644. [PubMed] [Google Scholar]
- Symons R. H. Modified procedure for the synthesis of 32P-labelled ribonucleoside 5'-monophosphates of high specific activity. Biochim Biophys Acta. 1968 Feb 26;155(2):609–610. doi: 10.1016/0005-2787(68)90205-0. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Weiss B., Richardson C. C. Enzymatic breadage and joining of deoxyribonucleic acid. 3. An enzyme-adenylate intermediate in the polynucleotide ligase reaction. J Biol Chem. 1967 Sep 25;242(18):4270–4272. [PubMed] [Google Scholar]
- Weiss B., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid, I. Repair of single-strand breaks in DNA by an enzyme system from Escherichia coli infected with T4 bacteriophage. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1021–1028. doi: 10.1073/pnas.57.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenden R., Sharpless T. K., Allan R. Substrate binding by adenosine deaminase. Specificity, pH dependence, and competition by mercurials. J Biol Chem. 1967 Mar 10;242(5):977–983. [PubMed] [Google Scholar]
- Wålinder O. Identification of a phosphate-incorporating protein from bovine liver as nucleoside diphosphate kinase and isolation of 1-32P-phosphohistidine, 3-32P-phosphohistidine, and N-epsilon-32P-phospholysine from erythrocytic nucleoside diphosphate kinase, incubated with adenosine triphosphate-32P. J Biol Chem. 1968 Jul 25;243(14):3947–3952. [PubMed] [Google Scholar]


