Abstract
Gynecologic cancers are a leading cause of morbidity and mortality for female patients, with an estimated 88,750 new cancer cases and 29,520 deaths in the United States in 2012. To offer the best treatment options to patients it is important that the radiologist, surgeon, radiation oncologist, and gynecologic oncologist work together with a multidisciplinary approach. Using the available diagnostic imaging modalities, the radiologist must give appropriate information to the surgeon in order to plan the best surgical approach and its timing.
Keywords: Gynecologic cancers, radiologic results, surgeons
Introduction
Gynecologic cancers are a leading causes of morbidity and mortality for female patients, with an estimated 88,750 new cancer cases and 29,520 deaths in the United States in 2012[1]. To offer the best treatment options to patients, it is important that the radiologist, surgeon, radiation oncologist, and gynecologic oncologist work together with a multidisciplinary approach. Using the available diagnostic imaging modalities, the radiologist must give appropriate information to the surgeon in order to plan the best surgical approach and its timing.
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy in women in the United States, with 47,130 new cases and 8010 estimated deaths in 2012[1]. Patients are usually of postmenopausal age and may have risk factors associated with increased estrogen exposure, such as nulliparity, chronic anovulation, and obesity. Prognosis of patients with endometrial cancer depends on a number of factors, including histologic type and grade, stage, lymphovascular space involvement, and nodal status.
Management of patients with endometrial cancer
Endometrial carcinomas are divided into two histologic subtypes. Type 1 includes endometrioid adenocarcinoma, the most common histologic subtype (90% of cases of endometrial cancer), further subdivided into 3 grades according to degree of differentiation. Type 2 endometrial carcinomas include serous papillary and clear-cell adenocarcinomas. Type 2 and high-grade type 1 are more aggressive tumors (50% pretest probability of locally advanced or distant disease at manifestation)[2].
The standard of care for patients with endometrial cancer is surgical removal, which may include hysterectomy, bilateral oophorectomy, pelvic and lomboaortic lymphadenectomy, and omental and peritoneal biopsies.
There is still no consensus about the necessity of performing extensive lymphadenectomy, because lymphadenectomy is associated with higher morbidity. A combination of preoperative imaging and intraoperative evaluation is therefore helpful in determining whether this surgical procedure is necessary in each patient[3]. Stratification of patients according to risk factors may guide the surgeon in avoiding unnecessary lymphadenectomies in low-risk patients. Indeed, low-risk patients may benefit from hysterectomy with bilateral oophorectomy, whereas high-risk patients may require pelvic and para-aortic lymphadenectomy, with the adjunct of omental and peritoneal biopsies in cases of type II endometrial cancer.
Preoperative evaluation
Staging, based on the revised International Federation of Gynecology and Obstetrics (FIGO) classification[4], still remains surgicopathologic. However, the European Society of Urogenital Imaging guidelines recommend magnetic resonance imaging (MRI) for staging, especially when high-risk endometrial carcinoma is suspected[5], such as in the case of type 2 tumors; in cases of suspected advanced disease, including cervical stroma extension, local or distant spread (stages III and IV); when lymph node enlargement is considered as a roadmap for lymph node sampling; and when there are medical contraindications for extensive surgical staging[5].
MRI is considered the most accurate imaging modality for preoperative assessment of endometrial carcinoma because of its excellent soft-tissue contrast resolution, whereas computed tomography (CT) is neither sensitive nor specific enough to assess the depth of myometrial or cervical involvement, owing to the lack of contrast difference between tumor and myometrium. The MR protocol for staging endometrial cancer must include T2-weighted sequences in the sagittal, axial oblique plane (perpendicular) and coronal oblique plane (parallel) to the uterine cavity. If cervical involvement is suspected an additional axial oblique sequence, perpendicular to the long axis of the endocervical canal, should be added.
Contrast-enhanced imaging is suggested for higher accuracy of the diagnosis of deep myometrial invasion. It is especially recommended for atrophic uteri, if there are associated adenomyosis or fibroids, or in suspected advanced tumors (bladder or rectal wall invasion). The degree of myometrial involvement is associated with higher risk of nodal metastases. In fact the prevalence of lymph node metastases increases from 3% with superficial myometrial invasion to 46% with deep myometrial invasion. Contrast-enhanced MRI of the pelvis shows an accuracy of about 91% in preoperative identification of deep myometrial invasion[6].
Diffusion-weighted imaging (DWI) is an adjunctive tool that gives information about water mobility, tissue cellularity, and integrity of cellular membranes[7]. At DWI endometrial cancer shows restricted diffusion on high b value (b = 1000 s/mm2) images (Fig. 1). The adjunct of DWI to T2-weighted images may aid in the detection of tumors. Higher-grade tumors demonstrate a trend to lower apparent diffusion coefficient (ADC) values compared with lower-grade tumors[8]. However, the role of DWI in assessing the depth of myometrial invasion is still debated, mainly because of low spatial resolution[9]. Cutoff ADC values able to distinguish normal tissue from cancerous endometrial tissue are not yet established[7].
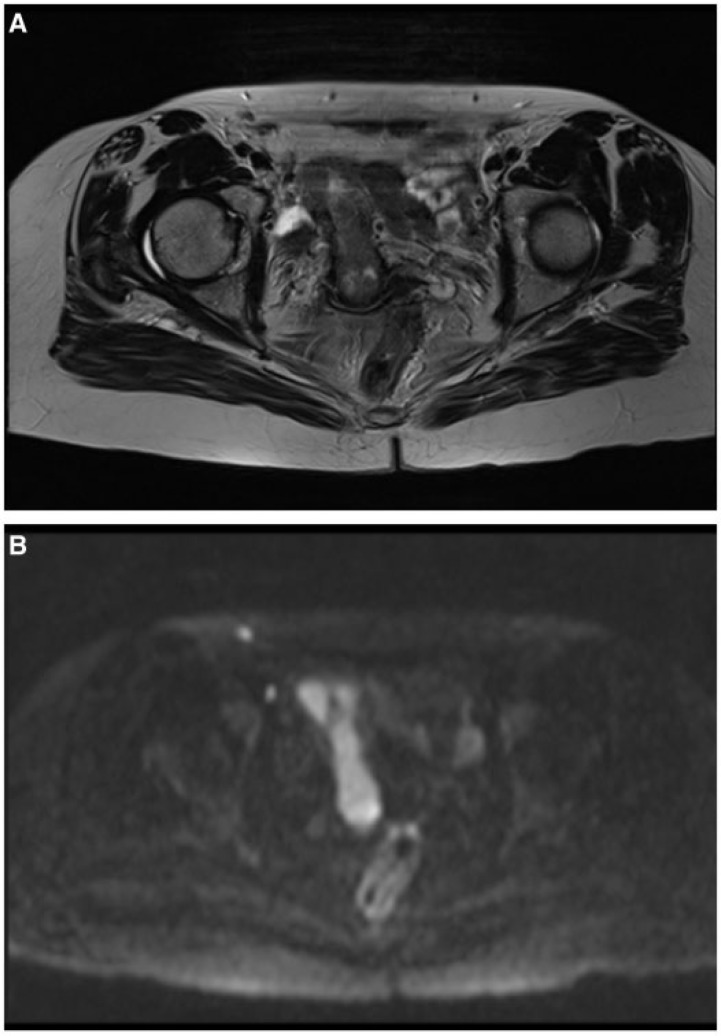
Figure 1.
Axial magnetic resonance (MR) T2-weighted image demonstrating endometrial cancer occupying the whole endometrial canal (A), with hyperintensity at high b value diffusion-weighted imaging (DWI) sequences (B).
MRI may also provide additional information, such as uterine size (especially transverse dimension), ascites, adnexal pathologic conditions, and the presence of peritoneal disease, thus helping to determine the surgical approach. Laparoscopic (robotic) surgery is the preferred approach for endometrial cancer, but is contraindicated in patients with peritoneal involvement, large-size uterus, and/or extrauterine disease[10].
In evaluation of nodal metastases, the role of positron emission tomography (PET)/CT is under consideration. Kitajima et al.[11] showed overall sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of PET/CT on a node-based analysis of patients who underwent pelvic, with or without lomboaortic, nodal dissection of 51.1%, 99.8%, 85.2%, 98.9%, and 98.7%, respectively. However, the sensitivity in detection of metastatic lesions 4 mm or less in short-axis diameter was as low as 12.5%[11].
PET/CT may be of particular value in high-risk patients. Indeed, Crivellaro et al.[12] showed moderate sensitivity (78.6%), and high specificity and accuracy (98.4% and 94.7%, respectively) of PET/CT in assessment of nodal status of 76 high-risk patients with clinical stage I endometrial cancer.
Dissemination routes
Nodal metastases from endometrial cancer involve pelvic and para-aortic nodes. Tumors from the middle and inferior uterus drain to the parametrial and obturator nodes, whereas those from the proximal body and fundus drain to the common iliac and para-aortic nodes[13]. Lymphatic drainage from the uterus also occurs to obturator nodes, and tumor can spread via the round ligament to inguinal nodes as well. The likelihood of nodal spread increases in the presence of greater than 50% invasion of the myometrium in comparison with those with a lesser amount of invasion[3].
Imaging reporting
The MRI report should include careful assessment of the following features: depth of myometrial invasion; cervical stromal invasion; local and/or regional spread and nodal status; bladder, bowel mucosa, and/or presence of distant metastases.
Depth of myometrial invasion (stage IA–IB)
The T2-weighted and contrast-enhanced sequences, parallel and perpendicular to the plane of the uterus, optimize visualization of the endometrial–myometrial interface. The normal endometrium is hyperintense on T2 images, whereas tumors are intermediate and heterogeneous in signal intensity[3]. Compared with tumors, the inner myometrium, also called the junctional zone (JZ), is hypointense on T2-weighted images (Fig. 2).
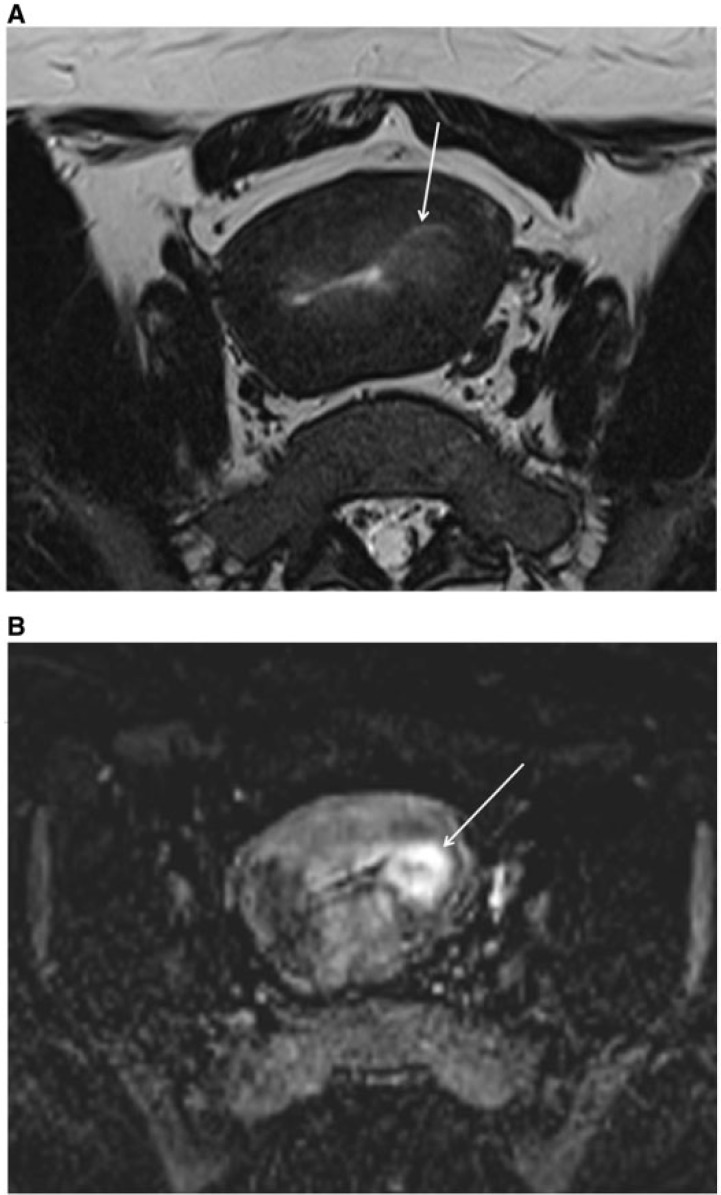
Figure 2.
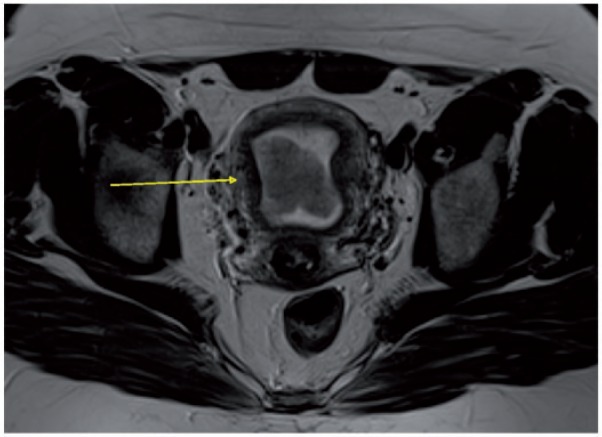
Axial MR T2-weighted image showing endometrial cancer hypointense to the endometrium and hyperintense to the junctional zone (arrow).
However, the JZ is not well seen in postmenopausal women (Fig. 3), who represent the vast majority of patients with endometrial cancer. In these cases contrast-enhanced scans, even with subtraction of native images, are helpful (Fig. 3) because the tumor enhances less than the normal myometrium, and the invasive hypointense tumor extends into the myometrium, causing irregularity and disruption of the enhancing JZ at the endometrial–myometrial interface (Fig. 4)[3]. Maximum contrast between hyperintense myometrium and hypointense endometrial tumor occurs 50–120 s after administration of contrast medium, and this is the most important phase for accurate assessment of the depth of myometrial invasion. Differential enhancement within the endometrial cavity can allow distinction between tumor, blood products, and debris.
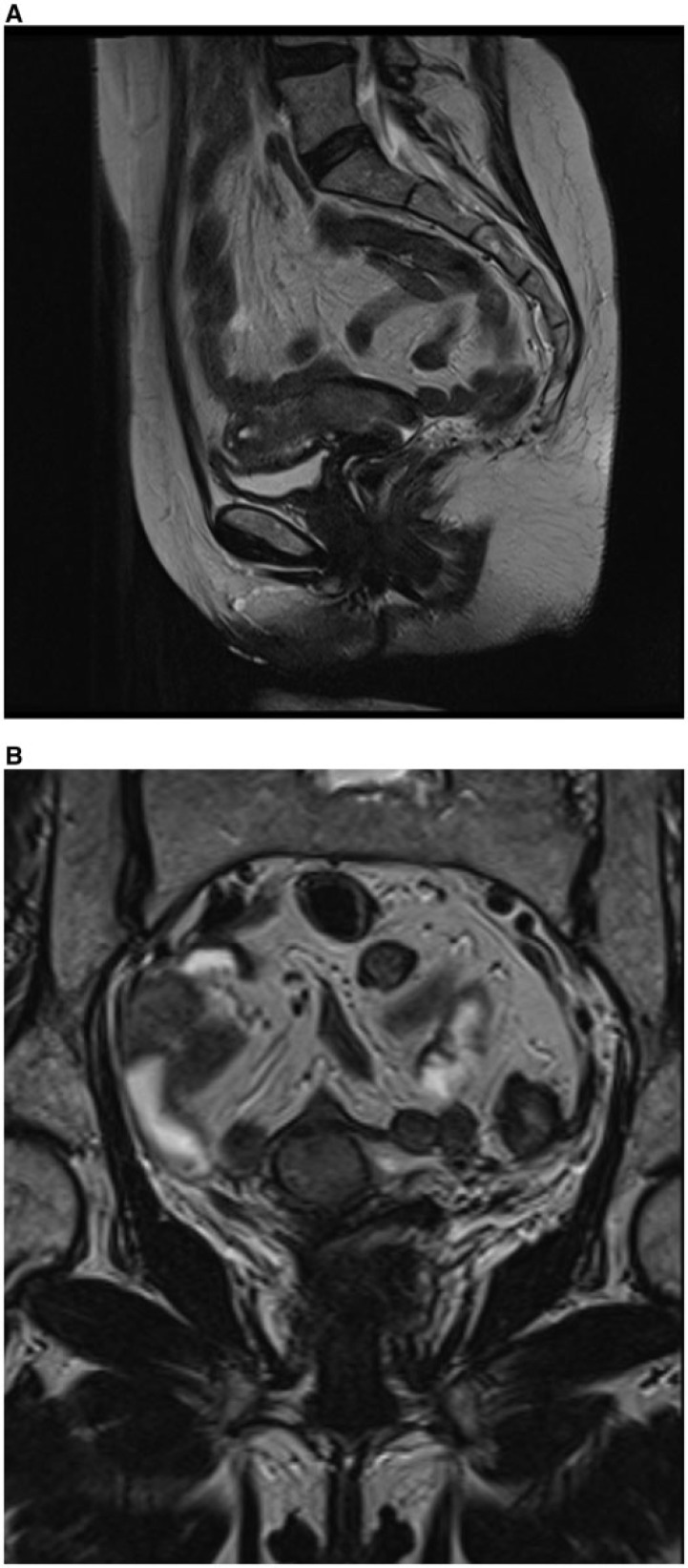
Figure 3.
(A) Axial MR T2-weighted image showing difficult distinction of the inner part of myometrium (also called the junctional zone) and consequent difficult delineation of tumor margins (arrow) in a postmenopausal woman with endometrial cancer. (B) Tumor is better delineated on subtracted postcontrast MR T1-weighted image (arrow).
Figure 4.
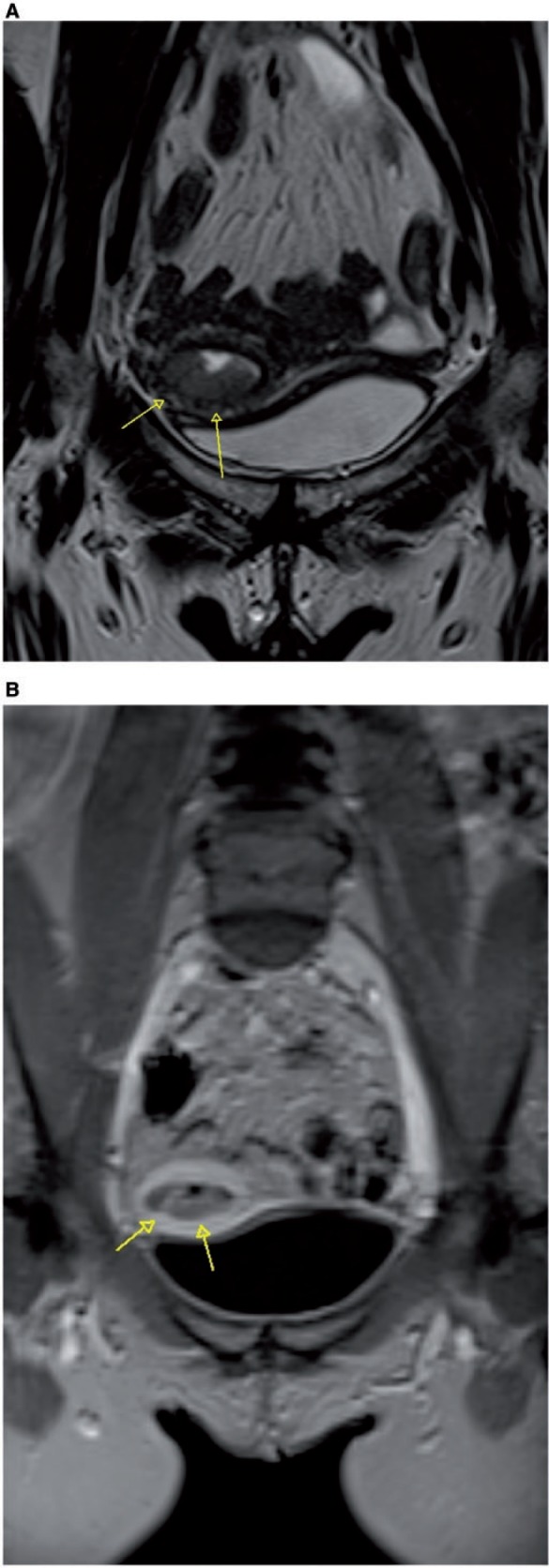
(A) Para-axial MR T2-weighted image shows endometrial cancer extending to the external part of the myometrium, with disruption of the enhancing junctional zone (arrows) at the endometrial-myometrial interface, well delineated also in the dynamic postcontrast MR T1-weighted image (arrows) (B).
In the revised FIGO staging system, tumors confined to the endometrium and tumors invading the inner half of the myometrium are staged as IA tumors, whereas tumors invading the outer half of the myometrium are staged as IB tumors[4].
Cervical stromal invasion (stage II)
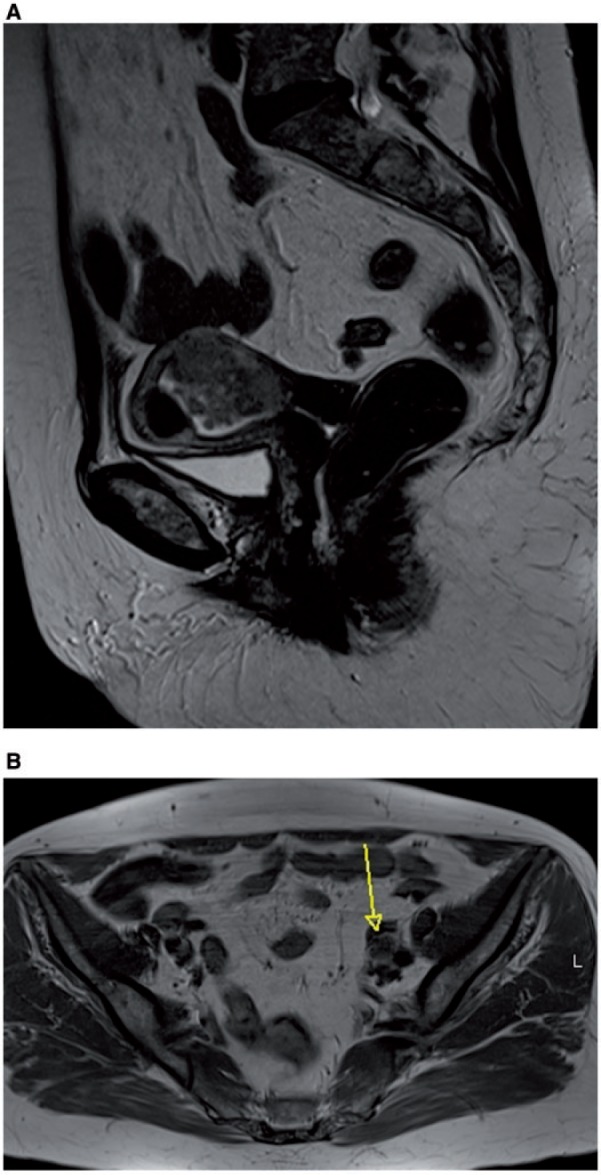
The normal cervical stroma is hypointense on T2-weighted images (Fig. 5) and is replaced by intermediate signal intensity tumor in the case of invasion. Thin-section axial oblique images perpendicular to the cervical canal improve the assessment of cervical invasion. Delayed-phase images obtained 3–4 min after administration of contrast medium may help the evaluation of cervical stromal invasion (FIGO stage II). The presence of an intact enhancing cervical mucosa excludes stromal invasion (Fig. 5). The new FIGO classification places endocervical glandular tumor extension into stage I, and cervical stromal invasion into stage II[4].
Figure 5.
Sagittal (A) and para-axial (B) MR T2-weighted images showing endometrial cancer extending to the cervix, with preserved hypointense stromal ring, as confirmed by regular enhancement at dynamic postcontrast T1-weighted image (C) in an endometrial carcinoma, International Federation of Gynecology and Obstetrics (FIGO) stage IIA.
Local and/or regional spread and nodal status (stage III)
Tumors that invade the serosa appear as an area of intermediate to high signal intensity that disrupts the contour of the outer myometrium. The use of DWI improves depiction of extrauterine metastatic deposits. When tumor extends into the vagina by direct invasion or metastatic spread, a segmental loss of low signal intensity in the vaginal wall on T2-weighted images may be seen at MRI.
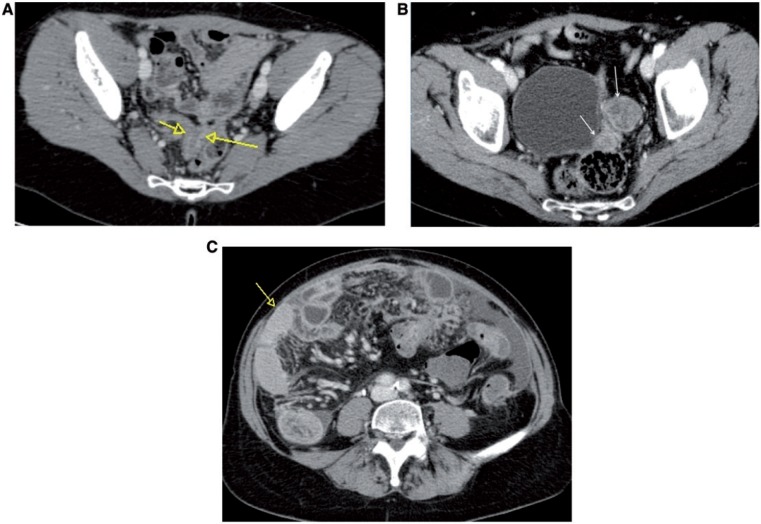
Tumors from the middle and inferior uterus drain to the parametrial, external, and obturator nodes (Fig. 6), whereas those from the proximal body and fundus drain to the common iliac and para-aortic nodes. Endometrial tumors may also spread to the inguinal nodes via the round ligament. Imaging findings suggestive of nodal involvement include a short-axis diameter greater than 10 mm, but size criteria have a wide range of sensitivities. Other features suggesting nodal involvement are presence of necrosis[3,14], multiplicity, irregular contour, and signal intensity similar to that of the primary tumor.
Figure 6.
Sagittal MR T2-weighted image showing endometrial cancer in the middle part of the uterine corpus, invading the outer part of myometrium (A), which led to nodal metastasis to obturator left lymph node (arrow), as shown in the axial unenhanced MR T1-weighted image (B).
DWI may be used to depict drop metastases in the cervix and vagina, and unexpected extrauterine spread of disease within the adnexa (Fig. 7) and peritoneum. It may also help the detection of positive nodes[15,16].
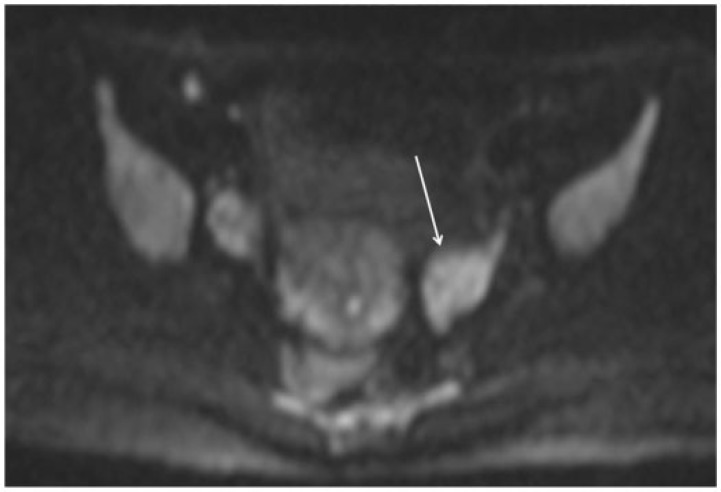
Figure 7.
Axial MR DWI image in a young patient undergoing staging for endometrial cancer shows hyperintense left ovary (arrow), indicating presence of disease.
CT performs as well as MRI in identifying extrauterine spread and identifying nodal metastases.
Bladder, bowel mucosa, and/or distant metastases (stage IV)
Extension of tumor directly into the normally hyperintense vesical or rectal mucosa is indicative of endometrial tumor invasion into the bladder or rectum[2]. Thickening of the high-signal-intensity mucosal layer is not indicative of mucosal invasion, whereas disruption of the hypointense muscularis layer does not indicate stage IV disease, because it cannot be visualized at subsequent cystoscopy or sigmoidoscopy[2].
In summary, the combination of dynamic contrast-enhanced and T2-weighted MRI, recommended by the European Society for Urologic Research (ESUR) in its guidelines for endometrial cancer staging, offers a “one-stop” examination that gives surgeons a good estimate of tumor burden that helps them tailor the necessary treatment of patients with endometrial cancer.
Ovarian cancer
Ovarian cancer is the second most common gynecologic malignancy in women in the United States after uterine corpus, with an estimated 22,280 new cases and 15,500 deaths in 2012[1].
Management of patients with ovarian cancer
The optimal treatment for patients with ovarian cancer is a complete cytoreduction followed by platinum-based chemotherapy. There is no universal agreement on the definition of optimal cytoreduction. However, recent studies have shown that no macroscopic residual disease best correlates with prognosis and long-term survival[17,18]. Extensive disease requires surgery by trained gynecologic oncology surgeons, at times supported by general surgeons for complex liver and/or bowel resections. Since it is more appropriate for patients to undergo an up-front debulking effort, instead of undergoing surgery as a diagnostic procedure, it is mandatory for the surgeon to know the exact extent of the disease prior to take the patient to the operative room.
Neoadjuvant chemotherapy is a treatment option in patients with medical comorbidities, stage IV disease, or extensive tumor load. The presence of difficult-to-reach sites that may preclude optimal debulking (Table 1) suggests that the patient should undergo primary chemotherapy. However, these criteria may vary, depending on the aggressiveness of the surgical procedure and the performance status of the patient; therefore, they should be used according to multidisciplinary consensus and be adapted to the single-institutional protocols[19].
Table 1.
Sites of disease that may preclude optimal debulking in patients with ovarian cancer
| Lymph node enlargement above the renal hilum and around the celiac axis |
| Abdominal wall invasion |
| Parenchymal liver metastases |
| Implants of >2 cm in diaphragm, lesser sac, porta hepatis, intersegmental fissure, gallbladder fossa, gastrosplenic ligament, gastrohepatic ligament |
| Extensive involvement of the small bowel or mesenteric root |
| Pleural infiltration |
| Pelvic side-wall invasion |
| Bladder trigone |
Preoperative evaluation
Ultrasonography (US), CT, and MRI may all be used in the evaluation of ovarian cancer. Recently, the use of PET/CT has also been considered of value in patients with ovarian cancer.
US usually represents the first-level examination to evaluate adnexal masses in both emergency and nonemergency settings. Once an ovarian cancer mass has been demonstrated, the next step is to stage the disease.
CT is currently considered the best imaging technique for staging ovarian cancer, since it is widely available and provides all the required information in a short examination time[27]. According to the ESUR guidelines[28], CT should be extended from the distal thorax to the inguinal region. Inclusion of the lung bases in the field of view is recommended because this enables assessment of cardiophrenic lymph nodes and pleural effusion[29].
CT provides rapid image acquisition, with an overall accuracy of 70–90% for the detection of implants at all disease stages[20,21]. The most important limitation of CT is its inability to reliably depict implants with a maximal diameter of less than 5 mm on the bowel serosa, mesentery, or peritoneum, especially in the absence of ascites[20]. Indeed, the detection rate for small peritoneal lesions is poor, with reported sensitivities of only 7–50% for those with a maximal diameter of less than 10 mm[21,22].
CT and MRI techniques have comparable overall sensitivity for the detection of malignant peritoneal deposits (92% and 95%, respectively)[23]. MRI performed with fat suppression, delayed contrast-enhanced acquisitions, oral contrast agents, and functional imaging techniques may allow diagnostic sensitivity higher than that achieved by CT. Specifically, the addition of diffusion-weighted sequences to gadolinium-enhanced MRI may improve the accuracy of tumor detection, especially for the assessment of mesentery, serosa of the small bowel and colon, and pelvis (surface of the uterus and bladder)[24], and may also help to detect very small malignant deposits[24].
However, given the cost, MRI is currently reserved for selected cases, to characterize complex adnexal masses and evaluate diffuse peritoneal spread[20].
Recently, PET/CT has been introduced as a diagnostic tool for patients with ovarian cancer. At staging, the contribution of PET is limited to the improved detection of lymphadenopathies on the basis of the degree of [18F]fluorodeoxyglucose uptake in metastatic but normal-sized pelvic and retroperitoneal lymph nodes[25].
In a recent meta-analysis, PET/CT was shown to have an important role in the evaluation of recurrent ovarian cancer, with the highest pooled sensitivity (92%) when compared with PET, CT, and MRI alone[26]. PET/CT is particularly recommended when recurrence is suspected (e.g., in the case of an increase in the cancer antigen 125 marker) and CT examination does not show disease.
Dissemination routes
The routes of spread of ovarian cancer are intraperitoneal dissemination, direct invasion of adjacent organs, lymphatic spread, and hematogenous spread[30].
Intraperitoneal dissemination generates seeding of malignant cells in the Douglas pouch and paravesical spaces, the paracolic gutters, the Morrison pouch, the subdiaphragmatic spaces, and the omentum (Fig. 8)[31]. Lymphatic spread may follow either the main lymphatic ducts along the ovarian veins reaching the para-aortic and paracaval nodes, the broad ligament reaching the pelvic lymph nodes, or the round ligament reaching the inguinal nodes[32]. Hematogenous dissemination is uncommon, the most common sites of disease being the colon (50%), liver (48%), small intestine (44%), and lung (34%).
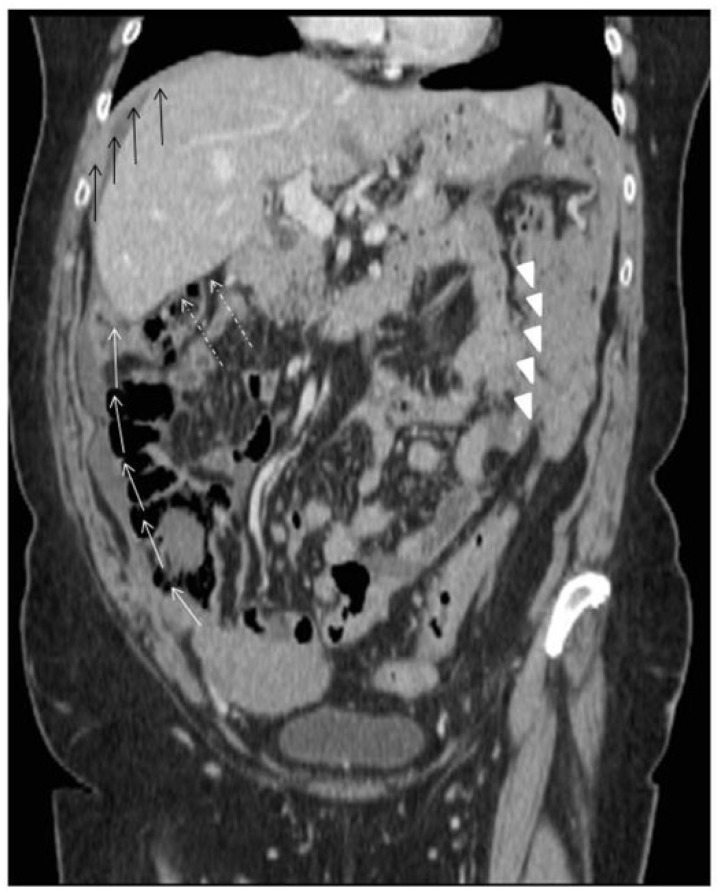
Figure 8.
Intraperitoneal dissemination generates seeding of malignant cells from the ovaries to the paracolic gutters (white arrows), in the Morrison pouch (dashed white arrows), in the subdiaphragmatic spaces (black arrows) and in the omentum (white arrowheads).
Imaging reporting
The goal of CT scanning at preoperative staging is to indicate the bulk of disease, with special attention paid to hard-to-reach sites (Table 1) such as extra-abdominal lesions, porta hepatis, or mesenteric root lymph nodes, to make clinicians aware of sites of disease that may preclude optimal debulking[33,34].
One way to constructively communicate imaging results to the surgeons is by analyzing the abdomen using a quadrant approach.
In the right upper quadrant, deposits on the liver surface and in the peritoneal spaces, such as the Morrison space and the gallbladder fossa, may be present (Fig. 9). Multiplanar reformatting, especially in presence of ascites, may facilitate the detection of diaphragmatic nodules[33,35]. The presence of disease in the subdiaphragmatic spaces does not preclude complete cytoreduction, but has to be indicated at preoperative imaging because it may be missed at a first laparoscopic approach. The presence of subcapsular implants in the region extending between the Morrison pouch and the inferior vena cava (Fig. 10) at the level of the right hepatic vein must be specifically documented. Indeed, these sites are at increased risk of intraoperative bleeding and, according to single-institutional experience, may preclude optimal debulking[33].
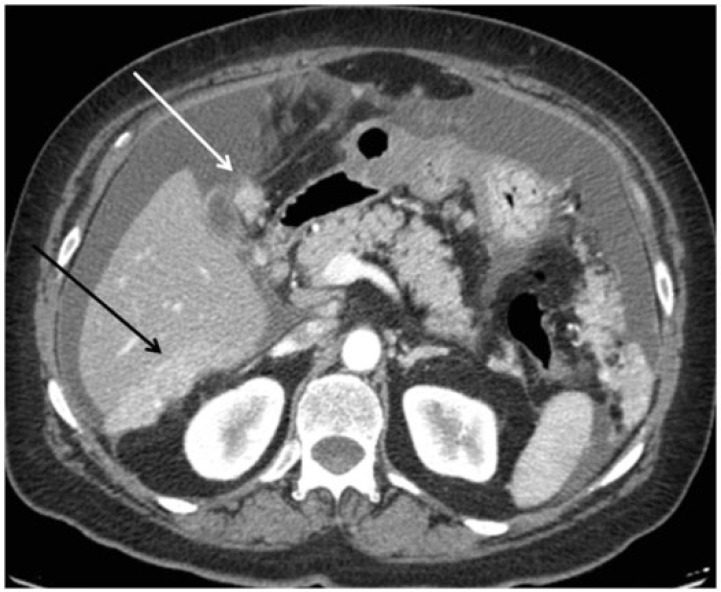
Figure 9.
Axial computed tomography (CT) image showing malignant deposits on the liver surface at the level of the Morrison space (black arrow) and the gallbladder fossa (white arrow).
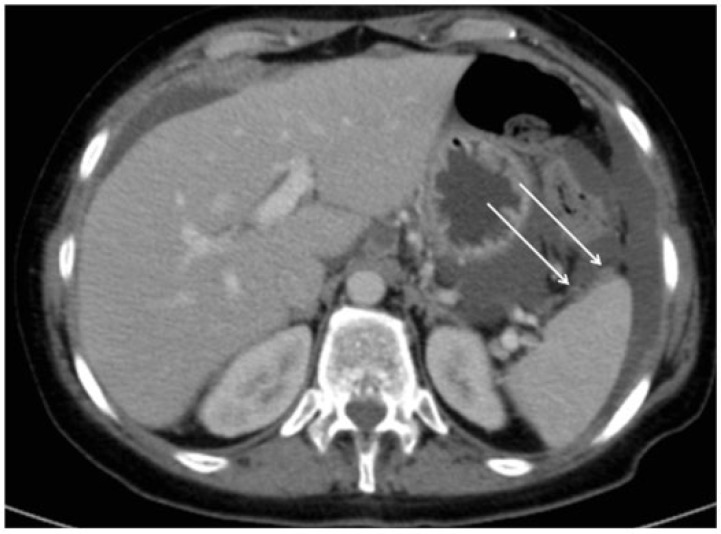
Figure 10.
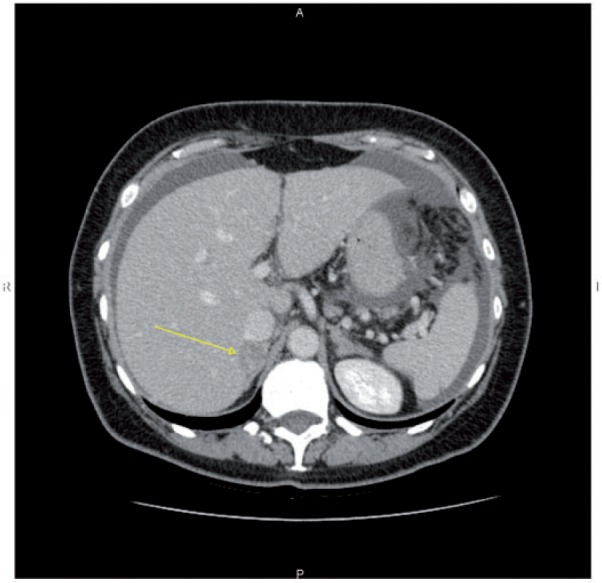
Axial CT image showing hypodense lesion on the posterior surface of the liver (arrow), close to the inferior vena cava.
In the left upper quadrant, attention should be paid to deposits within the spleen, the subdiaphragmatic space, and the lesser sac. Subcapsular splenic deposits produce a scalloped appearance of the underlying peripheral parenchyma and might be resected sparing the spleen (Fig. 11), whereas focal hilar implants are frequently associated with parenchymal invasion and may require splenectomy (Fig. 12). The lesser sac is delineated by the splenorenal ligament and the gastrosplenic ligament. Involvement of these ligaments (Fig. 13) should be reported because some surgeons consider these findings as precluding optimal debulking[33]. The evaluation of the upper quadrants should also include evaluation of lower thorax, where cardiophrenic lymph nodes and pleural effusion may be detected. Cardiophrenic lymph nodes (Fig. 14) are considered enlarged when the short axis is >5 mm[34]. According to different institutional protocols, these lymph nodes may or may not preclude optimal debulking. Pleural effusion may be reactive to the presence of malignant deposits on the abdominal diaphragmatic surface, although it might also indicate the presence of malignant cells on the pleural surface (Fig. 14).
Figure 11.
Axial CT image showing subcapsular splenic deposits (arrows), which produce a scalloped appearance of the peripheral splenic parenchyma.
Figure 12.
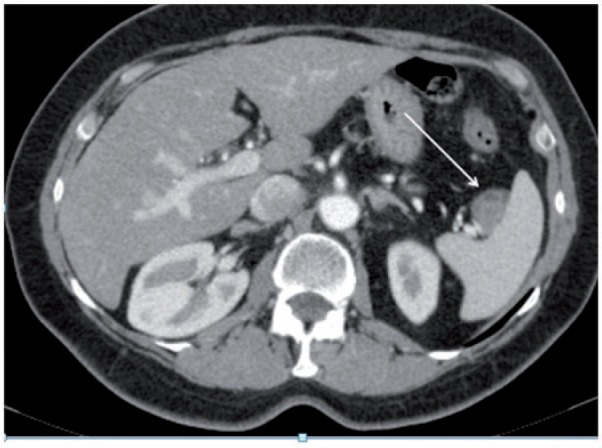
Axial CT image showing inhomogeneous contrast-enhanced lesion (arrow) infiltrating the splenic vessels at the level of the hilum.
Figure 13.
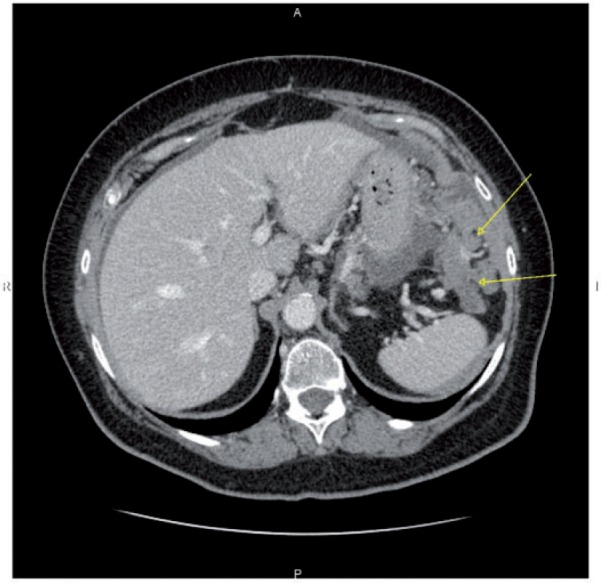
Axial CT image shows large and confluent peritoneal lesions of the gastrosplenic ligament of the lesser sac (arrows).
Figure 14.
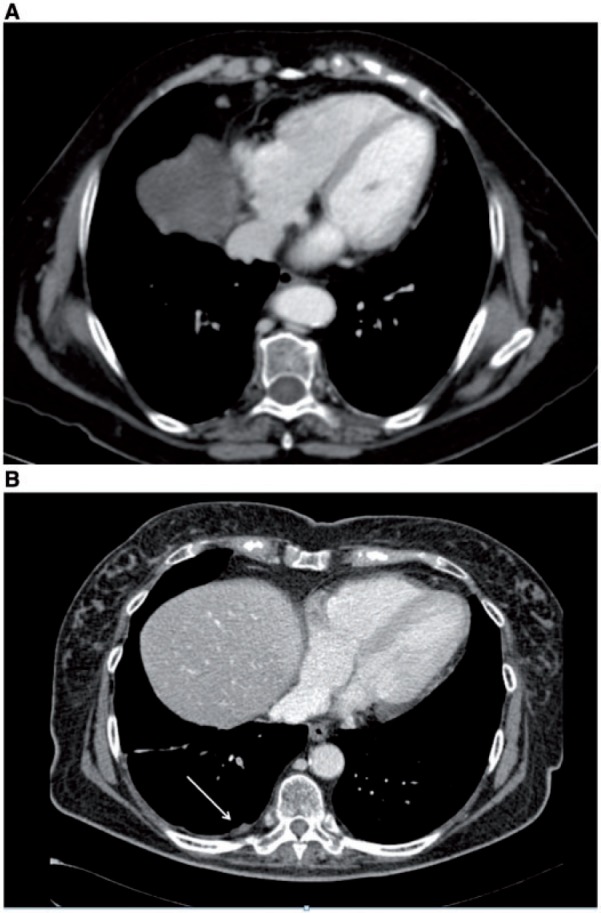
Axial enhanced CT images showing enlarged right cardiophrenic lymph nodes (A) and small enhancing nodules on the right pleural surface (arrow) suggesting stage IV disease (B).
The area of the central abdomen should be carefully evaluated for presence of lymphadenopathies, omental deposits, and mesenteric root deposits. Since lymphadenectomy is not always performed as routine in patients with ovarian cancer, indication of suspicious retroperitoneal lymph nodes may suggest that surgeons perform it. At present, a noninvasive method to recognize lymph node metastases with certainty does not exist. However, if CT shows the presence of lymph nodes with a short axis >10 mm, suspicion of metastasis should be raised[37]. Of note, retroperitoneal lymphadenopathies above the renal vessels and around the celiac axis (Fig. 15) may be difficult sites to resect, so their presence must be emphasized to the surgeon. Omental deposits are common in patients with ovarian cancer[23]. Imaging signs of such involvement range from subtle infiltrative stranding and discrete nodules to confluent masses, also known as omental cake (Fig. 16). Omental lesions are not considered a big challenge for surgeons, as they are routinely removed during debulking surgery. However, wide adhesions between the omentum and the small bowel (Fig. 17), as well as lesions of the lesser sac, may indicate potentially unresectable disease. The CT appearance of mesenteric disease may vary greatly, from clustered small rounded soft-tissue densities (Fig. 18) to confluent large irregular soft-tissue masses, distributed around the superior mesenteric vessels[33]. Extensive mesenteric involvement causes rigidity and retraction, drawing the bowel loops together. A careful assessment is required[21,23,38] because infiltration of the mesenteric root precludes adequate surgical resection[39]. This finding may also be detected and confirmed by DWI at MRI examination (Fig. 19).
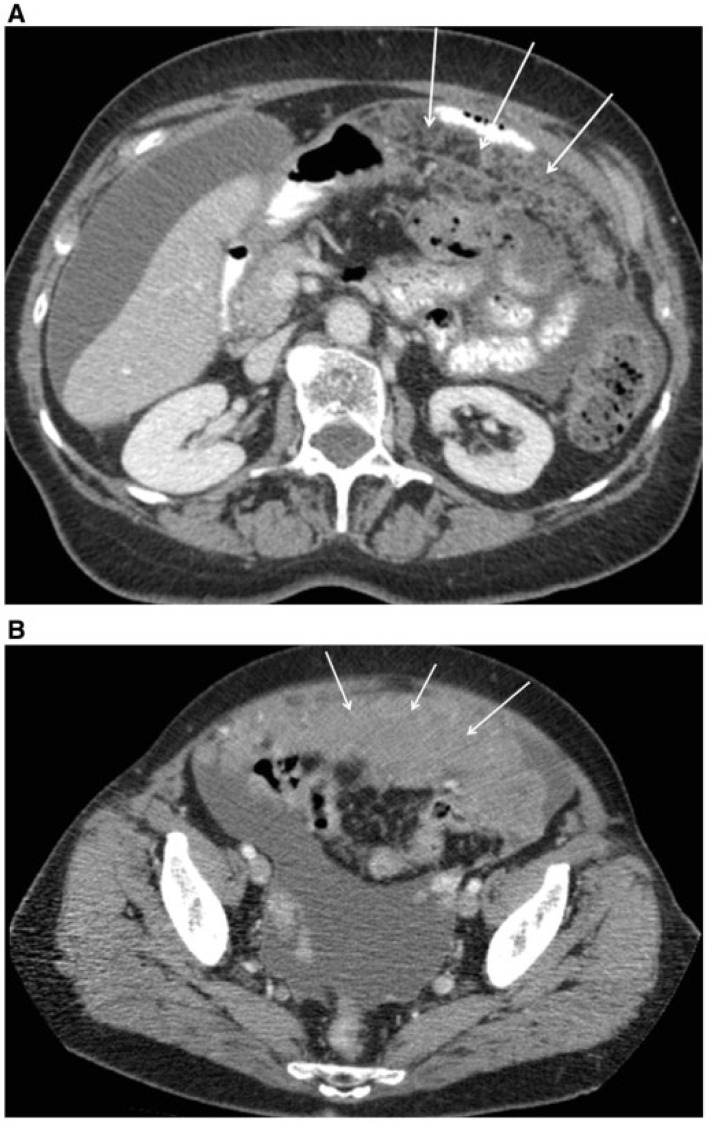
Figure 15.
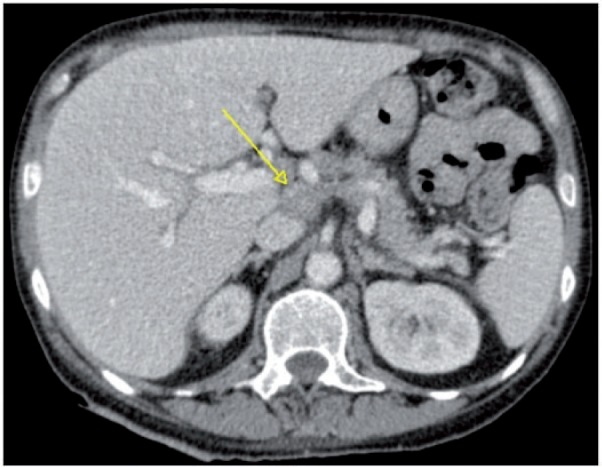
Axial CT image showing pathologic enlarged (short axis >10 mm) lymph node around the celiac axis (arrow), which may preclude optimal debulking.
Figure 16.
Axial CT images showing different appearances of omental deposits: arrows indicate subtle and tiny solid nodules infiltrating and stranding the hypodense omentum (A) and confluent solid masses, also known as omental cake (B).
Figure 17.
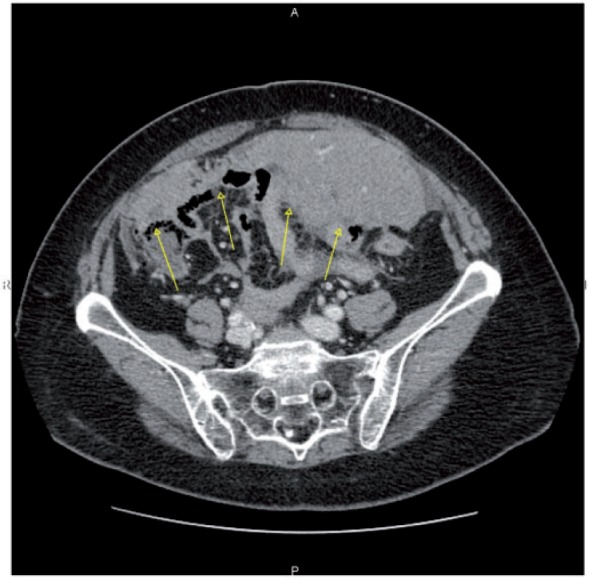
Axial CT image showing a wide adhesion between omental cake and the small bowel (arrows), which precluded optimal debulking.
Figure 18.
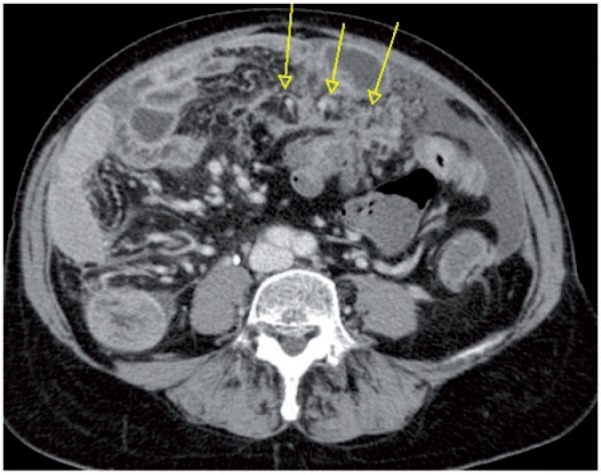
Axial CT image showing clustered small nodules partially confluent (arrows) along the mesenteric root. At surgery this infiltration precluded cytoreduction, and the patient underwent neoadjuvant chemotherapy.
Figure 19.
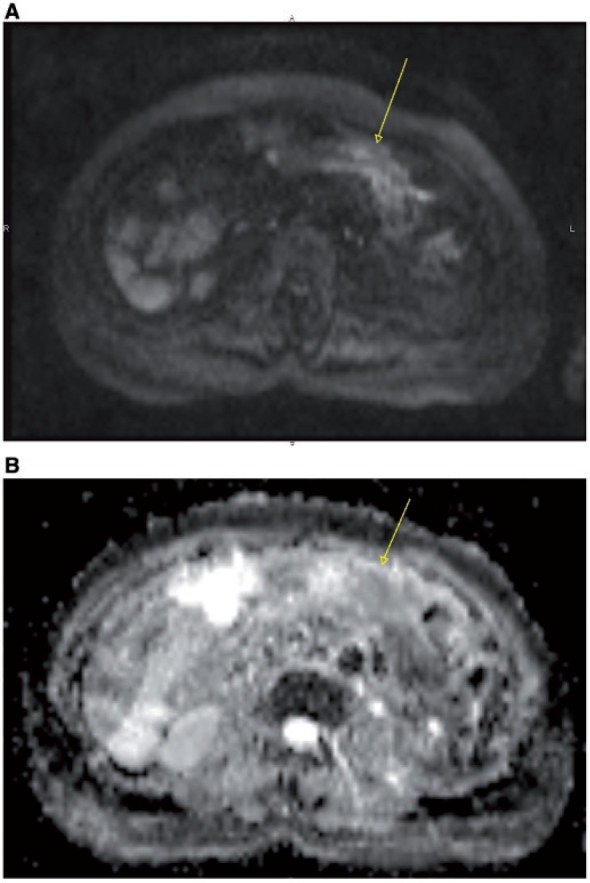
Axial DWI MR image at high b value (900) showing hyperintensity of the mesenteric root (arrow) (A), confirmed as diffusion-restricted area on apparent diffusion coefficient (ADC) map (arrow) (B).
The pelvis is a frequent site of ovarian masses. A finding suggestive of malignancy is the presence of solid components (Fig. 20) and contrast-enhanced intranodular septa (thicker than 3 mm). However, the presence of small ovaries does not exclude ovarian cancer, since cancer may arise from the serosal surface of the ovary, giving directly peritoneal spread with no large ovarian masses (Fig. 21). Intraperitoneal dissemination generates seeding of malignant cells in the Douglas pouch and paravesical spaces. Although rare, any evidence of invasion of the pelvic wall (suspected when the tumor lies 3 mm from the pelvic side wall) should be reported, because the patient may deserve neoadjuvant chemotherapy to reduce the bulk of the disease.
Figure 20.
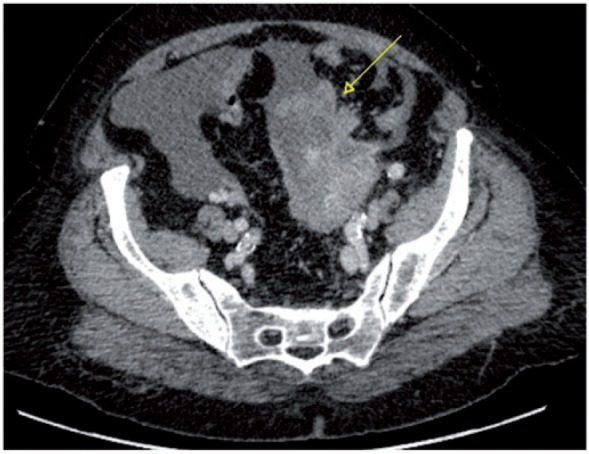
Axial CT image showing a large left adnexal mass (arrow) with solid components, infiltrating the adjacent bowel, associated to the presence of pelvic free fluid.
Figure 21.
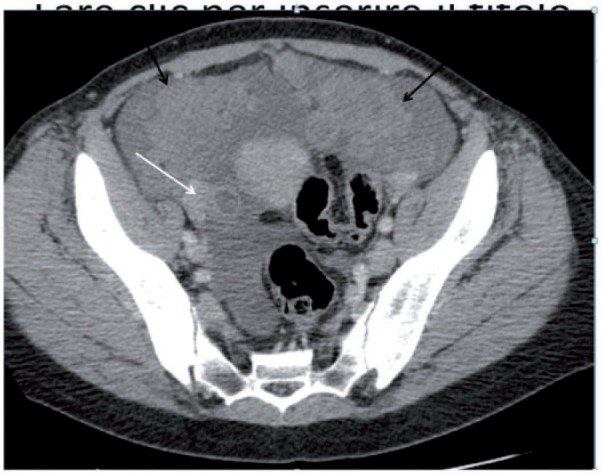
Axial CT image showing large pelvic nodules of carcinomatosis (black arrows), with a small solid right adnexal mass (white arrow).
Imaging features of bowel involvement in patients with ovarian cancer may include diffuse serosal infiltration (Fig. 22A), nodular foci (Fig. 22B), mural thickening (Fig. 22C), or masses involving the serosa and the adjacent mesentery. If the implants are tiny and located on the serosal surface of the bowel, they may be indistinguishable from the bowel wall itself, and only indirect signs of their presence may be seen, such as pelvic free fluid. Bowel serosal deposits, frequently seen on the sigmoid colon and the ileum, may be surgically resected[40] or, depending on the extent of the involved surface, may be considered an indication for neoadjuvant chemotherapy[33].
Figure 22.
Axial CT images showing different appearances of bowel infiltration by ovarian cancer: inhomogeneous enhancement of the bowel serosa (A); presence of enhancing nodular masses within the left pelvis (arrows) infiltrating the distal part of the large bowel (B); presence of abnormal mural thickening of small bowel (arrow) (C).
Cervical cancer
Cervical cancer is the third most common gynecologic malignancy in women in the United States, after uterine corpus and ovary, with an estimated 12,170 new cases and 4220 deaths in 2012[1], squamous carcinoma being the most frequent histologic type (85% and 15% of squamous cell carcinomas and adenocarcinomas, respectively).
A preponderance of evidence supports a strong causal link between human papillomavirus (HPV) (most commonly HPV types 16 and 18) infection and cervical neoplasia[41]. The introduction of the vaccine against high-risk HPV types will likely have a major impact on disease prevention and prevalence[42].
Management of patients with cervical cancer
Early-stage tumors can be managed with cone biopsy or simple hysterectomy (stage IA), whereas higher stage tumors can be treated with either radical surgery/radiotherapy (IB1–IIA, <4 cm) or concomitant chemoradiotherapy (IIB–IVB).
Extension into the parametrium represents a key factor for management because this is considered a contraindication to surgery. However, owing to newly introduced targeted radiotherapy techniques, in some centers large tumors, even those without parametrial invasion, have increasingly been treated by chemoradiation therapy[43].
In young patients willing to preserve fertility and with small invasive tumors, a conservative surgical procedure (such as trachelectomy) can be performed.
Preoperative evaluation
FIGO staging of cervical cancer is entirely clinical and does not rely on surgicopathologic findings. This is mainly due to the need of a staging system that is universally available. Indeed, cervical cancer is more prevalent in developing countries, where more sophisticated modalities are not available and where patients may not undergo staging via surgical or diagnostic imaging techniques.
The FIGO staging procedures include clinical examination of the cervix (under anesthesia, if necessary), colposcopy, lesion biopsy, cystoscopy, and rectoscopy when indicated[44]. Conventional radiographic techniques included in the FIGO staging are chest X-ray, barium enema, and intravenous urography.
Since the introduction of the revised FIGO staging[4] incorporation of cross-sectional imaging has been encouraged, if available, to assess important prognostic factors such as tumor size, parametrial involvement, adjacent organ and pelvic side-wall invasion, and evaluation of lymph node metastases. Specifically, MRI is considered the best imaging modality to stage early cervical carcinoma such as FIGO stage 1B1 or greater[45].
According to the ESUR guidelines, the essential protocol, preferably performed using a 1.5-T magnet, must include a combination of at least two T2-weighted sequences (sagittal and oblique planes, perpendicular to the long axis of the cervical canal), and one axial T1-weighted sequence of the pelvis. Many investigators also acquire at least one sequence from the level of the renal veins to the pelvic brim so as to obviate an additional CT examination for evaluation of retroperitoneal adenopathies and hydronephrosis[44,46].
The use of contrast-enhanced T1-weighted sequences is variable in different centers[45]. Evaluation of the earlier phases of contrast enhancement may help to accurately delineate small cervical tumors[47]. The use of intravenous contrast medium is also recognized as helpful in the posttreatment setting, to differentiate residual or recurrent tumor from radiation fibrosis[6].
DWI seems to be a promising technique in the evaluation of cervical cancer[48,49]. Cervical cancer shows a lower ADC in comparison with the normal cervix (Fig. 23), and the ADC increases after chemoradiotherapy[48,50]. DWI has been considered helpful in the detection of residual tumor or suspicious lymph nodes after chemoradiotherapy, and in some cases might be competitive with PET imaging[9,51]. However, there is still lack of standardization of the b values used in different centers, and some applications of DWI still remain confined to the research setting[9].
Figure 23.
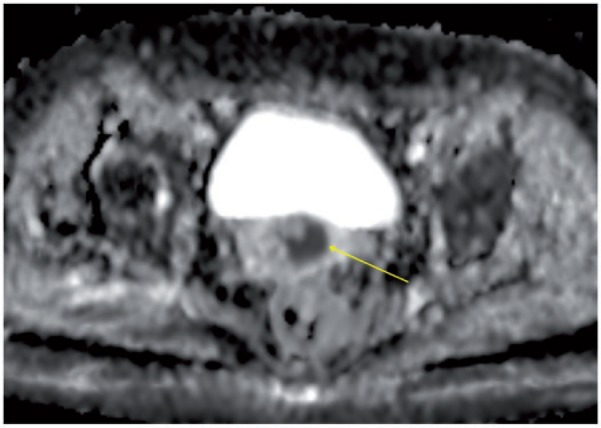
Axial MR ADC map showing hypointensity of cervical cancer (arrow), in comparison with the surrounding normal cervix.
PET/CT could be helpful at staging to detect lymph node and extranodal metastases. However, PET/CT has limited value in terms of detection of local spread because of its limited spatial resolution. Data show better detection of para-aortic involvement with PET than with MRI or CT[52]. In a meta-analysis, sensitivity of PET reached 73% when the prevalence of lymph node involvement was more than 15%[54]. However, in patients with negative morphologic imaging, sensitivity of PET/CT for detection of microscopic lymph node metastases was much lower (almost 34%)[53].
Dissemination routes
Cervical cancer spreads regionally by direct extension to contiguous structures, such as uterine corpus (stage I), vagina (stage IIA–IIIA), and parametrium (stage IIB). It can also spread through lymphatic channels to regional nodes (firstly to iliac and obturator nodes, secondarily to para-aortic lymph nodes) and, rarely, through hematogenous dissemination to distant organs (stage IVB)[54].
Imaging reporting
The ESUR committee has suggested a checklist to follow to provide a comprehensive and easy-to-read radiologic report, helpful in communication with the surgeons when tailoring treatment options[45]. Indeed, to better stage cervical cancer, MRI may add important information to the clinical FIGO staging.
Cervical carcinoma appears isointense to the cervical stroma on T1-weighted images, whereas it shows high signal intensity, surrounded by low signal intensity of the cervical stroma, on T2-weighted images (Fig. 24). Accurate description of the lesion, including size of the tumor (the largest size, transversal or longitudinal, indicates T parameter) and distance from the internal os, must be reported. MRI is particularly helpful in evaluation of patients being considered for fertility-preserving treatments (i.e., extended cone biopsy or trachelectomy). In these cases, specific information required comprises tumor size <2 cm, cervical length >2.5 cm, distance of the tumor from the internal os >1 cm[45].
Figure 24.
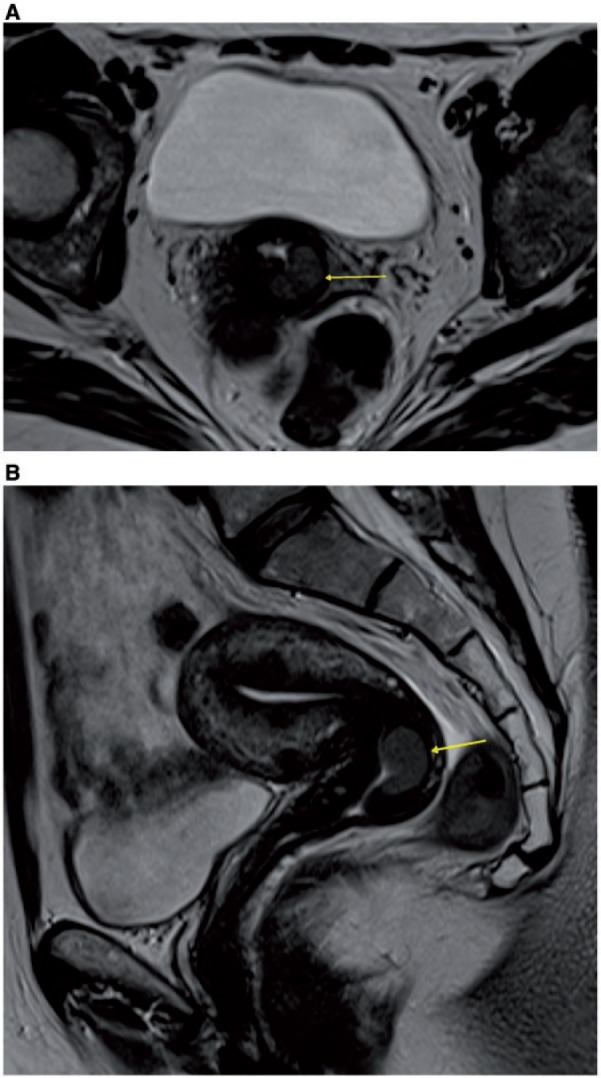
Axial (A) and sagittal (B) MR T2-weighted images showing hyperintense FIGO stage IB1 cervical tumor preserving the hypointense cervical stroma (arrows).
Vaginal invasion is detected when the normal low signal intensity of the vaginal wall is replaced by the high-signal-intensity tumor on T2-weighted images (Fig. 25).
Figure 25.
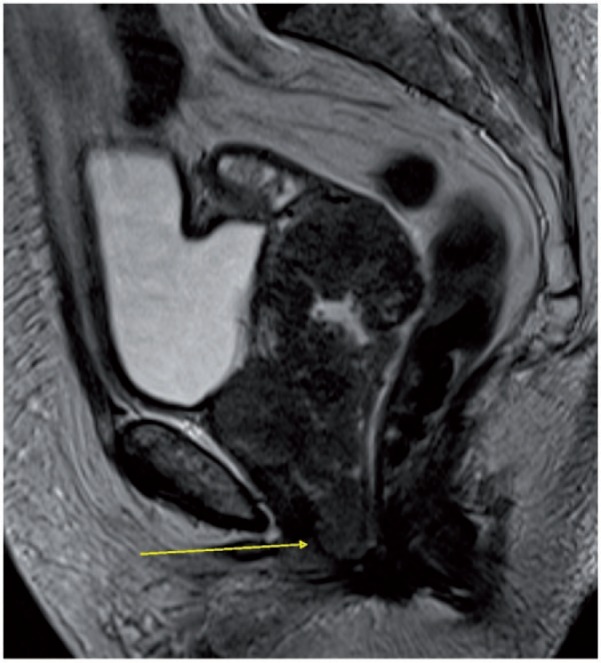
Sagittal MR T2-weighted image showing a large cervical cancer infiltrating the lower third of the vagina (arrow) and the upper third of the urethra.
Presence of parametrial invasion, suspected when there is disruption of the stromal ring (high NPV and moderate to high PPV), is best demonstrated on oblique axial high resolution images (Fig. 26). Additional features indicating parametrial invasion include: spiculated tumor-parametrium interface; soft-tissue extension into the parametria and along the cardinal or uterosacral ligaments (Fig. 26); encasement of the periuterine vessels; and hydro-ureteronephrosis (Fig. 27)[55].
Figure 26.
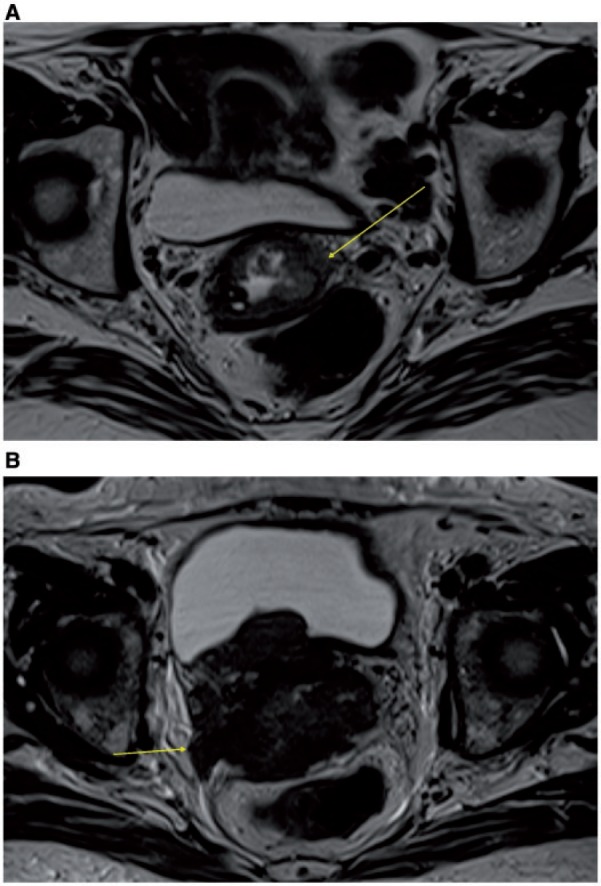
Axial MR T2-weighted images showing: disruption of the stromal ring on the left (arrow) and initial thickening of the ipsilateral parametrium by a FIGO stage IIB cervical cancer (A); large cervical cancer invading parametria associated with thickening of the right uterosacral ligament (arrow) (B).
Figure 27.
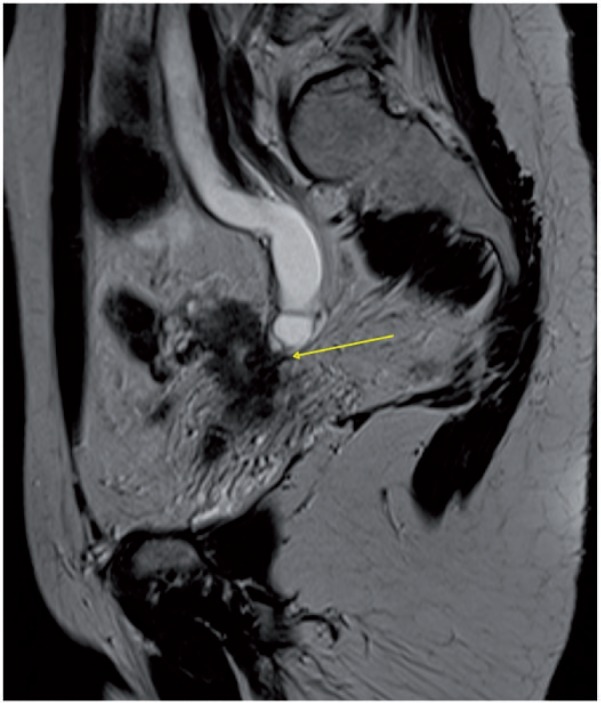
Sagittal MR T2-weighted image showing ureteronephrosis caused by encasement of the distal ureter by cervical cancer (arrow).
Isthmic invasion, difficult to evaluate by clinical evaluation, should be indicated because this information may suggest where to locate the brachytherapy coils[56].
Bladder and/or rectal involvement are diagnosed when there is disruption of the normal hypointense walls on T2-weighted images, with or without a mass protruding into the lumen. Pelvic side-wall invasion is likely when the tumor extends within 3 mm of the pelvic side wall (internal obturator, piriform, or levator ani muscles), with or without hydronephrosis.
Staging of lymph nodes, based on the size criterion (10 mm in short axis) still gives a low accuracy to MRI in the assessment of nodal status (reported ranges of sensitivity and specificity of 38–89% and 78–99%, respectively)[57,58]. The presence of intranodal necrosis may be an adjunctive feature suggesting nodal metastasis.
Conclusion
Imaging is crucial in gynecologic malignancies, for diagnosis as well as for arriving at the decision regarding the best treatment options available. Accordingly, if surgery is the best treatment option, accurate preoperative imaging assessment allows the surgeon to ensure the most appropriate surgical procedure for the patient. Greater involvement in multidisciplinary approaches and teamwork may reduce undertreatments and complications due to overtreatments, thus eventually increasing patient survival and quality of life.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Freeman SJ, Aly AM, Kataoka MY, Addley HC, Reinhold C, Sala E. The revised FIGO staging system for uterine malignancies: implications for MR imaging. Radiographics. 2012;32:1805–1827. doi: 10.1148/rg.326125519. [DOI] [PubMed] [Google Scholar]
- 3.Bell DJ, Pannu HK. Radiological assessment of gynecologic malignancies. Obstet Gynecol Clin North Am. 2011;38:45–68. doi: 10.1016/j.ogc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Kinkel K, Forstner R, Danza FM, et al. Staging of endometrial cancer with MRI: guidelines of the European Society of Urogenital Imaging. Eur Radiol. 2009;19:1565–1574. doi: 10.1007/s00330-009-1309-6. [DOI] [PubMed] [Google Scholar]
- 6.Kinkel K, Ariche M, Tardivon AA, et al. Differentiation between recurrent tumor and benign conditions after treatment of gynecologic pelvic carcinoma: value of dynamic contrast-enhanced subtraction MR imaging. Radiology. 1997;204:55–63. doi: 10.1148/radiology.204.1.9205223. [DOI] [PubMed] [Google Scholar]
- 7.Padhani AR, Liu G, Koh DM, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamai K, Koyama T, Saga T, et al. Diffusion-weighted MR imaging of uterine endometrial cancer. J Magn Reson Imaging. 2007;26:682–687. doi: 10.1002/jmri.20997. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi M, Matsuzaki K, Nishitani H. Diffusion-weighted magnetic resonance imaging of endometrial cancer: differentiation from benign endometrial lesions and preoperative assessment of myometrial invasion. Acta Radiol. 2009;50:947–953. doi: 10.1080/02841850903099981. [DOI] [PubMed] [Google Scholar]
- 10.Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology. 2013;266:717–740. doi: 10.1148/radiol.12120315. [DOI] [PubMed] [Google Scholar]
- 11.Kitajima K, Murakami K, Yamasaki E, Kaji Y, Sugimura K. Accuracy of integrated FDG-PET/contrast-enhanced CT in detecting pelvic and paraaortic lymph node metastasis in patients with uterine cancer. Eur Radiol. 2009;19:1529–1536. doi: 10.1007/s00330-008-1271-8. [DOI] [PubMed] [Google Scholar]
- 12.Crivellaro C, Signorelli M, Guerra L, et al. Tailoring systematic lymphadenectomy in high-risk clinical early stage endometrial cancer: the role of 18F-FDG PET/CT. Gynecol Oncol. 2013;130:306–311. doi: 10.1016/j.ygyno.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Manfredi R, Mirk P, Maresca G, et al. Local-regional staging of endometrial carcinoma: role of MR imaging in surgical planning. Radiology. 2004;231:372–378. doi: 10.1148/radiol.2312021184. [DOI] [PubMed] [Google Scholar]
- 14.Peungjesada S, Bhosale PR, Balachandran A, Iyer RB. Magnetic resonance imaging of endometrial carcinoma. J Comput Assist Tomogr. 2009;33:601–608. doi: 10.1097/RCT.0b013e31818d4279. [DOI] [PubMed] [Google Scholar]
- 15.Namimoto T, Awai K, Nakaura T, Yanaga Y, Hirai T, Yamashita Y. Role of diffusion-weighted imaging in the diagnosis of gynecological diseases. Eur Radiol. 2009;19:745–760. doi: 10.1007/s00330-008-1185-5. [DOI] [PubMed] [Google Scholar]
- 16.Lin G, Ng KK, Chang CJ, et al. Myometrial invasion in endometrial cancer: diagnostic accuracy of diffusion-weighted 3.0-T MR imaging—initial experience. Radiology. 2009;250:784–792. doi: 10.1148/radiol.2503080874. [DOI] [PubMed] [Google Scholar]
- 17.Aletti GD, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006;107:77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 19.Morice P, Dubernard G, Rey A, et al. Results of interval debulking surgery compared with primary debulking surgery in advanced stage ovarian cancer. J Am Coll Surg. 2003;197:955–963. doi: 10.1016/j.jamcollsurg.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Viswanathan A, Buttin BM, Kennedy AM. Oncodiagnosis Panel 2006. Ovarian, cervical, and endometrial cancer. Radiographics. 2008;28:289–307. doi: 10.1148/rg.281075134. [DOI] [PubMed] [Google Scholar]
- 21.Coakley FV, Choi PH, Gougoutas CA, et al. Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology. 2002;223:495–499. doi: 10.1148/radiol.2232011081. [DOI] [PubMed] [Google Scholar]
- 22.Kyriazi S, Collins DJ, Morgan VA, Giles SL, deSouza NM. Diffusion-weighted imaging of peritoneal disease for noninvasive staging of advanced ovarian cancer. Radiographics. 2010;30:1269–1285. doi: 10.1148/rg.305105073. [DOI] [PubMed] [Google Scholar]
- 23.Tempany CM, Zou KH, Silverman SG, Brown DL, Kurtz AB, McNeil BJ. Staging of advanced ovarian cancer: comparison of imaging modalities—report from the Radiological Diagnostic Oncology Group. Radiology. 2000;215:761–767. doi: 10.1148/radiology.215.3.r00jn25761. [DOI] [PubMed] [Google Scholar]
- 24.Low RN, Barone RM, Lacey C, Sigeti JS, Alzate GD, Sebrechts CP. Peritoneal tumor: MR imaging with dilute oral barium and intravenous gadolinium-containing contrast agents compared with unenhanced MR imaging and CT. Radiology. 1997;204:513–520. doi: 10.1148/radiology.204.2.9240546. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida Y, Kurokawa T, Kawahara K, et al. Incremental benefits of FDG positron emission tomography over CT alone for the preoperative staging of ovarian cancer. AJR Am J Roentgenol. 2004;182:227–233. doi: 10.2214/ajr.182.1.1820227. [DOI] [PubMed] [Google Scholar]
- 26.Gu P, Pan LL, Wu SQ, Sun L, Huang G. CA 125, PET alone, PET-CT, CT and MRI in diagnosing recurrent ovarian carcinoma: a systematic review and meta-analysis. Eur J Radiol. 2009;71:164–174. doi: 10.1016/j.ejrad.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Forstner R. Radiological staging of ovarian cancer: imaging findings and contribution of CT and MRI. Eur Radiol. 2007;17:3223–3235. doi: 10.1007/s00330-007-0736-5. [DOI] [PubMed] [Google Scholar]
- 28.Forstner R, Sala E, Kinkel K, Spencer JA European Society of Urogenital Radiology. ESUR guidelines: ovarian cancer staging and follow-up. Eur Radiol. 2010;20:2773–2780. doi: 10.1007/s00330-010-1886-4. [DOI] [PubMed] [Google Scholar]
- 29.Holloway BJ, Gore ME, A’Hern RP, Parsons C. The significance of paracardiac lymph node enlargement in ovarian cancer. Clin Radiol. 1997;52:692–697. doi: 10.1016/s0009-9260(97)80034-7. [DOI] [PubMed] [Google Scholar]
- 30.Woodward PJ, Hosseinzadeh K, Saenger JS. Radiologic staging of ovarian carcinoma with pathologic correlation. Radiographics. 2004;24:225–246. doi: 10.1148/rg.241035178. [DOI] [PubMed] [Google Scholar]
- 31.Meyers MA. Distribution of intra-abdominal malignant seeding: dependency on dynamics of flow of ascitic fluid. Am J Roentgenol Radium Ther Nucl Med. 1973;119:198–206. doi: 10.2214/ajr.119.1.198. [DOI] [PubMed] [Google Scholar]
- 32.Feldman GB, Knapp RC. Lymphatic drainage of the peritoneal cavity and its significance in ovarian cancer. Am J Obstet Gynecol. 1974;119:991–994. doi: 10.1016/0002-9378(74)90021-0. [DOI] [PubMed] [Google Scholar]
- 33.Nougaret S, Addley HC, Colombo PE, et al. Ovarian carcinomatosis: how the radiologist can help plan the surgical approach. Radiographics. 2012;32:1775–1800. doi: 10.1148/rg.326125511. [DOI] [PubMed] [Google Scholar]
- 34.Qayyum A, Coakley FV, Westphalen AC, Hricak H, Okuno WT, Powell B. Role of CT and MR imaging in predicting optimal cytoreduction of newly diagnosed primary epithelial ovarian cancer. Gynecol Oncol. 2005;96:301–306. doi: 10.1016/j.ygyno.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 35.Walkey MM, Friedman AC, Sohotra P, Radecki PD. CT manifestations of peritoneal carcinomatosis. AJR Am J Roentgenol. 1988;150:1035–1041. doi: 10.2214/ajr.150.5.1035. [DOI] [PubMed] [Google Scholar]
- 36.Kolev V, Mironov S, Mironov O, et al. Prognostic significance of supradiaphragmatic lymphadenopathy identified on preoperative computed tomography scan in patients undergoing primary cytoreduction for advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2010;20:979–984. doi: 10.1111/IGC.0b013e3181e833f5. [DOI] [PubMed] [Google Scholar]
- 37.Tangjitgamol S, Manusirivithaya S, Sheanakul C, et al. Can we rely on the size of the lymph node in determining nodal metastasis in ovarian carcinoma? Int J Gynecol Cancer. 2003;13:297–302. doi: 10.1046/j.1525-1438.2003.13192.x. [DOI] [PubMed] [Google Scholar]
- 38.de Bree E, Koops W, Kröger R, van Ruth S, Witkamp AJ, Zoetmulder FA. Peritoneal carcinomatosis from colorectal or appendiceal origin: correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J Surg Oncol. 2004;86:64–73. doi: 10.1002/jso.20049. [DOI] [PubMed] [Google Scholar]
- 39.Sugarbaker PH. Surgical responsibilities in the management of peritoneal carcinomatosis. J Surg Oncol. 2010;101:713–724. doi: 10.1002/jso.21484. [DOI] [PubMed] [Google Scholar]
- 40.Colombo PE, Mourregot A, Fabbro M, et al. Aggressive surgical strategies in advanced ovarian cancer: a monocentric study of 203 stage IIIC and IV patients. Eur J Surg Oncol. 2009;35:135–143. doi: 10.1016/j.ejso.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Syrjänen K, Mäntyjärvi R, Väyrynen M, et al. Human papillomavirus (HPV) infections involved in the neoplastic process of the uterine cervix as established by prospective follow-up of 513 women for two years. Eur J Gynaecol Oncol. 1987;8:5–16. [PubMed] [Google Scholar]
- 42.Stanley M. Human papillomavirus vaccines versus cervical cancer screening. Clin Oncol (R Coll Radiol) 2008;20:388–394. doi: 10.1016/j.clon.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Patel S, Liyanage SH, Sahdev A, Rockall AG, Reznek RH. Imaging of endometrial and cervical cancer. Insight Imaging. 2010;1:309–328. doi: 10.1007/s13244-010-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hancke K, Heilmann V, Straka P. Pretreatment staging of cervical cancer: is imaging better than palpation? Ann Surg Oncol. 2008;15:2856–2861. doi: 10.1245/s10434-008-0088-7. [DOI] [PubMed] [Google Scholar]
- 45.Balleyguier C, Sala E, Da Cunha T, et al. Staging of uterine cervical cancer with MRI: guidelines of the European Society of Urogenital Radiology. Eur Radiol. 2011;21:1102–1110. doi: 10.1007/s00330-010-1998-x. [DOI] [PubMed] [Google Scholar]
- 46.Choi SH, Kim SH, Choi HJ, Park BK, Lee HJ. Preoperative magnetic resonance imaging staging of uterine cervical carcinoma: results of prospective study. J Comput Assist Tomogr. 2004;28:620–627. doi: 10.1097/01.rct.0000138007.77725.0a. [DOI] [PubMed] [Google Scholar]
- 47.Akita A, Shinmoto H, Hayashi S, et al. Comparison of T2-weighted and contrast enhanced T1-weighted MR imaging at 1.5 T for assessing the local extent of cervical carcinoma. Eur Radiol. 2011;21:1850–1857. doi: 10.1007/s00330-011-2122-6. [DOI] [PubMed] [Google Scholar]
- 48.Harry VN, Semple SI, Gilbert FJ, Parkin DE. Diffusion weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol. 2008;111:213–220. doi: 10.1016/j.ygyno.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 49.Whittaker CS, Coady A, Culver L, Rustin G, Padwick M, Padhani AR. Diffusion-weighted MR imaging of female pelvic tumours: a pictorial review. Radiographics. 2009;29:759–774. doi: 10.1148/rg.293085130. [DOI] [PubMed] [Google Scholar]
- 50.Rizzo S, Summers P, Raimondi S, et al. Diffusion-weighted MR imaging in assessing cervical tumour response to nonsurgical therapy. Radiol Med. 2011;116:766–780. doi: 10.1007/s11547-011-0650-4. [DOI] [PubMed] [Google Scholar]
- 51.Park SO, Kim JK, Kim KA, et al. Relative apparent diffusion coefficient: determination of reference site and validation of benefit for detecting metastatic lymph nodes in uterine cervical cancer. J Magn Reson Imaging. 2009;29:383–390. doi: 10.1002/jmri.21635. [DOI] [PubMed] [Google Scholar]
- 52.Gouy S, Morice P, Narducci F, et al. Nodal-staging surgery for locally advanced cervical cancer in the era of PET. Lancet Oncol. 2012;13:e212–e220. doi: 10.1016/S1470-2045(12)70011-6. [DOI] [PubMed] [Google Scholar]
- 53.Kang S, Kim SK, Chung DC, et al. Diagnostic value of 18F-FDG PET for evaluation of paraaortic nodal metastasis in patients with cervical carcinoma: a meta-analysis. J Nucl Med. 2010;51:360–367. doi: 10.2967/jnumed.109.066217. [DOI] [PubMed] [Google Scholar]
- 54.Grigsby PW. The prognostic value of PET and PET/CT in cervical cancer. Cancer Imaging. 2008;8:146–155. doi: 10.1102/1470-7330.2008.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaur H, Silverman PM, Iyer RB, Verschraegen CF, Eifel PJ, Charnsangavej C. Diagnosis, staging, and surveillance of cervical carcinoma. AJR Am J Roentgenol. 2003;180:1621–1631. doi: 10.2214/ajr.180.6.1801621. [DOI] [PubMed] [Google Scholar]
- 56.Dimopoulos JC, Kirisits C, Petric P, et al. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: clinical feasibility and preliminary results. Int J Radiat Oncol Biol Phys. 2006;66:83–90. doi: 10.1016/j.ijrobp.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 57.Hawighorst H. Dynamic MR imaging in cervical carcinoma. Radiology. 1999;213:617–618. doi: 10.1148/radiology.213.2.r99se23617. [DOI] [PubMed] [Google Scholar]
- 58.Scheidler J, Hricak H, Yu KK, Subak L, Segal MR. Radiological evaluation of lymph node metastases in patients with cervical cancer. A meta-analysis. JAMA. 1997;278:1096–1101. [PubMed] [Google Scholar]