Abstract
Objective
To identify the cause of an adult-onset multisystemic disease with multiple deletions of mitochondrial DNA (mtDNA).
Design
Case report.
Setting
University hospitals.
Patient
A 65-year-old man with axonal sensorimotor peripheral neuropathy, ptosis, ophthalmoparesis, diabetes mellitus, exercise intolerance, steatohepatopathy, depression, parkinsonism, and gastrointestinal dysmotility.
Results
Skeletal muscle biopsy revealed ragged-red and cytochrome-c oxidase–deficient fibers, and Southern blot analysis showed multiple mtDNA deletions. No deletions were detected in fibroblasts, and the results of quantitative polymerase chain reaction showed that the amount of mtDNA was normal in both muscle and fibroblasts. Exome sequencing using a mitochondrial library revealed compound heterozygous MPV17 mutations (p.LysMet88-89MetLeu and p.Leu143*), a novel cause of mtDNA multiple deletions.
Conclusions
In addition to causing juvenile-onset disorders with mtDNA depletion, MPV17 mutations can cause adult-onset multisystemic disease with multiple mtDNA deletions.
Mitochondrial DNA (mtDNA) integrity requires nuclear DNA–encoded proteins to maintain deoxynucleotide and ribonucleotide balance, to replicate and repair the mitochondrial genome, and to generate the mtDNA-protein nucleoid complex. Disorders in the cross talk between the 2 genomes can compromise the integrity and quantity of mtDNA, leading to pathogenic multiple deletions, point mutations, and depletion of mtDNA.1
The clinical presentations of defects of intergenomic communication are heterogeneous. Depletion of mtDNA is associated with infantile-onset myopathy(OMIM #609560) or multisystemic diseases (OMIM #251880 and #203700), whereas multiple deletions of mtDNA have been observed in adult-onset autosomal dominant (ad) or autosomal recessive (ar) progressive external ophthalmoplegia (PEO) (OMIM #157640 and #258450).1,2 Although mtDNA depletion syndromes were initially considered clinically and etiologically distinct from adPEO and arPEO with multiple mtDNA deletions, the coexistence of multiple deletions, depletion, and point mutations of mtDNA in mitochondrial neurogastrointestinal encephalomyopathy (OMIM #603041) revealed that qualitative and quantitative defects of mtDNA were part of a spectrum ranging from depletion to multiple deletions.2,3 This concept was confirmed when mutations in genes initially considered typical causes of adPEO and arPEO with multiple mtDNA deletions, such as POLG, encoding mtDNA polymerase gamma, and C10orf2, encoding a mtDNA helicase, were later found to also cause depletion of mtDNA.4,5 Conversely, mutations in RRM2B and TK2, originally associated with mtDNA depletion and infantile myopathy, were found to cause late-onset arPEO with multiple mtDNA deletions.6,7
Mutations in MPV17 have been identified in patients with severe mtDNA depletion manifesting as early childhood-onset failure to thrive, hypoglycemia, encephalopathy, and hepatopathy progressing to liver failure.8–11 In addition, the p.R50Q MPV17 mutation was identified in homozygosity in Navajo Indian patients with infantile-, childhood-, or juvenile-onset neurohepatopathy (Navajo neurohepatopathy,OMIM#256810) and mtDNA depletion.8 We now report that MPV17 compound heterozygous mutations can cause multiple mtDNA deletions manifesting as adult-onset multisystemic disease.
REPORT OF A CASE
A 65-year-old man born of nonconsanguineous parents of European origin developed distal limb weakness and numbness at age 34 years. Nerve conduction studies and electromyographic results showed signs of an axonal sensorimotor peripheral neuropathy, initially diagnosed as Charcot-Marie-Tooth disease. In his 40s, he developed progressive proximal limb weakness, exercise intolerance, diabetes mellitus, ptosis, ophthalmoparesis, hearing loss, severe constipation due to gastrointestinal dysmotility, and depression. Ultrasonography of the abdomen revealed a fatty liver. At age 65 years, he was noted to have parkinsonism with bradykinesia, bilateral resting hand tremor, and mild rigidity.
His sister died from unexplained liver failure at 39 years of age. His mother reportedly had ptosis and weakness.
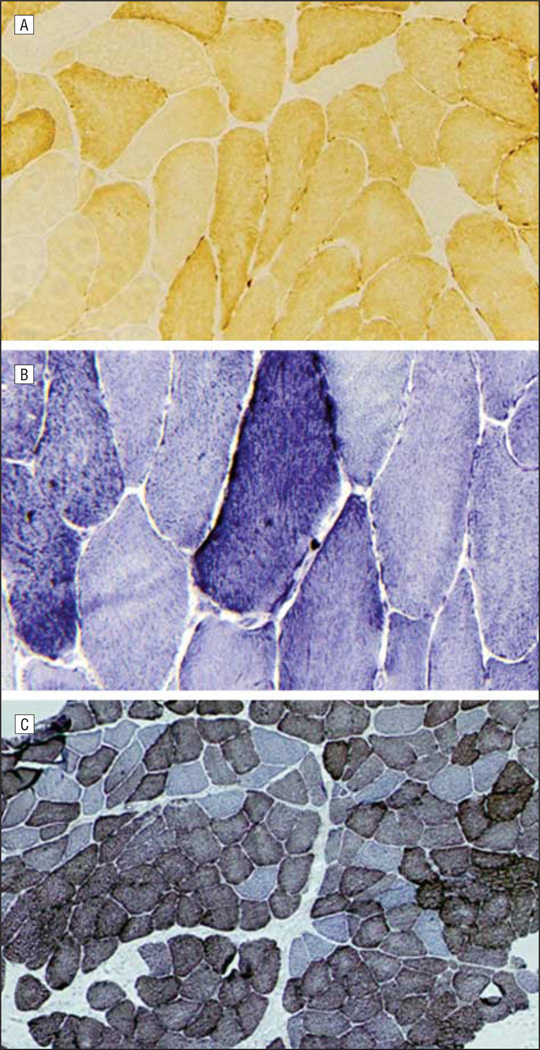
Laboratory studies revealed mildly elevated levels of serum creatine kinase (702 U/L; normal range, 30–220 U/L [to convert to microkatals per liter, multiply by 0.0167]) and venous lactate (2.3 mmol/L; normal range, 0.2–2.2 mmol/L [to convert to milligrams per deciliter, divide by 0.111]). Standard histological studies of a muscle biopsy performed at age 61 years showed chronic myopathy, mild neurogenic abnormalities, 10% of fibers with decreased or no cytochrome-c oxidase histochemical activity, and 3% of fibers with excessive subsarcolemmal succinate dehydrogenase staining. With cytochrome-c oxidase/succinate dehydrogenase combination staining, approximately half of the cytochrome-c oxidase–deficient fibers appeared blue, and scattered cytochrome-c oxidase–positive fibers showed increased subsarcolemmal succinate dehydrogenase staining (Figure 1). Muscle biochemistry results showed normal activities of all mitochondrial respiratory chain complexes and citrate synthase.
Figure 1.
Histochemical staining of the muscle biopsy showing (A) cytochrome-c oxidase–negative (original magnification ×200), (B) succinate dehydrogenase hyperreactive (original magnification ×400), and (C) ragged-red (original magnification ×100) fibers.
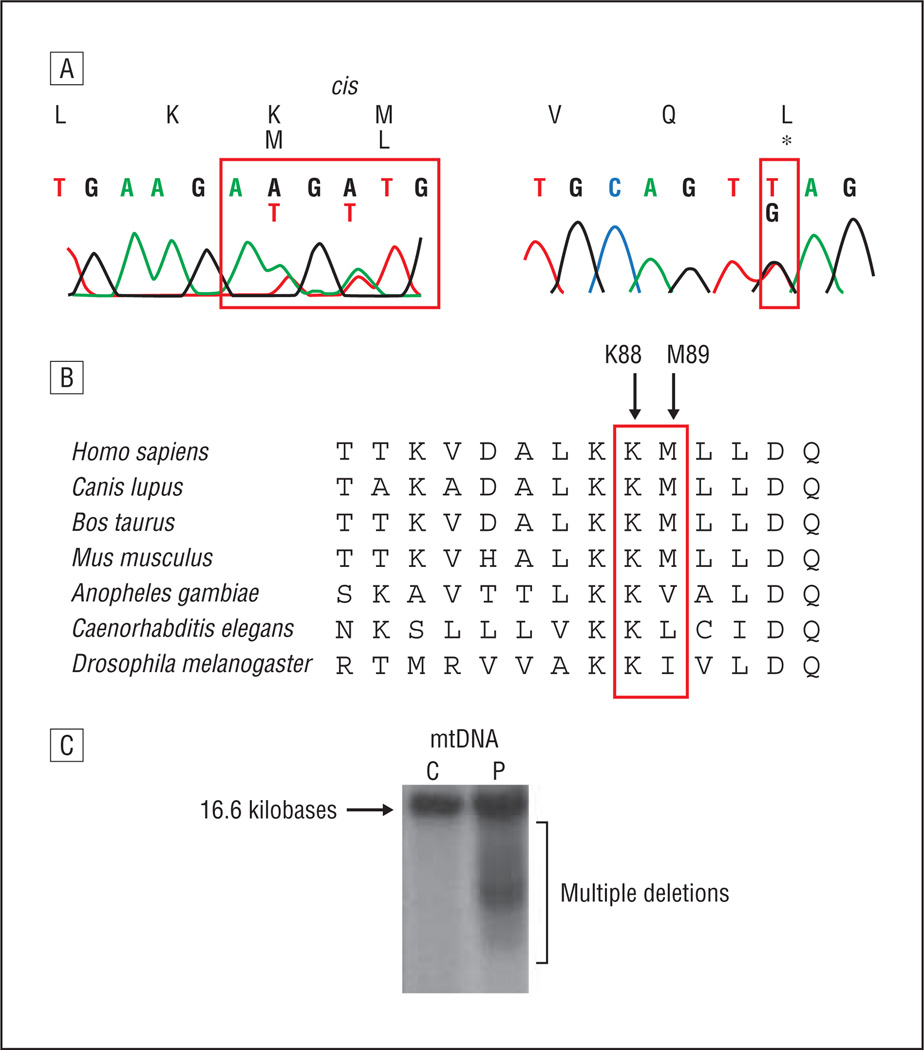
DNA extracted from skeletal muscle and fibroblasts using real-time polymerase chain reaction revealed a normal concentration of mtDNA. Southern blot analysis revealed multiple deletions in muscle but not in fibroblasts (Figure 2). Mutations in the POLG gene were excluded by sequencing all exons and flanking introns.
Figure 2.
Sequence of MPV17 showing the c.263A>T, c.265A>T, and c.428T>G pathogenic variants (A); evolutionary conservation of sites of mutations p.LysMet88-89MetLeu (B); Southern blot analysis showing mitochondrial DNA (mtDNA) multiple deletions in muscle biopsy (C); C indicates control; P, patient.
Targeted exome sequencing was performed on whole-genome–amplified DNA obtained from the muscle of the patient. A solution hybrid capture method was used to isolate 4.3 megabases of targeted DNA that included the 16-kilobase mtDNA, as well as all coding and untranslated exons of 1381 nuclear genes, 1013 mitochondrial genes from the MitoCarta database,12 21 genes with recent strong evidence of mitochondrial association, and 347 additional genes, which were then sequenced on Illumina GA-II platform.13
After filtering out common variants with high allele frequency (exceeding 0.005) within dbSNP132 and the “1000 Genomes Project,” we detected 2 heterozygous variants, c.263A>T (p.Lys88Met) and c.265A>T (p.Met89Leu), present in exon 3 of the MPV17 gene. These amino acid residues showed high evolutionary conservation, with identical amino acids observed in 37/37 aligned vertebrate species (p.Lys88) and 35/37 aligned vertebrate species (p.Met89). The Polyphen algorithm (http://genetics.bwh.harvard.edu/pph/) predicted the first variant to be “probably damaging” and the second variant to be “benign.” These variants are in cis, based on co-occurrence in sequence reads, and were both present in maternal DNA. Using Sanger sequencing, we screened all 7 exons of MPV17, confirming the exome sequencing data and identifying the third nonsynonymous variant in exon 6, c.428T>G (p.L143*) (Figure 2), that was absent in the mother and is likely inherited from the father (DNA not available). This heterozygous variant was supported by the targeted exome sequence data (3/10 aligned reads), although the depth and quality of the reads were insufficient for us to confidently detect a heterozygous variant using automated algorithms to detect a heterozygous variant.
All 3 sequence variants are extremely rare. They were not observed in 628 individuals with low-pass whole-genome sequence data available in the 1000 Genomes Project,14 371 healthy individuals of European ancestry from the National Institute of Mental Health control collection with available whole-exome data, 5379 individuals with available whole-exome data from the Exome Sequencing Project,15 or 100 control subjects whose MPV17 gene was sequenced.
COMMENT
MPV17 is a mitochondrial inner-membrane protein with unknown function. Mutations in MPV17 have been reported in 29 patients with infantile-onset progressive liver dysfunction, hypoglycemia, failure to thrive, or neurological symptoms leading to early death in the absence of a liver transplant.8–10
The homozygous p.R50Q mutation, first described in a large consanguineous Southern Italian family,9 causes a neurohepatopathy with high incidence (1 in 1600 live births) in the Navajos of Arizona and Utah.16–18 The exclusion of a recent common ancestor between the Italian family and Navajo neurohepatopathy families by single-nucleotide polymorphism analysis suggested to us that the p.R50Q mutation causes divergent phenotypes in the 2 ethnic groups owing to environmental or genetic modifiers.18
We detected 2 deleterious variants in MPV17 in a patient with adult-onset neurohepatopathy-plus syndrome with multiple deletions of mtDNA in muscle. MPV17 is an inner mitochondrial membrane protein, which is predicted to contain 4 transmembrane (TM) segments. A putative protein kinase C phosphorylation site is predicted to reside between TM2 and TM3 and is the hot spot for most previously described mutations.11 One of the patient’s mutations affects 2 adjacent codons, resulting in p.LysMet88-89MetLeu substitutions within the protein kinase C phosphorylation site, whereas the second mutation, p.143Leu*, is predicted to truncate the protein in TM4. The pathogenicity of these missense mutations is supported by the report of another mutation of the same amino acid (p.Lys88Glu).11 Maternal DNA was shown to contain the missense mutations but not the p.143Leu mutation, thus supporting the compound heterozygosity of these variants. The pathogenicity of these mutations in our patient is also supported by studies of the Sym1 (homologue of MPV17) mutant yeast model that showed mtDNA rearrangements, impairment of mitochondrial bioenergetics, and morphologically abnormal mitochondria under stress conditions.19 Owing to the severity of the observed mutations in the patient and the well-established link between recessive MPV17 mutations and mtDNA depletion, we posit that these mutations cause the observed phenotype in the patient.
The disease presentation in our patient is both similar to and different from the disease presentations in previously described patients with MPV17 defects. It is similar to the juvenile form of Navajo neurohepatopathy because the neurological manifestations (namely, axonal sensory-motor neuropathy and PEO) preceded liver dysfunction in our patient. Our patient had other clinical manifestations (including gastrointestinal dysmotility, depression, and parkinsonism) that are also common features of POLG or ANT1 mutations with multiple mtDNA deletions.20–22 Thus, our report reveals that MPV17 mutations can cause an adult-onset disorder with clinical features overlapping those of other defects of intergenomic communication, which may lead to diagnostic difficulties. In our patient, the presence of multiple deletions of mtDNA in the skeletal muscle reinforces the notion that the MPV17 protein is critical for the maintenance of mtDNA integrity.
Acknowledgments
Funding/Support: Dr Garone is supported by the Associazione Malattie Metaboliche Congenite ereditarie. Dr Hirano is supported by National Institutes of Health grants R01HD056103, 1R01HD057543, and U54NS078059, by a Muscular Dystrophy Association grant, and by the Marriott Mitochondrial Disorder Clinical Research Fund.
Footnotes
Author Contributions: Study concept and design: Garone, Mootha, and Hirano. Acquisition of data: Garone, Calvo, Naini, Tanji, Mootha, and Hirano. Analysis and interpretation of data: Garone, Rubio, Calvo, Tanji, DiMauro, Mootha, and Hirano. Drafting of the manuscript: Garone, Rubio, and Hirano. Critical revision of the manuscript for important intellectual content: Calvo, Naini, Tanji, Di-Mauro, Mootha, and Hirano. Statistical analysis: Calvo and Mootha. Obtained funding: Mootha and Hirano. Administrative, technical, and material support: Garone, Rubio, Naini, Tanji, Mootha, and Hirano. Study supervision: Naini, DiMauro, Mootha, and Hirano.
Financial Disclosure: None reported.
Additional Contributions: We are grateful to the patient and his relatives for participating in this study.
REFERENCES
- 1.Spinazzola A, Zeviani M. Mitochondrial diseases: a cross-talk between mitochondrial and nuclear genomes. Adv Exp Med Biol. 2009;652:69–84. doi: 10.1007/978-90-481-2813-6_6. [DOI] [PubMed] [Google Scholar]
- 2.Hirano M, Marti R, Ferreiro-Barros C, et al. Defects of intergenomic communication: autosomal disorders that cause multiple deletions and depletion of mitochondrial DNA. Semin Cell Dev Biol. 2001;12(6):417–427. doi: 10.1006/scdb.2001.0279. [DOI] [PubMed] [Google Scholar]
- 3.Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. 1999;283(5402):689–692. doi: 10.1126/science.283.5402.689. [DOI] [PubMed] [Google Scholar]
- 4.Naviaux RK, Nguyen KV. POLG mutations associated with Alpers’ syndrome and mitochondrial DNA depletion. Ann Neurol. 2004;55(5):706–712. doi: 10.1002/ana.20079. [DOI] [PubMed] [Google Scholar]
- 5.Spelbrink JN, Li FY, Tiranti V, et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat Genet. 2001;28(3):223–231. doi: 10.1038/90058. [DOI] [PubMed] [Google Scholar]
- 6.Tyynismaa H, Sun R, Ahola-Erkkilä S, et al. Thymidine kinase 2 mutations in autosomal recessive progressive external ophthalmoplegia with multiple mitochondrial DNA deletions. Hum Mol Genet. 2012;21(1):66–75. doi: 10.1093/hmg/ddr438. [DOI] [PubMed] [Google Scholar]
- 7.Fratter C, Raman P, Alston CL, et al. RRM2B mutations are frequent in familial PEO with multiple mtDNA deletions. Neurology. 2011;76(23):2032–2034. doi: 10.1212/WNL.0b013e31821e558b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinazzola A, Santer R, Akman OH, et al. Hepatocerebral form of mitochondrial DNA depletion syndrome: novel MPV17 mutations. Arch Neurol. 2008;65(8):1108–1113. doi: 10.1001/archneur.65.8.1108. [DOI] [PubMed] [Google Scholar]
- 9.Spinazzola A, Viscomi C, Fernandez-Vizarra E, et al. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat Genet. 2006;38(5):570–575. doi: 10.1038/ng1765. [DOI] [PubMed] [Google Scholar]
- 10.Wong LJ, Brunetti-Pierri N, Zhang Q, et al. Mutations in the MPV17 gene are responsible for rapidly progressive liver failure in infancy. Hepatology. 2007;46(4):1218–1227. doi: 10.1002/hep.21799. [DOI] [PubMed] [Google Scholar]
- 11.El-Hattab AW, Li FY, Schmitt E, Zhang S, Craigen WJ, Wong LJ. MPV17-associated hepatocerebral mitochondrial DNA depletion syndrome: new patients and novel mutations. Mol Genet Metab. 2010;99(3):300–308. doi: 10.1016/j.ymgme.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Pagliarini DJ, Calvo SE, Chang B, et al. Amitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134(1):112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvo SE, Compton AG, Hershman SG, et al. Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci Transl Med. 2012;4(118):118ra10. doi: 10.1126/scitranslmed.3003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing [published correction appears in Nature. 2011 May;473(7348):544] Nature. 2010;467(7319):1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NHLBI Exome Sequencing Project (ESP): exome variant server. National Heart, Lung, and Blood Institute (NHLBI) website. [Accessed January 1, 2012]; http://evs.gs.washington.edu/EVS/.
- 16.Karadimas CL, Vu TH, Holve SA, et al. Navajo neurohepatopathy is caused by a mutation in the MPV17 gene. Am J Hum Genet. 2006;79(3):544–548. doi: 10.1086/506913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holve S, Hu D, Shub M, Tyson RW, Sokol RJ. Liver disease in Navajo neuropathy. J Pediatr. 1999;135(4):482–493. doi: 10.1016/s0022-3476(99)70172-1. [DOI] [PubMed] [Google Scholar]
- 18.Vu TH, Tanji K, Holve SA, et al. Navajo neurohepatopathy: a mitochondrial DNA depletion syndrome? Hepatology. 2001;34(1):116–120. doi: 10.1053/jhep.2001.25921. [DOI] [PubMed] [Google Scholar]
- 19.Karasawa M, Zwacka RM, Reuter A, et al. The human homolog of the glomerulosclerosis gene Mpv17: structure and genomic organization. Hum Mol Genet. 1993;2(11):1829–1834. doi: 10.1093/hmg/2.11.1829. [DOI] [PubMed] [Google Scholar]
- 20.Davidzon G, Greene P, Mancuso M, et al. Early-onset familial parkinsonism due to POLG mutations. Ann Neurol. 2006;59(5):859–862. doi: 10.1002/ana.20831. [DOI] [PubMed] [Google Scholar]
- 21.Luoma P, Melberg A, Rinne JO, et al. Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet. 2004;364(9437):875–882. doi: 10.1016/S0140-6736(04)16983-3. [DOI] [PubMed] [Google Scholar]
- 22.Van Goethem G, Schwartz M, Löfgren A, Dermaut B, Van Broeckhoven C, Vissing J. Novel POLG mutations in progressive external ophthalmoplegia mimicking mitochondrial neurogastrointestinal encephalomyopathy. Eur J Hum Genet. 2003;11(7):547–549. doi: 10.1038/sj.ejhg.5201002. [DOI] [PubMed] [Google Scholar]