Abstract
Significance: Autophagy is a highly conserved eukaryotic cellular recycling process. Through the degradation of cytoplasmic organelles, proteins, and macromolecules, and the recycling of the breakdown products, autophagy plays important roles in cell survival and maintenance. Accordingly, dysfunction of this process contributes to the pathologies of many human diseases. Recent Advances: Extensive research is currently being done to better understand the process of autophagy. In this review, we describe current knowledge of the morphology, molecular mechanism, and regulation of mammalian autophagy. Critical Issues: At the mechanistic and regulatory levels, there are still many unanswered questions and points of confusion that have yet to be resolved. Future Directions: Through further research, a more complete and accurate picture of the molecular mechanism and regulation of autophagy will not only strengthen our understanding of this significant cellular process, but will aid in the development of new treatments for human diseases in which autophagy is not functioning properly. Antioxid. Redox Signal. 20, 460–473.
Introduction
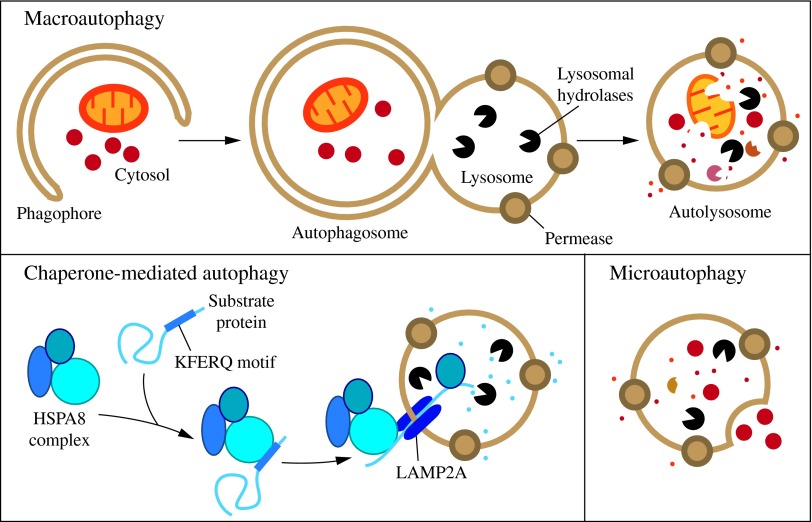
Autophagy is a cellular degradation and recycling process that is highly conserved in all eukaryotes. In mammalian cells, there are three primary types of autophagy: microautophagy, macroautophagy, and chaperone-mediated autophagy (CMA). While each is morphologically distinct, all three culminate in the delivery of cargo to the lysosome for degradation and recycling (Fig. 1) (154). During microautophagy, invaginations or protrusions of the lysosomal membrane are used to capture cargo (101). Uptake occurs directly at the limiting membrane of the lysosome, and can include intact organelles. CMA differs from microautophagy in that it does not use membranous structures to sequester cargo, but instead uses chaperones to identify cargo proteins that contain a particular pentapeptide motif; these substrates are then unfolded and translocated individually directly across the lysosomal membrane (95). In contrast to microautophagy and CMA, macroautophagy involves sequestration of the cargo away from the lysosome. In this case, de novo synthesis of double-membrane vesicles—autophagosomes—is used to sequester cargo and subsequently transport it to the lysosome (157).
FIG. 1.
Three types of autophagy in mammalian cells. Macroautophagy relies on de novo formation of cytosolic double-membrane vesicles, autophagosomes, to sequester and transport cargo to the lysosome. Chaperone-mediated autophagy transports individual unfolded proteins directly across the lysosomal membrane. Microautophagy involves the direct uptake of cargo through invagination of the lysosomal membrane. All three types of autophagy lead to degradation of cargo and release of the breakdown products back into the cytosol for reuse by the cell. See the text for details. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Of the three types of autophagy, macroautophagy is the best studied. Macroautophagy occurs at a low level constitutively and can be further induced under stress conditions, such as nutrient or energy starvation, to degrade cytoplasmic material into metabolites that can be used in biosynthetic processes or energy production, allowing for cell survival (157). Under normal growing conditions, macroautophagy aids in cellular maintenance by specifically degrading damaged or superfluous organelles (154). Thus, macroautophagy is primarily a cytoprotective mechanism; however, excessive self-degradation can be deleterious. Accordingly, autophagic dysfunction is associated with a variety of human pathologies, including lung, liver, and heart disease, neurodegeneration, myopathies, cancer, ageing, and metabolic diseases, such as diabetes (148).
This review provides an overview of the current state of knowledge of autophagy, with an emphasis on the morphology, molecular mechanism, regulation, and selectivity of mammalian macroautophagy.
Microautophagy
Microautophagy refers to a process by which cytoplasmic contents enter the lysosome through an invagination or deformation of the lysosomal membrane (94). In one early study, isolated rat liver lysosomes were shown by electron microscopy to engulf Percoll particles in vitro by way of protrusions or cup-like invaginations of the lysosomal membrane, forming vesicles within the lysosome. Some of these particles were seen free-floating within the lysosomal lumen, presumably through rupture/lysis of the vesicles (93). A very recent study presented evidence that a microautophagy-like process called endosomal microautophagy transports soluble cytosolic proteins to the vesicles of late endosomal multivesicular bodies (123). Due to the limited number of tools available for the study of microautophagy, we know relatively little about this process, including its regulation and possible roles in human health and disease (101).
Chaperone-Mediated Autophagy
A second type of autophagy, which has so far only been described in mammalian cells, is CMA. Unlike microautophagy and macroautophagy, which can both nonspecifically engulf bulk cytoplasm, CMA is highly specific; common to all CMA substrates is a pentapeptide targeting motif biochemically related to KFERQ (24). Based on sequence analysis and immunoprecipitation experiments, it is estimated that ∼30% of cytosolic proteins contain such a sequence (16). Target proteins containing the KFERQ consensus motif are unfolded through the action of cytosolic chaperones and translocated directly across the lysosomal membrane where they are degraded in the lumen (114). CMA degrades a wide range of substrate proteins, including certain glycolytic enzymes, transcription factors and their inhibitors, calcium and lipid binding proteins, proteasome subunits, and proteins involved in vesicular trafficking (3).
During CMA, the KFERQ motif is recognized by the heat shock 70 kDa protein 8 (HSPA8/HSC70), as well as other cochaperones (Fig. 1) (17). HSPA8 can then deliver the substrate to the lysosomal membrane, where it likely assists in substrate unfolding (1). At the lysosomal membrane, the substrate binds to monomers of the CMA substrate receptor, lysosomal-associated membrane protein 2A (LAMP2A) (18). This substrate-receptor binding leads to the multimerization of LAMP2A (8, 18). As the multimeric translocation complex forms, subunits of the complex are stabilized on the lumenal side of the lysosomal membrane by HSP90 (8). Following translocation of the substrate into the lysosomal lumen—in part, through the action of lumenal HSPA8—the translocation complex is actively disassembled by cytosolic HSPA8, and LAMP2A returns to a monomeric state where it can bind new substrate and initiate a new round of translocation (8).
Regulation of the translocation process occurs at the level of substrate binding to LAMP2A, which is rate-limiting for CMA (19). Changes in LAMP2A levels at the lysosomal membrane modulate the level of CMA activity and primarily result from changes in degradation and organization of LAMP2A rather than synthesis of the protein (8, 19, 20). Some data support the idea that redistribution of LAMP2A between fluid regions of the lysosomal membrane and lipid-enriched microdomains influences the degradation of LAMP2A (65). While much is known about translocation regulation, far less is clear about overall CMA regulation (3). Mild oxidative stress (66), protein-damaging toxins (21), and extended periods of nutrient deprivation all upregulate CMA (6, 22), but the intracellular signaling pathways that facilitate this change are not fully understood (3).
It is suggested that HSPA8 and LAMP2A also participate in a type of macroautophagy called chaperone-assisted selective autophagy. During this process, chaperones aid in the clearance of selectively ubiquitinated organelles and protein complexes (76). Association of these ubiquitinated targets with receptors, such as SQSTM1/p62 and NBR1, and with enzymes, including HDAC6, allows for recognition by the macroautophagy machinery, delivery to the lysosome, and degradation (74, 76, 81).
Macroautophagy
Basic morphological progression
As stated above, macroautophagy is distinct from microautophagy and CMA in part because the initial site of sequestration occurs away from the limiting membrane of the lysosome, and involves the formation of cytosolic vesicles that transport the cargo to this organelle. The morphological feature that makes macroautophagy unique from other intracellular vesicle-mediated trafficking processes is that the sequestering vesicles, termed autophagosomes, form de novo rather than through membrane budding; that is, the autophagosome forms by expansion, and does not bud from a preexisting organelle, already containing cargo (152). Upon induction of macroautophagy in yeast, formation of autophagosomes begins at a single perivacuolar site called the phagophore assembly site (PAS) (14). In mammalian systems, autophagosome generation is initiated at multiple sites throughout the cytoplasm rather than at a single PAS (14, 57). Several studies suggest that endoplasmic reticulum (ER)-associated structures called omegasomes may serve as initiation sites in mammals (41, 155).
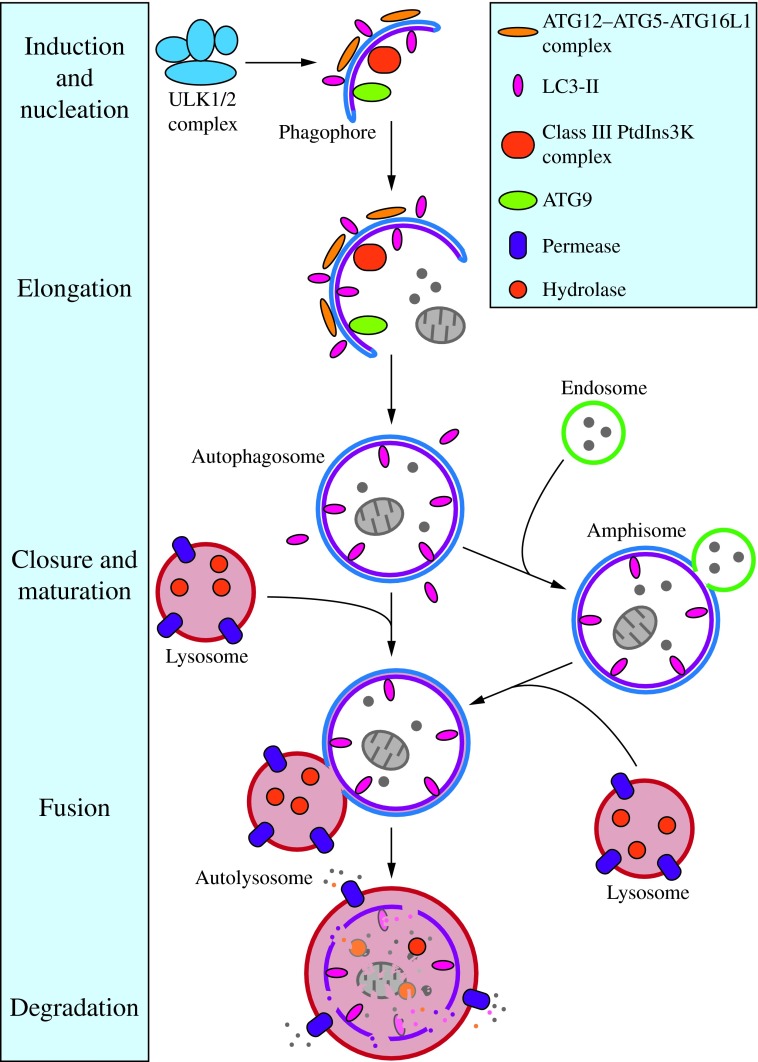
Following initiation, the membrane begins to expand. At this stage, it is called a phagophore, which is the primary double-membrane sequestering compartment (Fig. 2) (43). The source of membrane that makes up the phagophore is highly debated, but various studies have implicated the plasma membrane (120, 121), ER (41, 155), Golgi complex (134), and mitochondria (36) as possible sources (107, 145). As the phagophore expands, the membrane bends to ultimately generate a spherical autophagosome. The factors that drive curvature of the membrane during nonspecific macroautophagy are not known. In the case of selective macroautophagy, the membrane appears to essentially wrap around the cargo; thus, adjusting to fit the specific target (102). Upon completion, the phagophore fully surrounds its cargo and fuses to form the double-membrane autophagosome. The size of the autophagosome varies based on organism and cargo type. For example, the diameter of autophagosomes ranges from ∼0.4 to 0.9 μm in yeast, and 0.5 to 1.5 μm in mammals (104, 117, 127, 136).
FIG. 2.
Morphology of macroautophagy. Nucleation of the phagophore occurs following induction by the ULK1/2 complex. Elongation of the phagophore is aided by the ATG12–ATG5-ATG16L1 complex, the class III PtdIns3K complex, LC3-II, and ATG9. Eventually, the expanding membrane closes around its cargo to form an autophagosome and LC3-II is cleaved from the outer membrane of this structure. The outer membrane of the autophagosome will then fuse with the lysosomal membrane to form an autolysosome. In some instances, the autophagosome may fuse with an endosome, forming an amphisome, before fusing with the lysosome. The contents of the autolysosome are then degraded and exported back into the cytoplasm for reuse by the cell. See the text for details. This figure was modified from Figure 1 in Yang and Klionsky (153). ATG, autophagy-related; PtdIns3K, phosphatidylinositol 3-kinase; ULK, unc-51-like kinase (C. elegans). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Once the autophagosome is formed, it must deliver its cargo to the lysosome in mammals or the functionally related vacuole in yeast and plants. As it reaches its destination, the outer membrane of the autophagosome will fuse with the lysosomal/vacuolar membrane. In yeast and plants, due to the relatively large size of the vacuole, this releases a single-membrane autophagic body into the vacuolar lumen. Fusion between autophagosomes and lysosomes in mammals, however, does not generate autophagic bodies (23). The product of fusion between an autophagosome and lysosome in mammalian cells is referred to as an autolysosome (152). Exposed to the acidic lumen and resident hydrolases of the lysosome/vacuole, the autophagosome inner membrane and, subsequently, the autophagic cargo are degraded and the component parts are exported back into the cytoplasm through lysosomal permeases for use by the cell in biosynthetic processes or to generate energy (157). In mammals, macroautophagy often converges with the endocytic pathway. Hence, before fusion with lysosomes, autophagosomes may also fuse with early or late endosomes to form amphisomes, which then fuse with lysosomes to become autolysosomes (9, 140).
Macroautophagy machinery
Induction
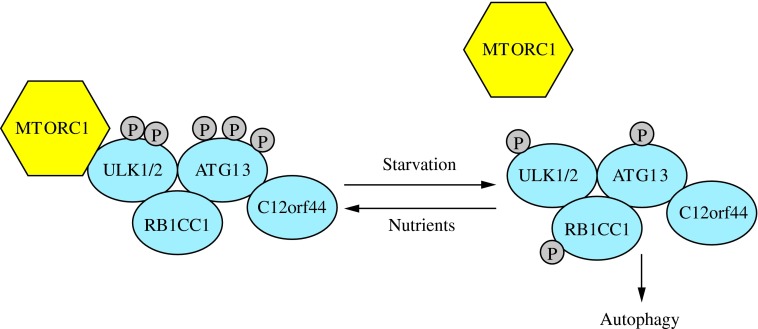
In yeast macroautophagy, induction of autophagosome formation is regulated by the Atg1-Atg13-Atg17-Atg31-Atg29 kinase complex (43). In mammalian cells, this complex is made up of an Atg1 homolog from the Unc-51-like kinase family (either ULK1 or ULK2), the mammalian homolog of Atg13 (ATG13), and RB1-inducible coiled-coil 1 (RB1CC1/FIP200), which is required for the induction of macroautophagy and may be an ortholog of yeast Atg17 (Fig. 3) (33, 38, 47, 62). Also in this complex is C12orf44/ATG101, which binds directly to ATG13, is essential for macroautophagy, and has no known yeast homolog (48, 100). The mammalian ULK1/2-ATG13-RB1CC1 complex is stable and forms regardless of nutrient status (47, 62).
FIG. 3.
The induction complex consists of ULK1/2, ATG13, RB1CC1, and C12orf44. Under nutrient-rich conditions, MTORC1 associates with the complex and inactivates ULK1/2 and ATG13 through phosphorylation. During starvation, MTORC1 dissociates from the complex and ATG13 and ULK1/2 become partially dephosphorylated by as yet unidentified phosphatases, allowing the complex to induce macroautophagy. RB1CC1/FIP200 and C12orf44/ATG101 are also associated with the induction complex and are essential for macroautophagy. RB1CC1/FIP200 may be the ortholog of yeast Atg17, whereas the function of C12orf44/ATG101 is not known. This figure was modified from Figure 1 in Yang and Klionsky (154). MTORC1, mechanistic target of rapamycin complex 1; RB1CC1, RB1-inducible coiled-coil 1. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The association of the mechanistic target of rapamycin complex 1 (MTORC1) with the induction complex is, however, influenced by nutrient status. Under nutrient-rich conditions, MTORC1 associates with the complex, but dissociates upon nutrient starvation (47). When MTORC1 is complex-associated, it phosphorylates ULK1/2 and ATG13, inactivating them. However, when cells are treated with rapamycin or starved for nutrients, MTORC1 dissociates from the induction complex, resulting in dephosphorylation at these sites and induction of macroautophagy (47, 62). The phosphatases responsible at this stage are as yet unknown. The involvement of MTORC1 in the regulation of macroautophagy is an active area of research and will be discussed in greater detail below, as well as in another review in this Forum series.
Nucleation
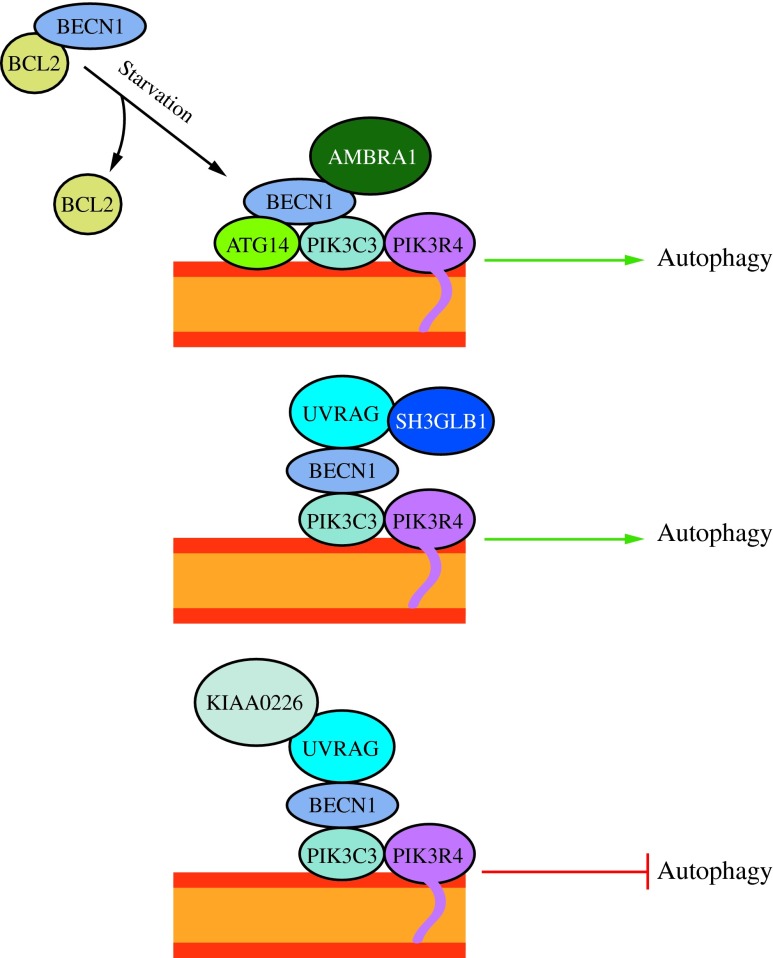
The next complex recruited to the putative site of autophagosome formation is the ATG14-containing class III phosphatidylinositol 3-kinase (PtdIns3K) complex (57). The PtdIns3K complex generates PtdIns3P, which is required for macroautophagy in both yeast and mammals (13). This complex is involved in the nucleation of the phagophore and consists of PIK3C3/VPS34, PIK3R4/p150 (Vps15 in yeast), and BECN1 (Vps30/Atg6 in yeast) (Fig. 4) (32, 55, 67, 87, 151). As in yeast, this complex can either function in macroautophagy by associating with ATG14 or in the endocytic pathway through an interaction with UVRAG (an ortholog of yeast Vps38) (55, 85, 132). While some data suggest that the UVRAG-associated PtdIns3K complex is involved in autophagosome formation (85), other reports suggest that it may act in later stages of autophagosome development (86). Another study found that siRNA knockdown of UVRAG in HeLa cells does not affect macroautophagy (55). It is clear that further work is required to fully understand the role of UVRAG in the endocytic and macroautophagic pathways.
FIG. 4.
The activity of the class III PtdIns3K complex is regulated by subunit composition. The ATG14 complex (ATG14-BECN1-PIK3C3-PIK3R4) is required for macroautophagy. It can be positively regulated by AMBRA1 and negatively regulated by BCL2 binding to BECN1 and preventing association with the complex. The UVRAG (UVRAG-BECN1-PIK3C3-PIK3R4) complex is involved in the endocytic pathway and also participates in macroautophagy. SH3GLB1/Bif-1 positively regulates this complex by binding UVRAG. The KIAA0226/Rubicon complex (KIAA0226-UVRAG-BECN1-PIK3C3-PIK3R4) negatively regulates macroautophagy. This figure was modified from Figure 1 in Yang and Klionsky (154). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Regulation of the PtdIns3K complex occurs largely through proteins that interact with BECN1, which is essential for macroautophagy (87, 160). The antiapoptotic protein BCL2 binds BECN1 and prevents its interaction with PIK3C3; thus, inhibiting macroautophagy (32, 88, 116). Another BECN1-binding protein, KIAA0226/Rubicon, inhibits PIK3C3 activity in UVRAG-associated PtdIns3K complexes (Fig. 4) (96, 163). Two positive regulators of the PtdIns3K complex are AMBRA1 (which directly binds BECN1) and SH3GLB1/Bif-1 (which interacts with BECN1 through UVRAG, and may be involved in generating membrane curvature) (29, 133, 135). Very little is known, however, about upstream events regulating the constituents of the various PtdIns3K complexes.
In yeast, there are several proteins that bind to PtdIns3P generated by the Vps34 complex. Of these, Atg18 and Atg21 have a role in macroautophagy and localize to the PAS (78). Mammalian cells express two Atg18 orthologs, WIPI1 and WIPI2, which are also involved in macroautophagy and associate with phagophores during amino acid starvation by binding to PtdIns3P (60, 118, 119). Another PtdIns3P-binding protein in mammalian cells is the zinc finger, FYVE domain containing 1 (ZFYVE1/DFCP1), which associates with PtdIns3P-enriched omegasomes (7). The precise functions of WIPI1/2 and ZFYVE1 in macroautophagy are still unknown.
Elongation
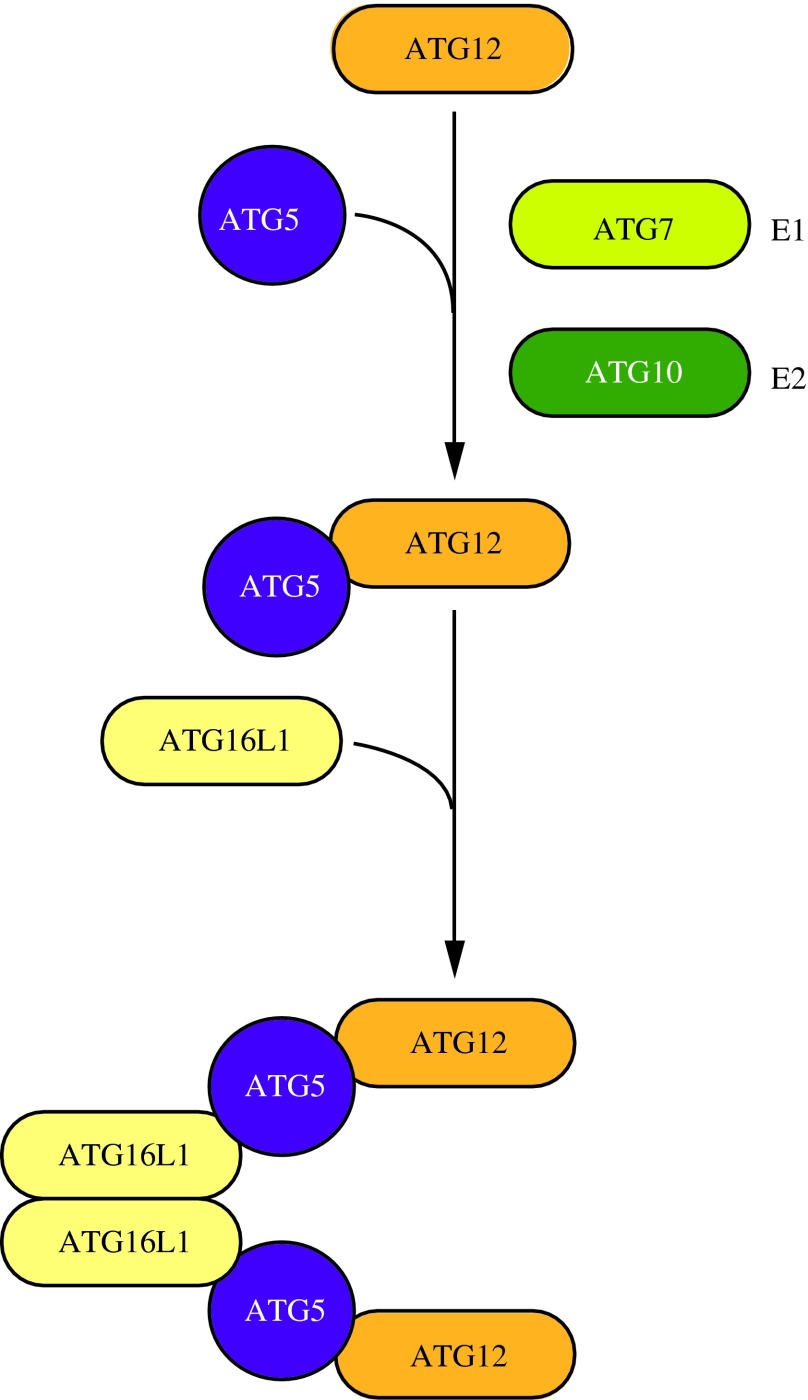
In both yeast and mammals, there are two conjugation systems involving ubiquitin-like (UBL) proteins that contribute to the expansion of the phagophore (145). The first system involves formation of the Atg12–Atg5-Atg16 complex. In yeast, the UBL protein Atg12 is covalently conjugated to Atg5 in a manner dependent on the E1 activating enzyme Atg7 and the E2 conjugating enzyme Atg10 (70, 113, 129). This process differs from ubiquitination in that the conjugation of Atg12 to Atg5 is irreversible and does not require an E3 ligase enzyme (34). Following Atg12–Atg5 conjugation, Atg16 binds to Atg5 noncovalently and dimerizes to form a larger complex (79). Mammalian orthologs of this system, ATG5, ATG12 and ATG16L1, have been identified, and function as in yeast (Fig. 5) (105, 113). The mammalian ATG12–ATG5-ATG16L1 complex associates with the phagophore membrane, but dissociates following autophagosome completion (105, 106). One way in which this complex is regulated is through the Golgi protein RAB33A, which can bind to and inhibit ATG16L1 (58). Additionally, ATG5, ATG7, and ATG12 are inhibited through acetylation by the acetyltransferase KAT2B/p300 (82).
FIG. 5.
ATG12–ATG5-ATG16L1 conjugation complex. The ubiquitin-like protein ATG12 is irreversibly conjugated to ATG5 in an ATG7- and ATG10-dependent manner. ATG7 and ATG10 function as E1 activating and E2 conjugating enzymes, respectively. The ATG12–ATG5 conjugate binds ATG16L1 through ATG5. ATG16L1 dimerizes and allows association with the phagophore, promoting membrane expansion. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
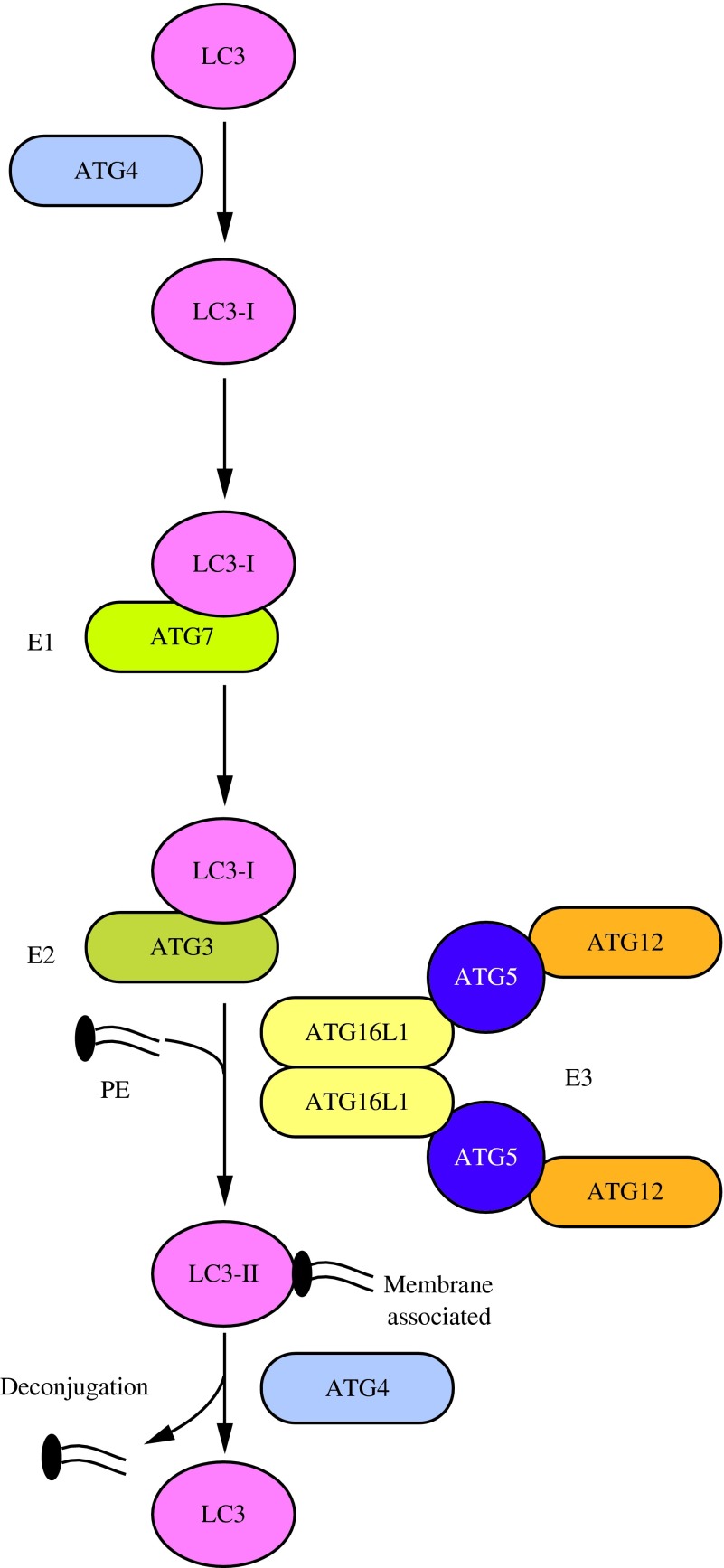
The second UBL system involved in phagophore expansion is the Atg8/LC3 system. This conjugation pathway in yeast begins with processing of Atg8 by the cysteine protease Atg4 to expose a glycine residue at the C terminus of Atg8 (73). The E1-like enzyme Atg7 activates the processed Atg8 and transfers it to the E2-like enzyme Atg3 (52). Finally, the C-terminal glycine of Atg8 is covalently conjugated to the lipid phosphatidylethanolamine (PE). The Atg12–Atg5 conjugate, which may act as an E3 ligase, facilitates this final step (30, 37, 52). Atg8–PE is membrane-associated, but can be released from membranes as a result of a second Atg4-mediated cleavage (73). The mechanism of regulation of the second Atg4-dependent processing event, referred to as deconjugation, is not known; however, this appears to be an important step in macroautophagy because defects in cleavage result in partial autophagic dysfunction (111).
Mammalian homologs of the Atg8/LC3 system function much like their yeast counterparts (Fig. 6) (34). Unlike yeast, which have only one Atg4 and one Atg8, mammals have four isoforms of ATG4 and several Atg8-like proteins, the latter of which are divided into the LC3 and GABARAP subfamilies (44, 91, 146). Whereas both subfamilies can localize with autophagosomes (64), it has been proposed that they function at different steps in phagophore elongation and completion, with the LC3 subfamily acting before the GABARAP subfamily (146). Among the Atg8-like proteins in mammals, LC3 has been the best characterized. The ATG4-processed form of LC3 is referred to as LC3-I and the PE-conjugated form is called LC3-II (34). Lipidation of LC3 in mammalian cells is accelerated under conditions of nutrient starvation or other types of stress (63). While the mechanism of the conjugation system of Atg8/LC3 is well understood, the precise role of Atg8/LC3 in macroautophagy is still unclear. Atg8, and to some extent LC3 (92, 138), shows a substantial increase in synthesis during macroautophagy induction (72), and in yeast this is a determining factor in autophagosome size (149).
FIG. 6.
The LC3 conjugation system. LC3 is processed by ATG4 to reveal a C-terminal glycine (LC3-I). ATG7, an E1-like enzyme, activates LC3-I and transfers it to the E2-like enzyme ATG3. The ATG12–ATG5-ATG16L1 complex may participate as an E3 ligase in the conjugation of PE to LC3-I to create LC3-II, which can associate with the phagophore. LC3-II can subsequently be cleaved by ATG4 to release LC3 (deconjugation). PE, phosphatidylethanolamine. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Another protein thought to function in elongation of the phagophore is the transmembrane protein ATG9. In yeast, Atg9 may cycle between the PAS and peripheral sites (122). These peripheral sites are referred to as Atg9 reservoirs or tubulovesicular clusters (TVCs). The TVCs may be direct membrane precursors to the PAS, and thus, to phagophores (90, 110). The movement of Atg9 is dependent on the Atg1-kinase complex, as well as multimerization of Atg9 (42, 122). The abilities of Atg9 to traffic and multimerize are necessary for autophagosome formation, suggesting that these properties of Atg9 contribute to a role for this protein in recruiting membrane to the expanding phagophore (42, 122).
The mammalian homolog of Atg9 (ATG9) is also seen to shift localization within the cell and is proposed to have a similar role in membrane recruitment (159). Under nutrient-rich conditions, ATG9 localizes to the trans-Golgi network and late endosomes (159). When cells are starved for nutrients, however, ATG9 colocalizes with autophagosomal markers (159). This cycling to autophagosomes is dependent on both ULK1 and PtdIns3K activity and is negatively regulated by MAPK14/p38α (144, 159). The exact functions of ATG9 in the cell, and how the ULK1 complex regulates ATG9 movement, are poorly understood.
Autophagosome completion and fusion
In what is perhaps the least understood step of macroautophagy, the expanding phagophore must eventually mature and close to form a completed autophagosome, which traffics to and fuses with an endosome and/or lysosome, becoming an autolysosome. Movement of autophagosomes to lysosomes is dependent on microtubules (108). Fusion of autophagosomes with endosomes involves the protein VTIlB (5). UVRAG, which can associate with the PtdIns3K complex, can activate the GTPase RAB7, which promotes fusion with lysosomes (59, 86). It has also been suggested that components of the SNARE machinery, such as VAM7 and VAM9, have a role in fusion (28, 31). Recent work has identified another SNARE, syntaxin 17, which localizes to completed autophagosomes and is required for fusion with the endosome/lysosome through an interaction with SNAP29 and the endosomal/lysosomal SNARE VAMP8 (56).
Regulation of macroautophagy
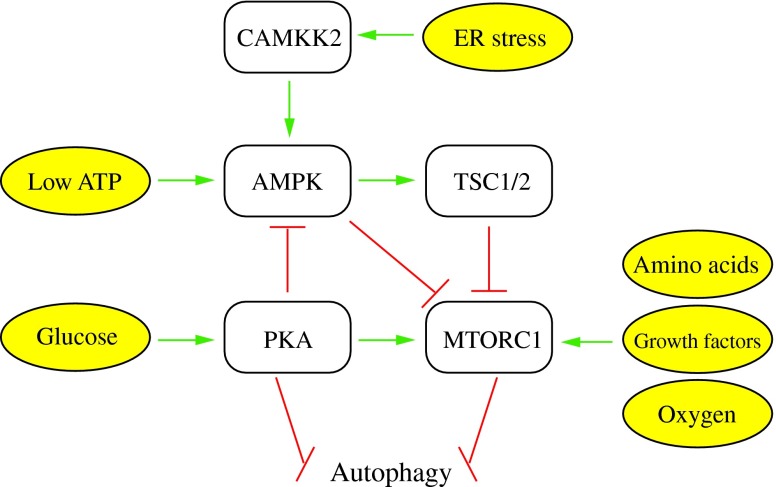
Macroautophagy helps cells respond to a wide range of extra- and intracellular stresses including nutrient starvation, the presence/absence of insulin and other growth factors, hypoxia, and ER stress (Fig. 7) (43). Two pathways involved in nutrient starvation are regulated by the cAMP-dependent protein kinase A (PKA) and TOR pathways, which sense primarily carbon and nitrogen, respectively (130). In yeast, PKA is an inhibitor of macroautophagy under nutrient-rich conditions (12). In mammals, this inhibition occurs at least partially through the phosphorylation of LC3 by PKA (15). For its role in nitrogen sensing, MTORC1 is positively regulated by the presence of amino acids. Amino acids regulate RAG proteins, RAS-related small GTPases that activate MTORC1 (68, 124). There is thought to be some crosstalk between the carbon- and nitrogen-sensing pathways, based on studies that demonstrated that mammalian PKA can phosphorylate, and thus activate, MTORC1 (11, 97). PKA can also indirectly activate MTORC1 through inactivation of the AMP-activated protein kinase (AMPK) (26).
FIG. 7.
Regulation of macroautophagy. Three of the major kinases that regulate macroautophagy are PKA, AMPK, and MTORC1. These kinases, along with proteins such as TSC1/2 and CAMKK2/CaMKKβ, respond to a variety of intracellular and extracellular signals to regulate macroautophagy. Green arrows indicate activation of a target and red bars indicate inhibition of a target. See the text for details. This figure was modified from Figure 4 of Chen and Klionsky (14). PKA, cAMP-dependent protein kinase A; AMPK, AMP-activated protein kinase; CAMKK2/CaMKKβ, calcium/calmodulin-dependent protein kinase kinase 2, beta. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
AMPK is not simply a substrate of PKA. It is the major energy-sensing kinase in the cell and responds to intracellular AMP/ATP levels to regulate a variety of cellular processes, including macroautophagy (2, 99). AMP and ATP have opposite effects on the activity of AMPK, with AMP binding activating the kinase activity of AMPK (40). When activated by low energy levels, AMPK can phosphorylate and activate the TSC1/2 complex, which indirectly inhibits the activity of MTORC1 (53). Alternatively, AMPK can directly inhibit MTORC1 (35, 154). Several studies have also reported that AMPK can phosphorylate and activate ULK1 to induce macroautophagy (27, 71, 84, 128). The modulation of macroautophagy by energy sensing is conserved in yeast where Snf1, the yeast ortholog of AMPK, serves as a positive regulator (50, 143).
It has also been observed that an increase in cytosolic Ca2+ concentrations resulting from ER stress causes calcium/calmodulin-dependent protein kinase kinase 2, beta (CAMKK2/CaMKKβ) to activate AMPK and induce macroautophagy (49). Another way in which ER stress can induce macroautophagy is through unfolded protein response (UPR) signaling. Accumulation of unfolded proteins in the ER can be caused by a variety of cellular stressors, and induces macroautophagy in both yeast and mammals. However, the role of macroautophagy in response to ER stress seems to vary, with some studies reporting that it enhances cell survival, while others suggest that it may result in autophagic cell death (25, 43).
Additional signals that cause the induction of macroautophagy include hypoxia and the absence of growth factors. Even in the presence of adequate nutrients, the absence of growth factors leads to the induction of macroautophagy (89). Both growth factor concentrations and hypoxia regulate macroautophagy at least in part through MTORC1, and hypoxia can inhibit MTORC1 even in the presence of adequate nutrients and growth factors (2, 4). Given its complex regulation by a variety of cellular signaling pathways, the involvement of MTORC1 in the regulation of macroautophagy is a very intriguing and active area of research, and is discussed in greater detail in another review in this Forum series.
Selective macroautophagy and cellular maintenance
While nonspecific macroautophagy can be induced in response to nutrient or energy deprivation to enable cell survival, macroautophagy can also be highly specific, and in this mode functions more in cell maintenance and homeostasis (14, 54). Specific autophagic cargoes can include, but are not limited to peroxisomes, mitochondria, and ubiquitinated proteins (83, 139, 145).
The selective macroautophagic degradation of peroxisomes, termed pexophagy, is important for a majority of the turnover of peroxisomes under normal growth conditions (51). For example, in mouse livers, macroautophagy is responsible for degradation of 70%–80% of the peroxisomal mass (156). Peroxisomes can also be degraded under starvation conditions, during which they can be specifically recognized by autophagosomes through binding of LC3-II to PEX14, a component of the peroxisomal translocon complex found on the peroxisomal membrane (39). Given the role of peroxisomes in a variety of metabolic functions and the negative effects of peroxisomal dysfunction on human health, pexophagy has an important role in maintaining proper cellular physiology (139).
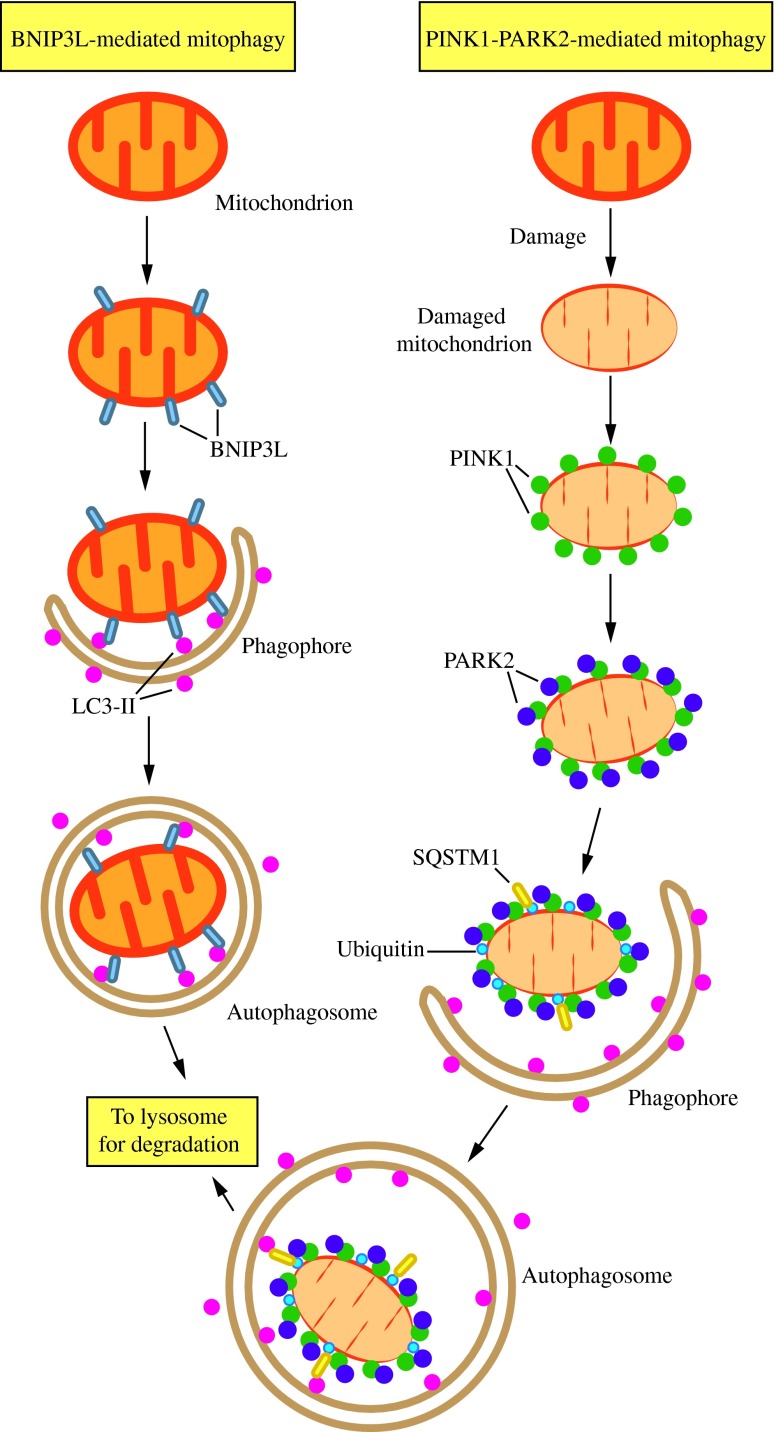
Mitophagy is another type of selective macroautophagy that involves the selective degradation of mitochondria, and has been shown to be important in mammals not only for steady-state turnover of these organelles (137), but also for the development of certain cell types and the clearance of damaged mitochondria (69, 80, 126). For example, in order for mammalian red blood cells to mature, mitophagy is used to remove mitochondria from the immature cells (80, 109, 161). During this process, it is thought that a mitochondrial outer membrane protein called BNIP3L/NIX interacts through a WXXL-like motif (also called the LC3-interacting region) with LC3 and GABARAP on the expanding phagophore, allowing for recognition (Fig. 8) (158).
FIG. 8.
Two mechanisms of mitophagy. Mitochondria are cleared from maturing red blood cells through a mechanism involving autophagic recognition of mitochondria through a BNIP3L–LC3 interaction. During removal of damaged mitochondria, PARK2 binds to PINK1 on the mitochondrial surface and ubiquitinates mitochondrial outer membrane proteins, which may then bind SQSTM1, a receptor that interacts with LC3. In either case, the interaction with LC3 leads to sequestration by the phagophore and eventual degradation. This figure was modified from Figure 2 of Youle and Narendra (158). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The clearance of damaged mitochondria, however, is thought to proceed in a slightly different way. In this case, the cytosolic E3 ubiquitin ligase PARK2/Parkin is recruited to damaged mitochondria by the mitochondrial outer membrane kinase PINK1, whereupon PARK2 ubiquitinates mitochondrial substrates, leading to mitophagy (158). In healthy mitochondria, PINK1 is imported into the mitochondrial inner membrane, and subsequent cleavage by the mitochondrial processing peptidase (PMPCB) and presenilin associated, rhomboid-like protease (PARL) leads to its eventual degradation. This prevents the accumulation of PINK1 on the mitochondrial outer membrane, which would otherwise lead to mitophagy of healthy mitochondria (61, 98). The genes encoding both PINK1 and PARK2 are mutated in autosomal recessive Parkinson disease (77, 142), emphasizing the importance of mitophagic clearance of damaged mitochondria in maintaining cellular and, thus, organismal health.
Another mechanism used by the cell to identify cargo for selective degradation by macroautophagy involves ubiquitination. The ubiquitin-binding protein SQSTM1/p62 targets intracellular bacteria for degradation by a specific type of macroautophagy called xenophagy (162). SQSTM1 is also important for the clearance of ubiquitinated protein aggregates by acting as an adaptor protein that interacts with LC3-II to target aggregates for macroautophagy-specific degradation in a process termed aggrephagy (10, 115, 141). NBR1 and OPTN are other receptors that function in targeting ubiquitinated proteins or pathogens to autophagosomes (75, 147).
Conclusions
Given the wide array of extra- and intracellular signals that can regulate autophagy and the range of possible cargos, it is not surprising to learn that autophagy has been implicated in various aspects of human health and pathophysiology. Several of these topics will be explored in depth in other reviews in this Forum series. One area that especially warrants further study is the regulatory network controlling macroautophagy. While several key regulators of macroautophagy have been identified, it is likely that many regulatory factors are not yet defined. Even in the case of relatively well-characterized regulators, such as MTORC1, the relevant downstream targets are not completely known, as is true for most of the kinases that control macroautophagy, and very little information is available with regard to the complementary phosphatases. Similarly, the crosstalk among the different regulatory pathways has not been well elucidated. The identification and characterization of such factors will be important in the development of therapeutics targeting regulatory proteins; without a deeper understanding of how the cell integrates various extracellular and intracellular signals into a cohesive macroautophagic response, it is difficult to predict how the regulatory network will function when perturbed by therapeutics.
Along these lines, potentially interesting targets for therapeutic applications include ULK1/2, ATG3, ATG4, ATG7, ATG10, and PIK3C3/VPS34. The crystal structures of most of these proteins have been determined from various organisms (45, 46, 103, 112, 125, 131, 150), and, importantly, they have clearly defined functions and functional motifs, making them interesting targets for rational drug design. Further elucidation of the individual steps of macroautophagy, additional structural studies, and a more complete knowledge of the role of this process in different disease conditions will provide a better understanding of this integral cellular process, and can guide the development of improved methods and/or drugs for the treatment of autophagy defects related to human disease.
Abbreviations Used
- AMPK
AMP-activated protein kinase
- ATG
autophagy-related
- CAMKK2/CaMKKβ
calcium/calmodulin-dependent protein kinase kinase 2, beta
- CMA
chaperone-mediated autophagy
- ER
endoplasmic reticulum
- HSPA8
heat shock 70 kDa protein 8
- LAMP2A
lysosomal-associated membrane protein 2A
- MTORC1
mechanistic target of rapamycin complex 1
- PAS
phagophore assembly site
- PE
phosphatidylethanolamine
- PKA
cAMP-dependent protein kinase A
- PtdIns3K
phosphatidylinositol 3-kinase
- RB1CC1
RB1-inducible coiled-coil 1
- TVC
tubulovesicular cluster
- UBL
ubiquitin-like
- ULK
unc-51-like kinase (C. elegans)
- UPR
unfolded protein response
- ZFYVE1
zinc finger, FYVE domain containing 1
References
- 1.Agarraberes FA. and Dice JF. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci 114: 2491–2499, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Alers S, Loffler AS, Wesselborg S, and Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 32: 2–11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arias E. and Cuervo AM. Chaperone-mediated autophagy in protein quality control. Curr Opin Cell Biol 23: 184–189, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arsham AM, Howell JJ, and Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem 278: 29655–29660, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Atlashkin V, Kreykenbohm V, Eskelinen E-L, Wenzel D, Fayyazi A, and Fischer von Mollard G. Deletion of the SNARE vti1b in mice results in the loss of a single SNARE partner, syntaxin 8. Mol Cell Biol 23: 5198–5207, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auteri JS, Okada A, Bochaki V, and Dice JF. Regulation of intracellular protein degradation in IMR-90 human diploid fibroblasts. J Cell Physiol 115: 167–174, 1983 [DOI] [PubMed] [Google Scholar]
- 7.Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, and Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182: 685–701, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandyopadhyay U, Kaushik S, Varticovski L, and Cuervo AM. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol Cell Biol 28: 5747–5763, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg TO, Fengsrud M, Stromhaug PE, Berg T, and Seglen PO. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem 273: 21883–21892, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Øvervatn A, Stenmark H, and Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171: 603–614, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blancquaert S, Wang L, Paternot S, Coulonval K, Dumont JE, Harris TE, and Roger PP. cAMP-dependent activation of mammalian target of rapamycin (mTOR) in thyroid cells. Implication in mitogenesis and activation of CDK4. Mol Endocrinol 24: 1453–1468, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budovskaya YV, Stephan JS, Reggiori F, Klionsky DJ, and Herman PK. The Ras/cAMP-dependent protein kinase signaling pathway regulates an early step of the autophagy process in Saccharomyces cerevisiae. J Biol Chem 279: 20663–20671, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burman C. and Ktistakis NT. Regulation of autophagy by phosphatidylinositol 3-phosphate. FEBS Lett 584: 1302–1312, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Chen Y. and Klionsky DJ. The regulation of autophagy—unanswered questions. J Cell Sci 124: 161–170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherra SJ, III, Kulich SM, Uechi G, Balasubramani M, Mountzouris J, Day BW, and Chu CT. Regulation of the autophagy protein LC3 by phosphorylation. J Cell Biol 190: 533–539, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang H-L. and Dice JF. Peptide sequences that target proteins for enhanced degradation during serum withdrawal. J Biol Chem 263: 6797–6805, 1988 [PubMed] [Google Scholar]
- 17.Chiang H-L, Terlecky SR, Plant CP, and Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science 246: 382–385, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Cuervo AM. and Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science 273: 501–503, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Cuervo AM. and Dice JF. Regulation of lamp2a levels in the lysosomal membrane. Traffic 1: 570–583, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Cuervo AM. and Dice JF. Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci 113 Pt 24: 4441–4450, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Cuervo AM, Hildebrand H, Bomhard EM, and Dice JF. Direct lysosomal uptake of α2-microglobulin contributes to chemically induced nephropathy. Kidney Int 55: 529–545, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Cuervo AM, Knecht E, Terlecky SR, and Dice JF. Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. Am J Physiol 269: C1200–C1208, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Devenish RJ. and Klionsky DJ. Autophagy: mechanism and physiological relevance ‘brewed’ from yeast studies. Front Biosci (Schol Ed) 4: 1354–1363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dice JF. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci 15: 305–309, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, Stolz DB, Shao ZM, and Yin XM. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem 282: 4702–4710, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, Vaahtomeri K, Auchli Y, Rechsteiner H, Brunisholz RA, Viollet B, Mäkelä TP, Wallimann T, Neumann D, and Krek W. PKA phosphorylates and inactivates AMPKα to promote efficient lipolysis. EMBO J 29: 469–481, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, and Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331: 456–461, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fader CM, Sanchez DG, Mestre MB, and Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta 1793: 1901–1916, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Fimia GM, Stoykova A, Romagnoli A, Giunta L, Di Bartolomeo S, Nardacci R, Corazzari M, Fuoco C, Ucar A, Schwartz P, Gruss P, Piacentini M, Chowdhury K, and Cecconi F. Ambra1 regulates autophagy and development of the nervous system. Nature 447: 1121–1125, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Fujita N, Itoh T, Omori H, Fukuda M, Noda T, and Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell 19: 2092–2100, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuta N, Fujita N, Noda T, Yoshimori T, and Amano A. Combinational soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins VAMP8 and Vti1b mediate fusion of antimicrobial and canonical autophagosomes with lysosomes. Mol Biol Cell 21: 1001–1010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuya N, Yu J, Byfield M, Pattingre S, and Levine B. The evolutionarily conserved domain of Beclin 1 is required for Vps34 binding, autophagy and tumor suppressor function. Autophagy 1: 46–52, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Ganley IG, Lam du H, Wang J, Ding X, Chen S, and Jiang X. ULK1·ATG13·FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem 284: 12297–12305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geng J. and Klionsky DJ. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. EMBO Rep 9: 859–864, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, and Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, and Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 141: 656–667, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, Inagaki F, and Ohsumi Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem 282: 37298–37302, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan J-L, and Mizushima N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol 181: 497–510, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hara-Kuge S. and Fujiki Y. The peroxin Pex14p is involved in LC3-dependent degradation of mammalian peroxisomes. Exp Cell Res 314: 3531–3541, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8: 774–785, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Hayashi-Nishino M, Fujita N, Noda T, Yamaguchi A, Yoshimori T, and Yamamoto A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol 11: 1433–1437, 2009 [DOI] [PubMed] [Google Scholar]
- 42.He C, Baba M, Cao Y, and Klionsky DJ. Self-interaction is critical for Atg9 transport and function at the phagophore assembly site during autophagy. Mol Biol Cell 19: 5506–5516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He C. and Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43: 67–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hemelaar J, Lelyveld VS, Kessler BM, and Ploegh HL. A single protease, Apg4B, is specific for the autophagy-related ubiquitin-like proteins GATE-16, MAP1-LC3, GABARAP, and Apg8L. J Biol Chem 278: 51841–51850, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Hong SB, Kim BW, Kim JH, and Song HK. Structure of the autophagic E2 enzyme Atg10. Acta Crystallogr D Biol Crystallogr 68: 1409–1417, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Hong SB, Kim BW, Lee KE, Kim SW, Jeon H, Kim J, and Song HK. Insights into noncanonical E1 enzyme activation from the structure of autophagic E1 Atg7 with Atg8. Nat Struct Mol Biol 18: 1323–1330, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan J-L, Oshiro N, and Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20: 1981–1991, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hosokawa N, Sasaki T, Iemura S, Natsume T, Hara T, and Mizushima N. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy 5: 973–979, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, and Jäättelä M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-β, and Bcl-2. Mol Cell 25: 193–205, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Huang KM. and Snider MD. Isolation of protein glycosylation mutants in the fission yeast Schizosaccharomyces pombe. Mol Biol Cell 6: 485–496, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huybrechts SJ, Van Veldhoven PP, Brees C, Mannaerts GP, Los GV, and Fransen M. Peroxisome dynamics in cultured mammalian cells. Traffic 10: 1722–1733, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, and Ohsumi Y. A ubiquitin-like system mediates protein lipidation. Nature 408: 488–492, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Inoki K, Zhu T, and Guan K-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Isakson P, Holland P, and Simonsen A. The role of ALFY in selective autophagy. Cell Death Differ 20: 12–20, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Itakura E, Kishi C, Inoue K, and Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 19: 5360–5372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Itakura E, Kishi-Itakura C, and Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151: 1256–1269, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Itakura E. and Mizushima N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy 6: 764–776, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Itoh T, Fujita N, Kanno E, Yamamoto A, Yoshimori T, and Fukuda M. Golgi-resident small GTPase Rab33B interacts with Atg16L and modulates autophagosome formation. Mol Biol Cell 19: 2916–2925, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, and Eskelinen E-L. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci 117: 4837–4848, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Jeffries TR, Dove SK, Michell RH, and Parker PJ. PtdIns-specific MPR pathway association of a novel WD40 repeat protein, WIPI49. Mol Biol Cell 15: 2652–2663, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, and Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 191: 933–942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, and Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20: 1992–2003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, and Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19: 5720–5728, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, and Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci 117: 2805–2812, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Kaushik S, Massey AC, and Cuervo AM. Lysosome membrane lipid microdomains: novel regulators of chaperone-mediated autophagy. EMBO J 25: 3921–3933, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiffin R, Christian C, Knecht E, and Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell 15: 4829–4840, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kihara A, Noda T, Ishihara N, and Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol 152: 519–530, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, and Guan K-L. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 10: 935–945, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim I, Rodriguez-Enriquez S, and Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462: 245–253, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim J, Dalton VM, Eggerton KP, Scott SV, and Klionsky DJ. Apg7p/Cvt2p is required for the cytoplasm-to-vacuole targeting, macroautophagy, and peroxisome degradation pathways. Mol Biol Cell 10: 1337–1351, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J, Kundu M, Viollet B, and Guan K-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, Noda T, and Ohsumi Y. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J Cell Biol 147: 435–446, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, Ohsumi M, Takao T, Noda T, and Ohsumi Y. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol 151: 263–276, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kirkin V, Lamark T, Johansen T, and Dikic I. NBR1 cooperates with p62 in selective autophagy of ubiquitinated targets. Autophagy 5: 732–733, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Kirkin V, Lamark T, Sou YS, Bjørkøy G, Nunn JL, Bruun JA, Shvets E, McEwan DG, Clausen TH, Wild P, Bilusic I, Theurillat JP, Øvervatn A, Ishii T, Elazar Z, Komatsu M, Dikic I, and Johansen T. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol Cell 33: 505–516, 2009 [DOI] [PubMed] [Google Scholar]
- 76.Kirkin V, McEwan DG, Novak I, and Dikic I. A role for ubiquitin in selective autophagy. Mol Cell 34: 259–269, 2009 [DOI] [PubMed] [Google Scholar]
- 77.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, and Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392: 605–608, 1998 [DOI] [PubMed] [Google Scholar]
- 78.Krick R, Henke S, Tolstrup J, and Thumm M. Dissecting the localization and function of Atg18, Atg21 and Ygr223c. Autophagy 4: 896–910, 2008 [DOI] [PubMed] [Google Scholar]
- 79.Kuma A, Mizushima N, Ishihara N, and Ohsumi Y. Formation of the approximately 350-kDa Apg12-Apg5·Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J Biol Chem 277: 18619–18625, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Kundu M, Lindsten T, Yang CY, Wu J, Zhao F, Zhang J, Selak MA, Ney PA, and Thompson CB. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 112: 1493–1502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lamark T, Kirkin V, Dikic I, and Johansen T. NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell Cycle 8: 1986–1990, 2009 [DOI] [PubMed] [Google Scholar]
- 82.Lee IH. and Finkel T. Regulation of autophagy by the p300 acetyltransferase. J Biol Chem 284: 6322–6328, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee J, Giordano S, and Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441: 523–540, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee JW, Park S, Takahashi Y, and Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS One 5: e15394, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, and Jung JU. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol 8: 688–699, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Liang C, Lee JS, Inn KS, Gack MU, Li Q, Roberts EA, Vergne I, Deretic V, Feng P, Akazawa C, and Jung JU. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol 10: 776–787, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, and Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402: 672–676, 1999 [DOI] [PubMed] [Google Scholar]
- 88.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, and Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol 72: 8586–8596, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, and Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120: 237–248, 2005 [DOI] [PubMed] [Google Scholar]
- 90.Mari M, Griffith J, Rieter E, Krishnappa L, Klionsky DJ, and Reggiori F. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J Cell Biol 190: 1005–1022, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mariño G, Uría JA, Puente XS, Quesada V, Bordallo J, and López-Otín C. Human autophagins, a family of cysteine proteinases potentially implicated in cell degradation by autophagy. J Biol Chem 278: 3671–3678, 2003 [DOI] [PubMed] [Google Scholar]
- 92.Martinet W, De Meyer GR, Andries L, Herman AG, and Kockx MM. In situ detection of starvation-induced autophagy. J Histochem Cytochem 54: 85–96, 2006 [DOI] [PubMed] [Google Scholar]
- 93.Marzella L, Ahlberg J, and Glaumann H. In vitro uptake of particles by lysosomes. Exp Cell Res 129: 460–466, 1980 [DOI] [PubMed] [Google Scholar]
- 94.Marzella L, Ahlberg J, and Glaumann H. Autophagy, heterophagy, microautophagy and crinophagy as the means for intracellular degradation. Virchows Arch B Cell Pathol Incl Mol Pathol 36: 219–234, 1981 [DOI] [PubMed] [Google Scholar]
- 95.Massey A, Kiffin R, and Cuervo AM. Pathophysiology of chaperone-mediated autophagy. Int J Biochem Cell Biol 36: 2420–2434, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, Maejima I, Shirahama-Noda K, Ichimura T, Isobe T, Akira S, Noda T, and Yoshimori T. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol 11: 385–396, 2009 [DOI] [PubMed] [Google Scholar]
- 97.Mavrakis M, Lippincott-Schwartz J, Stratakis CA, and Bossis I. Depletion of type IA regulatory subunit (RIα) of protein kinase A (PKA) in mammalian cells and tissues activates mTOR and causes autophagic deficiency. Hum Mol Genet 15: 2962–2971, 2006 [DOI] [PubMed] [Google Scholar]
- 98.Meissner C, Lorenz H, Weihofen A, Selkoe DJ, and Lemberg MK. The mitochondrial intramembrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking. J Neurochem 117: 856–867, 2011 [DOI] [PubMed] [Google Scholar]
- 99.Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, and Meijer AJ. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem 281: 34870–34879, 2006 [DOI] [PubMed] [Google Scholar]
- 100.Mercer CA, Kaliappan A, and Dennis PB. A novel, human Atg13 binding protein, Atg101, interacts with ULK1 and is essential for macroautophagy. Autophagy 5: 649–662, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Mijaljica D, Prescott M, and Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy 7: 673–682, 2011 [DOI] [PubMed] [Google Scholar]
- 102.Mijaljica D, Prescott M, and Devenish RJ. The intriguing life of autophagosomes. Int J Mol Sci 13: 3618–3635, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miller S, Tavshanjian B, Oleksy A, Perisic O, Houseman BT, Shokat KM, and Williams RL. Shaping development of autophagy inhibitors with the structure of the lipid kinase Vps34. Science 327: 1638–1642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mizushima N. and Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr 27: 19–40, 2007 [DOI] [PubMed] [Google Scholar]
- 105.Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, Takao T, Natsume T, Ohsumi Y, and Yoshimori T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci 116: 1679–1688, 2003 [DOI] [PubMed] [Google Scholar]
- 106.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, and Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol 152: 657–668, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mizushima N, Yoshimori T, and Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 27: 107–132, 2011 [DOI] [PubMed] [Google Scholar]
- 108.Monastyrska I, Rieter E, Klionsky DJ, and Reggiori F. Multiple roles of the cytoskeleton in autophagy. Biol Rev Camb Philos Soc 84: 431–448, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mortensen M, Ferguson DJ, Edelmann M, Kessler B, Morten KJ, Komatsu M, and Simon AK. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci U S A 107: 832–837, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nair U, Jotwani A, Geng J, Gammoh N, Richerson D, Yen W-L, Griffith J, Nag S, Wang K, Moss T, Baba M, McNew JA, Jiang X, Reggiori F, Melia TJ, and Klionsky DJ. SNARE proteins are required for macroautophagy. Cell 146: 290–302, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nair U, Yen W-L, Mari M, Cao Y, Xie Z, Baba M, Reggiori F, and Klionsky DJ. A role for Atg8-PE deconjugation in autophagosome biogenesis. Autophagy 8: 780–793, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Noda NN, Satoo K, Fujioka Y, Kumeta H, Ogura K, Nakatogawa H, Ohsumi Y, and Inagaki F. Structural basis of Atg8 activation by a homodimeric E1, Atg7. Mol Cell 44: 462–475, 2011 [DOI] [PubMed] [Google Scholar]
- 113.Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol 2: 211–216, 2001 [DOI] [PubMed] [Google Scholar]
- 114.Orenstein SJ. and Cuervo AM. Chaperone-mediated autophagy: molecular mechanisms and physiological relevance. Semin Cell Dev Biol 21: 719–726, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Øverbye A, Fengsrud M, and Seglen PO. Proteomic analysis of membrane-associated proteins from rat liver autophagosomes. Autophagy 3: 300–322, 2007 [DOI] [PubMed] [Google Scholar]
- 116.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, and Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122: 927–939, 2005 [DOI] [PubMed] [Google Scholar]
- 117.Pfeifer U. Inhibition by insulin of the formation of autophagic vacuoles in rat liver. A morphometric approach to the kinetics of intracellular degradation by autophagy. J Cell Biol 78: 152–167, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Polson HE, de Lartigue J, Rigden DJ, Reedijk M, Urbe S, Clague MJ, and Tooze SA. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy 6: 506–522, 2010 [DOI] [PubMed] [Google Scholar]
- 119.Proikas-Cezanne T, Waddell S, Gaugel A, Frickey T, Lupas A, and Nordheim A. WIPI-1α (WIPI49), a member of the novel 7-bladed WIPI protein family, is aberrantly expressed in human cancer and is linked to starvation-induced autophagy. Oncogene 23: 9314–9325, 2004 [DOI] [PubMed] [Google Scholar]
- 120.Ravikumar B, Moreau K, Jahreiss L, Puri C, and Rubinsztein DC. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol 12: 747–757, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ravikumar B, Moreau K, and Rubinsztein DC. Plasma membrane helps autophagosomes grow. Autophagy 6: 1184–1186, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reggiori F, Tucker KA, Stromhaug PE, and Klionsky DJ. The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev Cell 6: 79–90, 2004 [DOI] [PubMed] [Google Scholar]
- 123.Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Potolicchio I, Nieves E, Cuervo AM, and Santambrogio L. Microautophagy of cytosolic proteins by late endosomes. Dev Cell 20: 131–139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, and Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Satoo K, Noda NN, Kumeta H, Fujioka Y, Mizushima N, Ohsumi Y, and Inagaki F. The structure of Atg4B-LC3 complex reveals the mechanism of LC3 processing and delipidation during autophagy. EMBO J 28: 1341–1350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, Kundu M, Opferman JT, Cleveland JL, Miller JL, and Ney PA. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci U S A 104: 19500–19505, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schworer CM, Shiffer KA, and Mortimore GE. Quantitative relationship between autophagy and proteolysis during graded amino acid deprivation in perfused rat liver. J Biol Chem 256: 7652–7658, 1981 [PubMed] [Google Scholar]
- 128.Shang L, Chen S, Du F, Li S, Zhao L, and Wang X. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc Natl Acad Sci U S A 108: 4788–4793, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shintani T, Mizushima N, Ogawa Y, Matsuura A, Noda T, and Ohsumi Y. Apg10p, a novel protein-conjugating enzyme essential for autophagy in yeast. EMBO J 18: 5234–5241, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stephan JS, Yeh YY, Ramachandran V, Deminoff SJ, and Herman PK. The Tor and cAMP-dependent protein kinase signaling pathways coordinately control autophagy in Saccharomyces cerevisiae. Autophagy 6: 294–295, 2010 [DOI] [PubMed] [Google Scholar]
- 131.Sugawara K, Suzuki NN, Fujioka Y, Mizushima N, Ohsumi Y, and Inagaki F. Structural basis for the specificity and catalysis of human Atg4B responsible for mammalian autophagy. J Biol Chem 280: 40058–40065, 2005 [DOI] [PubMed] [Google Scholar]
- 132.Sun Q, Fan W, Chen K, Ding X, Chen S, and Zhong Q. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A 105: 19211–19216, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mule JJ, Pledger WJ, and Wang HG. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol 9: 1142–1151, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takahashi Y, Meyerkord CL, Hori T, Runkle K, Fox TE, Kester M, Loughran TP, and Wang HG. Bif-1 regulates Atg9 trafficking by mediating the fission of Golgi membranes during autophagy. Autophagy 7: 61–73, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Takahashi Y, Meyerkord CL, and Wang HG. Bif-1/endophilin B1: a candidate for crescent driving force in autophagy. Cell Death Differ 16: 947–955, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takeshige K, Baba M, Tsuboi S, Noda T, and Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol 119: 301–311, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tal R, Winter G, Ecker N, Klionsky DJ, and Abeliovich H. Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. J Biol Chem 282: 5617–5624, 2007 [DOI] [PubMed] [Google Scholar]
- 138.Tanida I, Minematsu-Ikeguchi N, Ueno T, and Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 1: 84–91, 2005 [DOI] [PubMed] [Google Scholar]
- 139.Till A, Lakhani R, Burnett SF, and Subramani S. Pexophagy: the selective degradation of peroxisomes. Int J Cell Biol 2012: 512721, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tooze J, Hollinshead M, Ludwig T, Howell K, Hoflack B, and Kern H. In exocrine pancreas, the basolateral endocytic pathway converges with the autophagic pathway immediately after the early endosome. J Cell Biol 111: 329–345, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vadlamudi RK, Joung I, Strominger JL, and Shin J. p62, a phosphotyrosine-independent ligand of the SH2 domain of p56lck, belongs to a new class of ubiquitin-binding proteins. J Biol Chem 271: 20235–20237, 1996 [DOI] [PubMed] [Google Scholar]
- 142.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, and Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304: 1158–1160, 2004 [DOI] [PubMed] [Google Scholar]
- 143.Wang Z, Wilson WA, Fujino MA, and Roach PJ. Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol Cell Biol 21: 5742–5752, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Webber JL. and Tooze SA. Coordinated regulation of autophagy by p38α MAPK through mAtg9 and p38IP. EMBO J 29: 27–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weidberg H, Shvets E, and Elazar Z. Biogenesis and cargo selectivity of autophagosomes. Annu Rev Biochem 80: 125–156, 2011 [DOI] [PubMed] [Google Scholar]
- 146.Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, and Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J 29: 1792–1802, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, Richter B, Korac J, Waidmann O, Choudhary C, Dotsch V, Bumann D, and Dikic I. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333: 228–233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wirawan E, Vanden Berghe T, Lippens S, Agostinis P, and Vandenabeele P. Autophagy: for better or for worse. Cell Res 22: 43–61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xie Z, Nair U, and Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Mol Biol Cell 19: 3290–3298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yamada Y, Suzuki NN, Hanada T, Ichimura Y, Kumeta H, Fujioka Y, Ohsumi Y, and Inagaki F. The crystal structure of Atg3, an autophagy-related ubiquitin carrier protein (E2) enzyme that mediates Atg8 lipidation. J Biol Chem 282: 8036–8043, 2007 [DOI] [PubMed] [Google Scholar]
- 151.Yan Y, Flinn RJ, Wu H, Schnur RS, and Backer JM. hVps15, but not Ca2+/CaM, is required for the activity and regulation of hVps34 in mammalian cells. Biochem J 417: 747–755, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang Z. and Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol 335: 1–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang Z. and Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol 12: 814–822, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang Z. and Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 22: 124–131, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ylä-Anttila P, Vihinen H, Jokitalo E, and Eskelinen E-L. 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy 5: 1180–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 156.Yokota S. and Dariush Fahimi H. Degradation of excess peroxisomes in mammalian liver cells by autophagy and other mechanisms. Histochem Cell Biol 131: 455–458, 2009 [DOI] [PubMed] [Google Scholar]
- 157.Yorimitsu T. and Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ 12: 1542–1552, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Youle RJ. and Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12: 9–14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Young ARJ, Chan EYW, Hu XW, Köchl R, Crawshaw SG, High S, Hailey DW, Lippincott-Schwartz J, and Tooze SA. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci 119: 3888–3900, 2006 [DOI] [PubMed] [Google Scholar]
- 160.Zeng X, Overmeyer JH, and Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci 119: 259–270, 2006 [DOI] [PubMed] [Google Scholar]
- 161.Zhang J, Randall MS, Loyd MR, Dorsey FC, Kundu M, Cleveland JL, and Ney PA. Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation. Blood 114: 157–164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zheng YT, Shahnazari S, Brech A, Lamark T, Johansen T, and Brumell JH. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol 183: 5909–5916, 2009 [DOI] [PubMed] [Google Scholar]
- 163.Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, Heintz N, and Yue Z. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol 11: 468–476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]