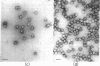
Abstract
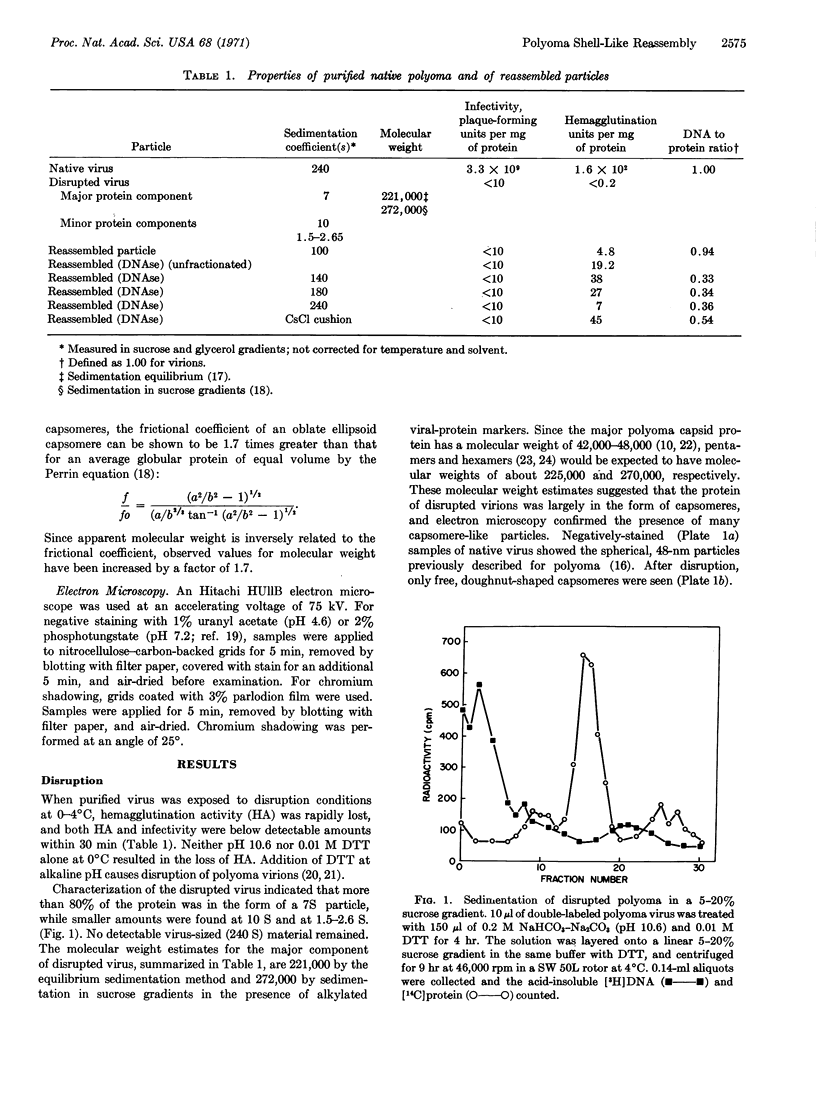
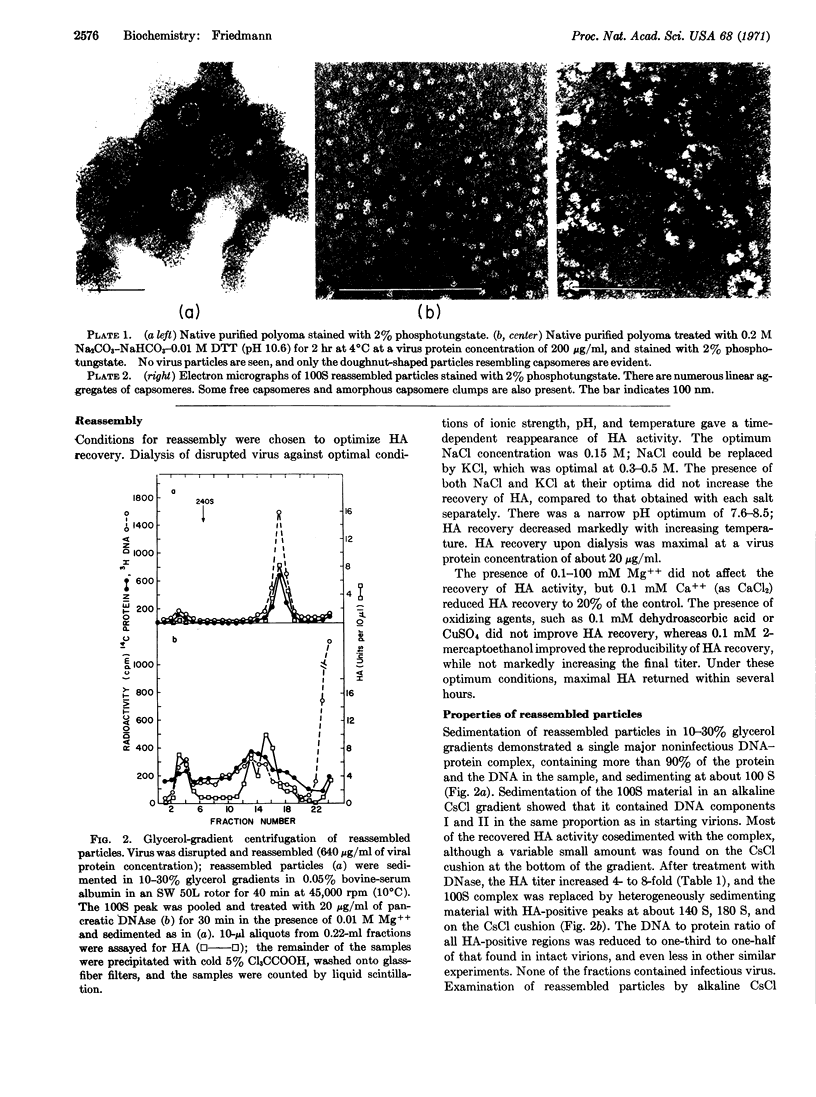
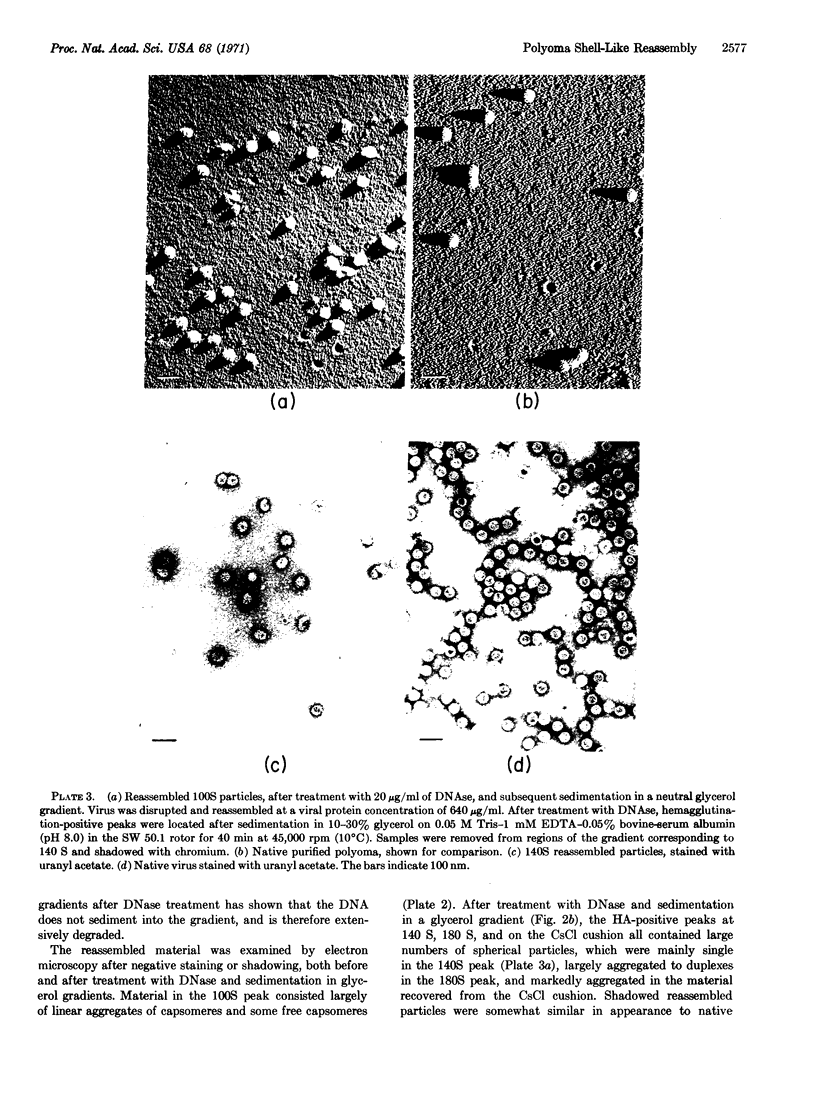
When purified polyoma virus is exposed to 0.01 M dithiothreitol in the presence of 0.2 M Na2CO3-NaHCO3 (pH 10.6) at 0-4°C, the capsids are rapidly disrupted to protein subunits of capsomere size, as judged by density gradient centrifugation, sedimentation equilibrium centrifugation, and electron microscopy. Hemagglutination activity and infectivity of disrupted virus are reduced to below detectable amounts. Removal of the disruption reagents by dialysis at 4°C against 0.05 M Tris-0.14 M NaCl-1 mM EDTA and 0.1 mM 2-mercaptoethanol (pH 8.0) results in a time-dependent reappearance of up to 17% of the starting hemagglutination titer, under optimum conditions of ionic strength, pH, temperature, and virus protein concentration. The recovered hemagglutination activity is found in glycerol gradients associated with a 100S DNA-protein complex consisting mostly of linear aggregates of capsomeres. When the linear complex is treated with pancreatic DNase, the complex is converted into spherical particles, of approximately virus size, that sediment at 140 S (with aggregates at 180 S), as well as on the cushion of half-saturated CsCl at the bottom of the gradients. All reassembled particles are not infectious and have markedly reduced DNA to protein ratios.
Keywords: hemagglutination, infectivity, electron microscopy, density-gradient centrifugation
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Bancroft J. B., Wagner G. W., Bracker C. E. The self-assembly of a nucleic-acid free pseudo-top component for a small spherical virus. Virology. 1968 Sep;36(1):146–149. doi: 10.1016/0042-6822(68)90126-8. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., CRAWFORD E. M., WATSONDH The physical characteristics of polyoma virus. I. Two types of particle. Virology. 1962 Oct;18:170–176. doi: 10.1016/0042-6822(62)90002-8. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V. The adsorption of polyoma virus. Virology. 1962 Oct;18:177–181. doi: 10.1016/0042-6822(62)90003-x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. EVIDENCE FOR A RING STRUCTURE OF POLYOMA VIRUS DNA. Proc Natl Acad Sci U S A. 1963 Aug;50:236–243. doi: 10.1073/pnas.50.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel-Conrat H. Reconstitution of viruses. Annu Rev Microbiol. 1970;24:463–478. doi: 10.1146/annurev.mi.24.100170.002335. [DOI] [PubMed] [Google Scholar]
- Fraenkel-Conrat H., Williams R. C. RECONSTITUTION OF ACTIVE TOBACCO MOSAIC VIRUS FROM ITS INACTIVE PROTEIN AND NUCLEIC ACID COMPONENTS. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):690–698. doi: 10.1073/pnas.41.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., Haas M. Rapid concentration and purification of polyoma virus and SV40 with polyethylene glycol. Virology. 1970 Sep;42(1):248–250. doi: 10.1016/0042-6822(70)90263-1. [DOI] [PubMed] [Google Scholar]
- Hare J. D., Chan J. C. Role of hydrogen and disulfide bonds in polyoma capsid structure. Virology. 1968 Mar;34(3):481–491. doi: 10.1016/0042-6822(68)90068-8. [DOI] [PubMed] [Google Scholar]
- Hiebert E., Bancroft J. B., Bracker C. E. The assembly in vitro of some small spherical viruses, hybrid viruses, and other nucleoproteins. Virology. 1968 Mar;34(3):492–508. doi: 10.1016/0042-6822(68)90069-x. [DOI] [PubMed] [Google Scholar]
- Hohn T. Selfassembly of defective particles of the bacteriophage fr. Eur J Biochem. 1967 Sep;2(2):152–155. doi: 10.1111/j.1432-1033.1967.tb00119.x. [DOI] [PubMed] [Google Scholar]
- KLUG A. STRUCTURE OF VIRUSES OF THE PAPILLOMA-POLYOMA TYPE. II. COMMENTS ON OTHER WORK. J Mol Biol. 1965 Feb;11:424–431. doi: 10.1016/s0022-2836(65)80067-5. [DOI] [PubMed] [Google Scholar]
- Kass S. J. Chemical studies on polyoma and Shope papilloma viruses. J Virol. 1970 Mar;5(3):381–387. doi: 10.1128/jvi.5.3.381-387.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A., Finch J. T. Structure of viruses of the papilloma-polyoma type. IV. Analysis of tilting experiments in the electron microscope. J Mol Biol. 1968 Jan 14;31(1):1–12. doi: 10.1016/0022-2836(68)90050-8. [DOI] [PubMed] [Google Scholar]
- Ling C. M., Hung P. P., Overby L. R. Specificity in self-assembly of bacteriophages Q beta and MS2. Biochemistry. 1969 Nov;8(11):4464–4469. doi: 10.1021/bi00839a036. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Michel M. R., Hirt B., Weil R. Mouse cellular DNA enclosed in polyoma viral capsids (pseudovirions). Proc Natl Acad Sci U S A. 1967 Oct;58(4):1381–1388. doi: 10.1073/pnas.58.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami W. T., Fine R., Harrington M. R., Sassan Z. B. Properties and amino acid composition of polyoma virus purified by zonal ultracentrifugation. J Mol Biol. 1968 Aug 28;36(1):153–166. doi: 10.1016/0022-2836(68)90226-x. [DOI] [PubMed] [Google Scholar]
- Perry J. L., To C. M., Consigli R. A. Alkaline degradation of polyoma virus. J Gen Virol. 1969 Apr;4(3):403–411. doi: 10.1099/0022-1317-4-3-403. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Steitz J. E. The reconstitution of infective bacteriophage R17. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1416–1421. doi: 10.1073/pnas.58.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Reynolds D. A. Assembly of protein and nucleoprotein particles from extracted tobacco rattle virus protein and RNA. Science. 1969 May 2;164(3879):559–560. doi: 10.1126/science.164.3879.559. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIL R., VINOGRAD J. THE CYCLIC HELIX AND CYCLIC COIL FORMS OF POLYOMA VIRAL DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:730–738. doi: 10.1073/pnas.50.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]