Abstract
Aims: Sepsis, a systemic inflammatory response to infection, represents the leading cause of death in critically ill patients. However, the pathogenesis of sepsis remains incompletely understood. Carbon monoxide (CO), when administered at low physiologic doses, can modulate cell proliferation, apoptosis, and inflammation in pre-clinical tissue injury models, though its mechanism of action in sepsis remains unclear. Results: CO (250 ppm) inhalation increased the survival of C57BL/6J mice injured by cecal ligation and puncture (CLP) through the induction of autophagy, the down-regulation of pro-inflammatory cytokines, and by decreasing the levels of bacteria in blood and vital organs, such as the lung and liver. Mice deficient in the autophagic protein, Beclin 1 (Becn1+/−) were more susceptible to CLP-induced sepsis, and unresponsive to CO therapy, relative to their corresponding wild-type (Becn1+/+) littermate mice. In contrast, mice deficient in autophagic protein microtubule-associated protein-1 light chain 3B (LC3B) (Map1lc3b−/−) and their corresponding wild-type (Map1lc3b+/+) mice showed no differences in survival or response to CO, during CLP-induced sepsis. CO enhanced bacterial phagocytosis in Becn1+/+ but not Becn1+/− mice in vivo and in corresponding cultured macrophages. CO also enhanced Beclin 1-dependent induction of macrophage protein signaling lymphocyte-activation molecule, a regulator of phagocytosis. Innovation: Our findings demonstrate a novel protective effect of CO in sepsis, dependent on autophagy protein Beclin 1, in a murine model of CLP-induced polymicrobial sepsis. Conclusion: CO increases the survival of mice injured by CLP through systemic enhancement of autophagy and phagocytosis. Taken together, we suggest that CO gas may represent a novel therapy for patients with sepsis. Antioxid. Redox Signal. 20, 432–442.
Introduction
Sepsis, a systemic inflammatory response to an infectious insult, represents the leading cause of death in critically ill patients (19). Intra-abdominal infections, often leading to polymicrobial sepsis, account for 20% of sepsis cases, which have substantial mortality of approximately 60% (2). Sepsis-associated mortality primarily results from multiple organ dysfunction with subsequent organ failure (5). Invading microorganisms can activate immune cells, resulting in the production of pro-inflammatory mediators that trigger cellular defense mechanisms to fight the infection (5). If therapy is not initiated in the first incipient stage, and the pathogen is not eliminated, multiple organ dysfunction ensues, followed by refractory hypotension and hemodynamic abnormalities, which lead to decreased oxygen delivery to tissues and death (4, 33, 34). Therefore, additional investigation is urgently needed to understand the mechanisms underlying the pathogenesis of sepsis and to develop new therapeutic alternatives.
Autophagy, an evolutionarily conserved cellular process, facilitates the turnover of damaged proteins and organelles such as mitochondria and plays an important role in the clearance of intracellular pathogens, including bacteria, viruses, and protozoa (25, 42). During autophagy, cytosolic constituents are engulfed into double membrane-bound vesicles called autophagosomes, which are subsequently delivered to the lysosomes for degradation (16). Autophagic dysfunction is associated with aging and human diseases, including cancer and neurodegenerative disorders (25). Moreover, autophagy affects innate and adaptive immunity such as antigen presentation, lymphocyte development, and cytokine secretion by immune cells (42).
Innovation.
We have previously shown that carbon monoxide (CO) may act as a potential therapeutic, which modulates cell proliferation, cell death, immunity, and autophagy in pre-clinical models of tissue injury in vitro or in vivo. Here, we have shown that CO increased the survival of mice injured by cecal ligation and puncture through systemic enhancement of phagocytosis. These protective effects of CO are dependent on autophagic protein, Beclin 1. The Beclin 1 dependency also affects the induction of signaling lymphocyte-activation molecule and enhancement of phagocytosis by CO. Our study suggests that CO gas may represent a new and powerful therapeutic option for sepsis.
To date, a number of genes that are critical for the regulation of autophagy (Atg) have been identified in mammals, each with distinct roles in the regulation of the autophagic pathway (28). Of these, Beclin 1, the mammalian homolog of yeast Atg6, plays a critical upstream regulatory function in the initiation of the autophagic pathway. Beclin 1 forms a multi-protein complex that includes the Vps34 class III phosphatidylinositol 3-kinase (PI3KC3), Atg14L, and p150. On cellular stimulation, the increased production of phosphatidylinositol-3-phosphate by PI3KC3 regulates the formation of nascent autophagosomes. The conversion of microtubule associated protein-1 light chain 3B (LC3B) from LC3B-I (free form) to LC3B-II (phosphatidylethanolamine-conjugated form) represents another major step in autophagosome formation (7, 28). Moreover, Beclin 1 is involved in diverse biological effects. Beclin 1 binds to Bcl-2, an important regulator of apoptosis, and this interaction results in inhibition of autophagy. The biallelic loss of Beclin 1 results in early embryonic lethality, which demonstrates that Beclin 1 is essential for early embryonic development (49). Beclin 1 is well characterized as a haplo-insufficient tumor suppressor gene in humans and mice (1, 26). In the nervous system, Beclin 1 can activate autophagy and death of cerebellar Purkinje cells through complex formation with the glutamate receptor δ2 (48).
Phagocytosis is a vital process by which macrophages eliminate microorganisms after recognition by pathogen sensors (40). Recent studies have shown that a subset of autophagy-related proteins, including Beclin 1 (Atg6) and LC3 (Atg8), are also involved in the regulation of phagocytosis (3, 27, 38, 45).
Carbon monoxide (CO), a low-molecular-weight diatomic gas, can cause clinical toxicity such as hypoxemia as the result of competitive binding to the heme-iron centers of the oxygen carrier protein, hemoglobin, and inhibition of mitochondrial respiratory chain enzymes (i.e., cytochrome c oxidase) by complex formation at heme-copper centers at a high concentration. However, recent studies have revealed the cytoprotective effects of CO when applied at low doses in animal models of acute inflammation and lung injury caused by hyperoxia, mechanical ventilation, and ischemia/reperfusion (I/R) (13, 30, 50). Inhaled CO enhances bacterial clearance as well as mitochondrial energy metabolism and confers anti-inflammatory effects through the modulation of p38 MAPK and NF-κB signaling in rodent models of sepsis (10, 30, 46). These numerous biological effects of CO are associated with cell-type specific responses and molecular targets depending on the physiological state of the cells and tissues (47).
We describe here that CO rescues mice from cecal ligation and puncture (CLP)-induced sepsis through the systemic induction of autophagy and enhancement of phagocytosis. The induction of autophagy and phagocytosis by CO was dependent on the autophagic protein, Beclin 1.
Results
CO rescues mice from CLP-induced sepsis through systemic enhancement of autophagy
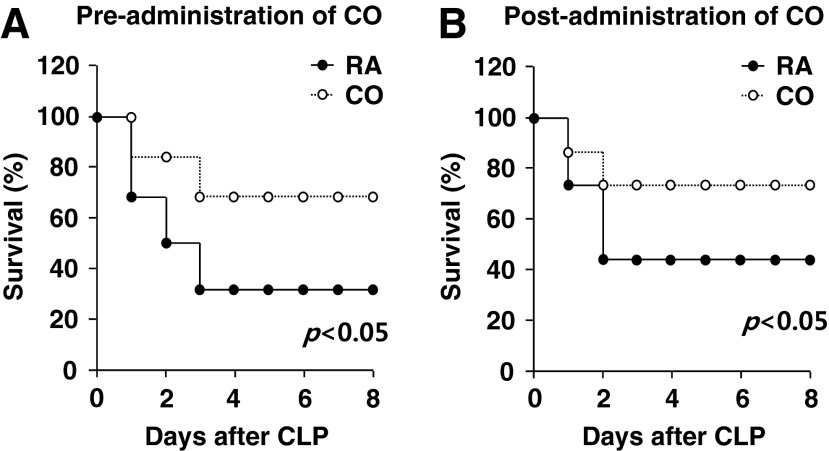
We determined the effect of CO on the survival rate of C57BL/6J mice subjected to CLP, a murine model of peritonitis-induced polymicrobial sepsis. CO (250 ppm) was administered by inhalation in mice for 24 h before CLP surgery, or, alternatively, was post-administered to mice continuously after CLP surgery. Both pre- and post-administration of CO significantly improved the survival rates of mice subjected to CLP-induced sepsis compared with room air exposure of CLP-treated mice (Fig. 1A, B).
FIG. 1.
CO rescues mice from CLP-induced sepsis through systemic enhancement of autophagy. C57BL/6J mice were subjected to CLP surgery. CO (250 ppm) was administered by inhalation for 24 h before (A) or continuously after (B) CLP surgery. Room air (RA) served as the control. Rates of survival were determined. n=6–7 per condition. p<0.05, log-rank test. CLP, cecal ligation and puncture; CO, carbon monoxide.
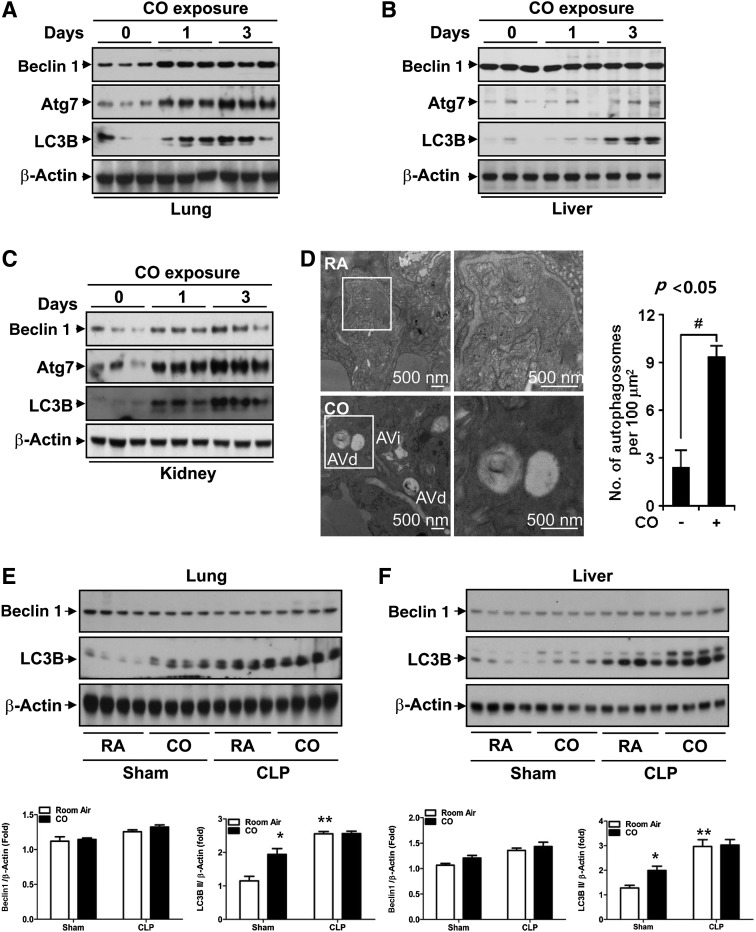
Sepsis or lipopolysaccharide (LPS)-induced autophagy can protect against cell death in the liver (6). Since we have previously shown that CO can induce autophagy in cultured endothelial cells and mice (12, 24), we hypothesized that autophagy could play a role in the protective effects of CO in CLP. First, we confirmed that CO inhalation can induce autophagy proteins in various mouse tissues. We exposed C57BL/6J mice to CO (250 ppm) for 24 and 72 h and determined the expression level of autophagy proteins by western blot analysis. CO inhalation efficiently induced the expression of autophagy proteins, Beclin 1, Atg7, and LC3B, in a time-dependent manner in lung (Fig. 2A), liver (Fig. 2B), and kidney (Fig. 2C) tissue. Furthermore, we analyzed the ultrastructure of mouse lung tissue exposed to CO (250 ppm) using electron microscopic (EM) analysis. Both immature (AVi) and degradative (AVd) autophagic vacuoles were increased in the lung exposed to CO compared with the normoxic control group (Fig. 2D).
FIG. 2.
CO induces systemic autophagy. The C57BL/6J mice were exposed to 250 ppm CO for 24 and 72 h. The expression of autophagy proteins was determined in the lung (A), liver (B), and kidney (C) by western blot analysis. β-actin served as the standard. CO efficiently induced autophagic proteins, Beclin 1, Atg7, and LC3B in a time-dependent manner. (D) The number of autophagosomes was increased in the lungs of mice exposed to CO by transmission electron microscopy. Representative micrograph depicts immature (AVi) and degradative (AVd) autophagic vacuoles. Scale bar=500 nm. Autophagosomes were quantified per unit area, from n=25 images (Right) #p<0.05. (E, F) C57BL/6J mice were exposed to CLP surgery or sham operation. After 6 h of recovery time, animals were exposed to 250 ppm CO or room air (RA) for 24 h. The expression of autophagy proteins Beclin 1 and LC3B was determined in lung (E) and liver (F) tissue by western blot analysis. β-actin served as the standard. LC3B, microtubule associated protein-1 light chain 3B.
Next, we evaluated whether CO could modulate autophagy proteins in the context of the CLP model in vivo. The autophagy protein Beclin 1 was not significantly modulated by CLP in the absence or presence of CO, relative to sham controls, indicating that Beclin 1 expression was not altered under these experimental conditions. The autophagy protein, LC3B, and its conversion from the cytosolic form LC3B-1 to its lipid-conjugated form LC3-II was induced by CO inhalation (250 ppm) in the mouse lung (Fig. 2E) and liver (Fig. 2F) of wild-type mice subjected to sham surgery. After CLP, the accumulation of LC3B was also significantly induced in the mouse liver relative to sham surgery controls. The levels of LC3-II remained high in mice treated with CLP with CO, but were not further increased relative to CLP-treated mice.
CO-dependent autophagy is impaired in Becn1+/− mice
We hypothesized that the enhancement of autophagy by CO was responsible for the increased survival rate after CLP-induced sepsis in mice. To test this hypothesis, we implemented a functional study using autophagic protein Beclin 1 heterozygous knockout mice (Becn1+/−).
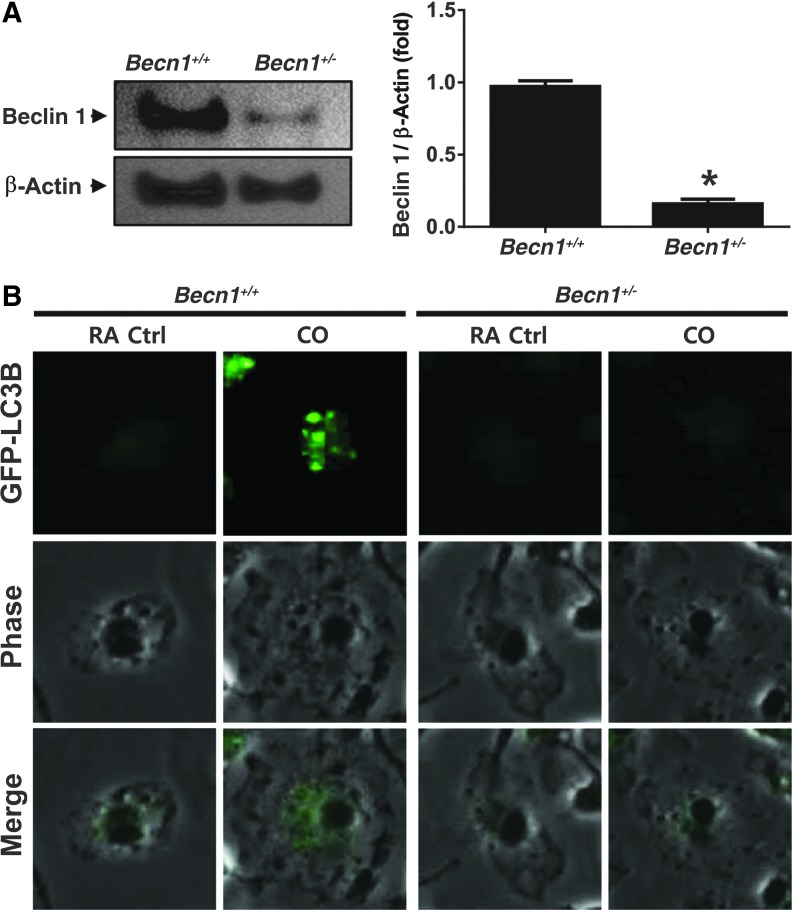
We exposed bone marrow-derived macrophage (BMDM) cells derived from Becn1+/+ and Becn1+/− mice to CO (250 ppm), and determined whether CO can modulate the function of autophagy. The monoallelic deficiency of Becn1 was validated in Becn1+/− BMDM. The Beclin 1 protein was decreased by 80% in Becn1+/− BMDMs (Fig. 3A). To further confirm the role of Beclin 1 in CO-induced autophagy, we also assessed the effect of CO on LC3B puncta formation, an indicator of autophagosome formation, in LC3-green fluorescence protein (GFP) transfected BMDMs isolated and differentiated from Becn1+/+ and Becn1+/− mice. CO significantly induced LC3B puncta in Becn1+/+ but not Becn1+/− BMDM (Fig. 3B). Autophagosome formation in response to CO exposure could also be demonstrated in pulmonary vascular cells. The CO effectively increased the number of autophagosomes in both endothelial cells and smooth muscle cells derived from the pulmonary vasculature (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/ars). Consistent with the results observed in BMDM, LC3B puncta formation in response to CO was impaired in Becn1+/− endothelial cells (Supplementary Fig. S1B).
FIG. 3.
The Becn1+/− mice are autophagy deficient and susceptible to CLP. The deficiency of Becn1 was validated in Becn1+/+ or Becn1+/− BMDMs via western blot analysis. *p<0.05 (A) The effect of CO on the autophagic function of isolated Becn1+/+ or Becn1+/− BMDMs was determined by assessing GFP-LC3B puncta formation (B) in transfected cells by confocal microscopy. BMDM, bone marrow-derived macrophage; GFP, green fluorescence protein. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
CO rescues Becn1+/+ mice, but not Becn1+/− mice, from CLP-induced sepsis
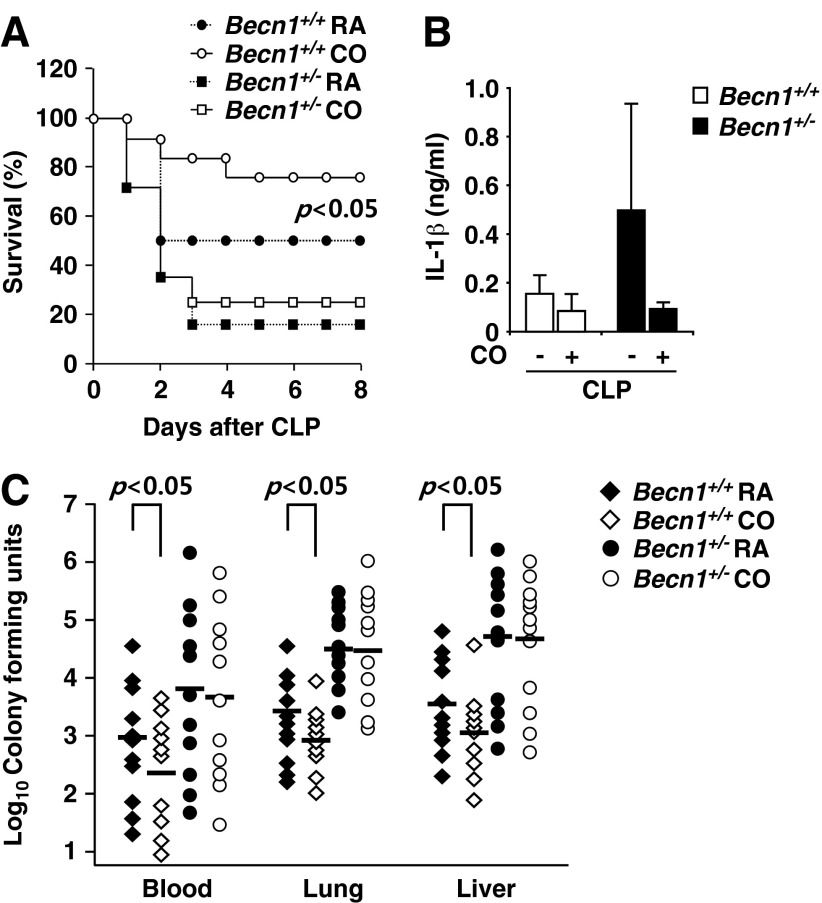
CO is known to exhibit biological effects, including the modulation of vascular tone (37), the inhibition of inflammation by suppressing pro-inflammatory cytokines (e.g., TNF-α, IL-1β), and up-regulating the anti-inflammatory cytokine IL-10 (8). Furthermore, our recent study demonstrated that CO induces autophagy proteins in cultured endothelial cells (24). We have shown here that CO activates autophagy, and that this response is also enhanced as an endogenous response to CLP-induced sepsis (Fig. 2). We investigated whether autophagic deficiency affects CLP mortality. We performed CLP in wild-type (Becn1+/+) and heterozygous (Becn1+/−) littermate mice. The Becn1+/− mice displayed higher mortality rates than those of Becn1+/+ after CLP-induced sepsis (Fig. 4A). Further, we assessed whether CO would improve the survival rates of Becn1+/+ and Becn1+/− mice after CLP-induced sepsis. C57BL/6J mice were exposed to CO (250 ppm) for 24 h after CLP. The Becn1+/+ mice exposed to CO demonstrated a significant improvement of survival rate compared with room air-exposed control mice (Fig. 4A). However, CO failed to rescue Becn1+/− mice from CLP-induced sepsis.
FIG. 4.
CO significantly rescues Becn1+/+ mice from CLP but not Becn1+/− mice. (A) Becn1+/+ or Becn1+/− mice were subjected to CO that was pre-administrated for 24 h before or continuously after CLP surgery. Rates of survival were determined. p<0.05, log-rank test. (B) After CLP, the plasma levels of inflammatory cytokines in plasma, IL-1β, and IL-18 were measured by ELISA. (C) For determination of CFU, blood and organ homogenates (lung and liver) were serially diluted and then plated on LB plates. The LB plates were incubated for 24 h at 37°C. Statistical significance was determined by nonparametric Mann–Whitney U analysis. *p<0.05.
We also assessed whether another autophagy protein, LC3B, could function in the same manner as Beclin 1 during CO-dependent rescue of CLP. Therefore, LC3B-deficient mice (Map1lc3b−/−) were exposed to CO or room air after CLP. The LC3B-deficient mice showed no difference in survival rate compared with wild-type littermate mice (Map1lc3b+/+) during recovery from CLP. In the CO exposure group, the survival rates of both LC3B-deficient and homozygote wild-type mice were improved (Supplementary Fig. S2). These data, taken together, indicate that the protective effects of CO are dependent on Beclin 1 but independent of LC3B, demonstrating specificity in the physiologic functions of select autophagy proteins.
CO rescues wild-type mice from CLP-induced sepsis through enhancement of Beclin 1-phagocytosis
After CLP (24 h), we collected plasma from the mice injured by CLP and measured the level of cytokines. Mice treated with CO showed a reduced level of pro-inflammatory cytokine IL-1β in the serum after CLP, relative to the room air-exposed control group (Fig. 4B). These data indicate that CO can down-regulate inflammation in the CLP model. To further elucidate the mechanisms by which CO improves mice survival rate and the differential mortality between the strains, we cultured circulating blood and tissue extracts from the lung and liver after CLP for bacterial count. The number of bacterial colonies in the blood, lung, and liver from Becn1+/+ mice were significantly lower than that of Becn1+/− mice (Fig. 4C). Interestingly, the bacterial colony number was decreased in the blood, lung, and liver of Becn1+/+ mice that inhaled CO (250 ppm) after CLP (Fig. 4C). In the case of Becn1+/− mice subjected to CLP, CO did not reduce the bacterial number effectively (Fig. 4C). These data suggest that CO improves survival rate after CLP through enhancement of bacterial clearance. These data suggest that the efficiency of phagocytosis is based on Beclin 1-dependent processes promoted by CO.
CO enhances phagocytosis through up-regulation of SLAM
We next examined the possibility that the Beclin 1-dependent protective effects of CO in this model also involve phagocytosis. Phagocytosis is a crucial process by which innate immune cells (e.g., macrophages, neutrophils) eliminate microorganisms after recognition by pathogen sensors (40). We have previously reported that CO-releasing molecules (CORMs) can enhance total phagocytic activity in C57BL/6J mice (10).
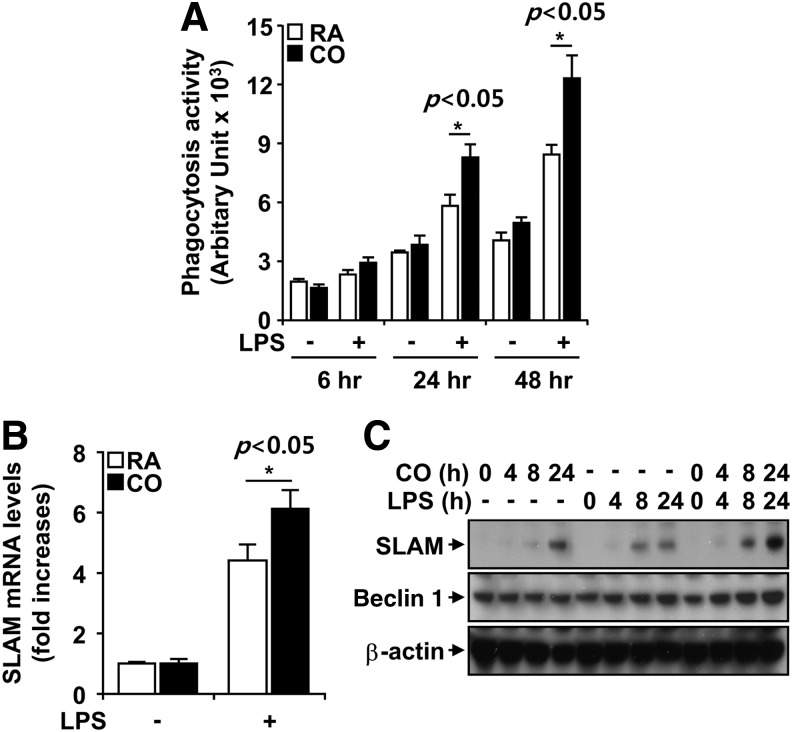
We assessed the effect of CO on phagocytosis activity of BMDM subjected to LPS stimulation. The phagocytic activity of BMDM was enhanced by LPS stimulation in a time-dependent manner (Fig. 5A). CO treatment (250 ppm) further enhanced the phagocytic activity of LPS-stimulated BMDM (Fig. 5A).
FIG. 5.
CO enhances phagocytosis by up-regulation of SLAM/CD150. (A) BMDM isolated from wild-type C57BL/6J mice were stimulated with 100 ng/ml LPS, in the absence and presence of CO (250 ppm) at the indicated times. Phagocytosis of Escherichia coli by BMDM was determined by spectrofluorometry after exposure to FITC-labeled E. coli for 1 h. (B, C) The expression of SLAM/CD150 was evaluated at the mRNA level by quantitative PCR (B) and at the protein level by western blot analysis. β-actin served as the standard. (C) Results shown are the mean±SD of three experiments *p<0.05. LPS, lipopolysaccharide, polymerase chain reaction; SLAM/CD150, signaling lymphocyte-activation molecule.
Recently, it has been shown that the self-ligand and cell-surface receptor, signaling lymphocyte-activation molecule (SLAM/CD150), functions not only as a co-stimulatory molecule but also as a microbial sensor that controls the killing of Gram-negative bacteria by macrophages. SLAM recruits a complex containing the intracellular class III phosphatidylinositol kinase Vps34, its regulatory protein kinase Vps15, and the autophagy-associated molecule Beclin 1 to the phagosome (3). We, therefore, assessed the role of CO in SLAM induction. We exposed BMDM stimulated by LPS to room air or CO (250 ppm) for 8 h. The mRNA level of SLAM was determined by quantitative polymerase chain reaction (PCR) in the cells stimulated by LPS. SLAM mRNA was increased fourfold after LPS stimulation (Fig. 5B). CO exposure significantly induced SLAM mRNA expression in BMDM compared with that of room air-exposed BMDM after LPS stimulation (Fig. 5B).
To determine whether CO regulates SLAM protein expression, we exposed BMDM to CO. CO exposure induced SLAM protein expression in a time-dependent manner in BMDM. LPS treatment also induced SLAM protein expression in a time-dependent manner. When CO exposure was combined with LPS stimulation, the induction of SLAM was greatly enhanced (Fig. 5C). These results indicated that CO exposure enhances macrophage SLAM expression during LPS stimulation, which may account for CO-induced phagocytosis.
CO increases Escherichia coli phagocytosis in a Becn1-dependent fashion
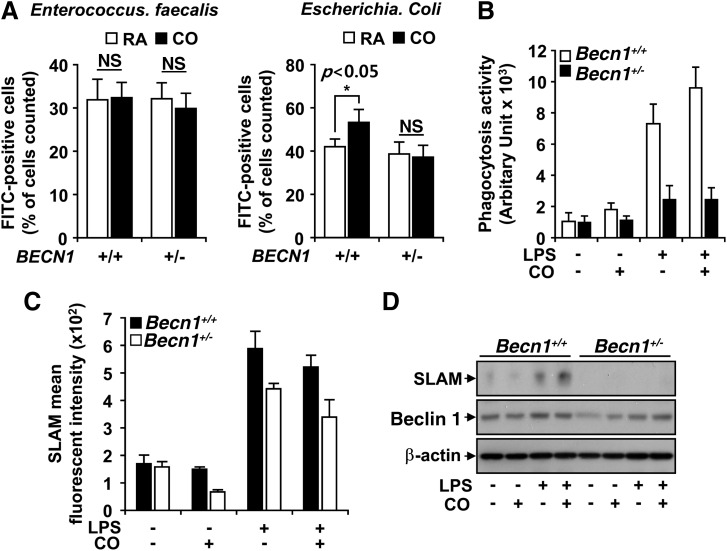
It has been previously reported that SLAM mediates the killing of Gram-negative bacteria by macrophages (3). Therefore, we injected FITC-labeled Escherichia coli and Enterococcus faecalis into the peritoneum of Becn1+/+ and Becn1+/− mice to determine whether a difference was evident in the ability of inflammatory cells to sense and phagocytose the bacteria. After 24 h, peritoneal lavage was performed and phagocytic rates were determined using flow cytometry. The phagocytosis was not different between Becn1+/+ and Becn1+/− mice exposed to both E. faecalis and E. coli in the room air group. However, CO exposure caused a ∼15% increase in phagocytosis in Becn1+/+ mice exposed to Gram-negative E. coli (Fig. 6A), with no apparent effect against E. faecalis.
FIG. 6.
CO increases phagocytosis of E. coli in a Becn1-dependent fashion. The Becn1+/− and Becn1+/+ mice were injected with FITC-labeled Enterococcus faecalis (A, left) or E. coli (A, right). Subsequently, mice (n=6, injected with E. faecalis; n=8, injected with E. coli) were exposed to CO (250 ppm) or RA. After 24 h, phagocytosis was analyzed. (B) BMDM isolated from Becn1+/− or Becn1+/+ mice were stimulated with 100 ng/ml LPS, in the absence and presence of CO (250 ppm) at the indicated times. Phagocytosis of E. coli by BMDM was determined by spectrofluorometry after exposure to FITC-labeled E. coli for 1 h. The expression of SLAM/CD150 was evaluated at the mRNA level (C) by quantitative PCR, and at the protein level (D) by western blot analysis. β-Actin served as the standard. Results shown are the mean±SD of three experiments, *p<0.05, NS, non significance; RA, room air; PCR, polymerase chain reaction.
In vitro, the phagocytosis activity of Becn1+/+ BMDM was increased after LPS stimulation. CO exposure increased phagocytosis ∼25% in Becn1+/+ macrophages but not in Becn1+/− macrophages (Fig. 6B).
We also checked the expression of SLAM in Becn1+/+ and Becn1+/− macrophages. First, we evaluated the mRNA levels of SLAM via quantitative PCR. When Becn1+/+ cells stimulated by LPS were exposed to CO, SLAM expression was increased threefold (Fig. 6C). However, this induction of SLAM by CO was attenuated by Beclin 1 deficiency (Fig. 6C). SLAM protein was also up-regulated by LPS and by the addition of CO in Becn1+/+ macrophages; however, Becn1 deficiency abolished SLAM protein expression under these conditions (Fig. 6D).
Discussion
In this study, we have demonstrated a protective effect of CO against sepsis-induced mortality using a murine model of CLP-induced polymicrobial sepsis. Our findings emphasize the potential of CO as a new therapeutic option for sepsis. CO increases the survival of mice injured by CLP, in part, through the systemic enhancement of autophagy and phagocytosis.
Gaseous compounds such as CO and nitric oxide (NO) are important physiological mediators that have been implicated in progression of clinical illness such as sepsis and I/R injury. Both CO and NO share similar properties, such as activation of soluble guanylate cyclase to increase cyclic GMP, resulting in the inhibition of platelet aggregation, and regulation of vascular tone. Despite these similarities, CO is preferred as a therapeutic method because of its chemical properties, including high diffusivity, portability (via hemoglobin), and biochemical stability (integrity). The underlying mechanism for the therapeutic effect of CO in sepsis, where high levels of endogenous NO may be present, remains incompletely understood. Further studies are needed to determine the relative role of guanylate cyclase activation in our model.
The therapeutic effects of CO related to the modulation of inflammation, pro-/anti-apoptotic effects, pro-/anti-oxidative effects, and effects on systemic circulation (21, 22, 35, 43) have been previously studied in models of acute lung injury and inflammation, I/R injury, endotoxemia, organ transplantation, and others (13, 31, 50). Specifically, inhaled CO shows remarkable effects on the regulation of innate immunity in a murine sepsis model. CO enhances anti-inflammatory responses and inhibits pro-inflammatory signaling. These effects may be driven by redox modulation in certain cell types, though other mechanisms cannot be excluded. Furthermore, CO can enhance the anti-bacterial response, by augmenting bacterial clearance through TLR4-dependent pathways, leading to rapid resolution of infection (32).
CO can also be produced systemically from heme degradation by the heme oxygenase (HO) enzyme system (39). The inducible form of HO-1, a major cellular protein, has a protective function under chemical and physical stress, such as induced by xenobiotics, hyperoxia, pro-inflammatory cytokines, and bacterial endotoxin (17, 18, 20). Chung et al. previously demonstrated that HO-1-derived CO and treatment with CORMs promote host defense responses to sepsis through enhancement of bacterial clearance by increasing phagocytosis and the endogenous antibacterial response (10). Furthermore, Carchman et al. reported that HO-1 protects against hepatocyte death and hepatic injury from infection/sepsis in mice through induction of autophagy via the p38 MAPK pathway (6). This study indicated that endogenous CO produced by HO-1 is potentially involved in autophagy.
To explore the role of autophagy in CO-dependent protection against sepsis, we used Beclin 1 heterozygous knockout mice (Becn1+/−). Yue et al. have shown that heterozygous deletion of Beclin 1 is critical for not only spontaneous tumor formation but also autophagy in vivo (49). We report here that Becn1+/− mice were much more susceptible than wild-type littermate mice (Becn1+/+) during CLP-induced sepsis. However, CO inhalation enhanced the survival of Becn1+/+ wild-type littermate mice injured with CLP through the induction of autophagy, the down-regulation of inflammation, and the decreased level of bacteria in blood and vital organs, such as lung and liver. These salutary effects of CO did not work effectively in Beclin 1-deficient mice.
Recent studies have elucidated that autophagy contributes to the regulation and function of innate and adaptive immunity. During infection, autophagic processes target intracellular bacteria and viruses to autophagosomes for degradation and also regulate an unconventional pathway for secretion of cytokines (e.g., IL-1β) (14). Autophagy also plays critical roles in adaptive immunity such as antigen presentation and lymphocyte development. Autophagosomes can fuse with major-histocompatibility-complex class II loading compartments (11). Furthermore, macrophage-specific deletion of Atg5 in mice increases their susceptibility to Mycobacterium tuberculosis infection (45). On the other hand, IL-1β and IL-18 were excessively produced in response to LPS and other pathogen-associated molecular patterns in Atg16L1-deleted mice (36). Furthermore, depletion of the autophagy proteins LC3B and Beclin 1 enhanced the activation of NLRP3 inflammasome and secretion of IL-1β and IL-18 in stimulated macrophages (29).
In the current study, we demonstrate a differential sensitivity of Becn1+/− mice to CLP mortality that was not evident in Map1lc3b−/− mice. Although both Beclin 1 and LC3 represent core autophagy proteins, they differ substantially in their relative role in autophagy regulation (16, 28). Beclin 1 represents a critical upstream signaling mediator of autophagosome nucleation, which interacts with a multi-protein regulatory signalosome, whereas LC3B acts downstream of these events by participating in autophagosome elongation, a function that may be potentially compensated by other ATG8 homologues (16, 28). We also cannot exclude the possibility that the phenotypes observed in association with Becn1 heterozygous mice are related to loss of cellular functions of Beclin 1 that are independent of its role in autophagic regulation.
In this study, we also demonstrated Beclin 1-dependent enhancement of phagocytosis by CO. Interestingly, in the current study, the protective effects of CO, which depended on Beclin 1, and the induction of macrophage phagocytosis, had an apparent selectivity for Gram-negative bacteria. We hypothesized that the selectivity for Gram-negative bacteria in CO-treated macrophages was caused by induction of SLAM, which was induced by CO in wild-type but not Beclin 1-deficient macrophages. The current work differs from that of Chung et al., which noted preferential protection in vivo against E. faecalis but not E. coli-induced sepsis in HO-1 expressing transgenic mice (10). Although HO-1 produces endogenous CO as one of its biological products, HO-1 overexpression cannot be directly compared with inhalation of CO, as it may involve additional factors (e.g., changes in iron and redox status).
In general, bacteria are recognized by low-specificity, high-affinity receptors such as integrins, lectins, and scavenger receptors that initiate the formation of the phagocytic synapse and intracellular signaling events. However, SLAM recognizes bacterial surface proteins that are embedded in the outer membrane of most Gram-negative bacteria such as OmpC and OmpF. Once SLAM engages the bacteria, it is actively incorporated into the developing phagosome, where it is responsible for recruiting a complex containing Vps34, Vps15, and Beclin 1 to the early phagosome. Thus, SLAM supports phagosome maturation by sharing the ubiquitous autophagy machinery. There is also an overlap between the elements used in phagosome maturation and autophagy, as TLR-dependent triggering of phagocytosis recruits the autophagy proteins Beclin 1 and LC3B to the phagosome (3).
Here, we uncovered the fact that CO enhances phagocytosis through up-regulation of SLAM. Interestingly, the induction of SLAM by CO was depending on Becn1. Recent studies suggest that LC3-associated phagocytosis (41) and enhancement of resolution of inflammation and macrophage efferocytosis by inhaled CO (9) support our finding. Further studies are needed to determine the relative role of Beclin 1-dependent autophagy versus phagocytosis in the protective effects of CO, as both processes can contribute to bacterial clearance. Furthermore, we suggest that induction of autophagy in systemic tissues by CO may contribute to organ preservation during sepsis.
We conclude that CO gas has a protective effect in a murine model of CLP-induced polymicrobial sepsis. CO increases the survival of mice injured by CLP through systemic enhancement of autophagy and phagocytosis. The autophagic protein, Beclin 1 is crucial for induction of autophagy and enhancement of phagocytosis by CO. Beclin 1-dependent SLAM induction and enhancement of phagocytosis were also up-regulated by CO. SLAM provides selectivity for Gram-negative bacteria. Taken together, these data strongly suggest that CO gas may present a new and powerful therapeutic option for sepsis through modulation of bacterial autophagy and phagocytosis.
Materials and Methods
Reagents
Rabbit anti-mouse Beclin 1 and anti-mouse ATG7 were purchased from Santa Cruz Biotechnology, Inc. Rabbit anti-mouse LC3B and anti-β-actin were from Sigma-Aldrich. Sheep anti-mouse SLAM was from R&D Systems. LPS (E. coli) was from Invivogen. GFP-LC3B expression plasmid was a gift of Dr. Noboru Mizushima (Tokyo Medical and Dental University). All other reagents were from Sigma-Aldrich.
Cell isolation and culture
BMDMs were isolated and cultured as previously described (28). Bone marrow collected from mouse femurs and tibias was plated on sterile Petri dishes and was incubated for 7 days in Dulbecco's modified Eagle's medium (DMEM) containing 10% (vol/vol) heat-inactivated FCS, penicillin and streptomycin, and 25% (vol/vol) conditioned medium from L929 mouse fibroblasts. Mouse lung endothelial cells (mLECs) were isolated and cultured as previously described (43, 44).
Animals
All animals were housed in accordance with the guidelines from the American Association for Laboratory Animal Care. The Animal Research Committee of Brigham and Women's Hospital approved all protocols. Becn1+/− mice were obtained from Beth Levine (UT Southwestern Medical Center) (26).
CLP model of polymicrobial sepsis
The CLP model of polymicrobial sepsis was performed with 10–12 week-old mice. Anesthesia was induced in mice by i.p. administration of 100 mg/kg ketamine HCl and 43 mg/kg xylazine HCl. The mouse cecum was exposed through a 1.5-cm incision, and 60% of the cecum was ligated below the ileocecal valve. Once ligated, the cecum was punctured once with a 23-gauge needle. A 23-gauge, one-hole injury was performed in studies using Becn1+/+ and Becn1+/− mice, respectively. The cecum was repositioned, and the abdominal incision was closed in layers with 6-0 surgical sutures. Sham-operated mice underwent the same procedure, including opening of the peritoneum and exposing the bowel, but without ligation and needle perforation of the cecum. After surgery, the mice were injected with 1 ml of physiologic saline solution subcutaneously for fluid resuscitation. No antibiotics were administered to the mice after surgery to assess the effect of Beclin 1 on bacterial levels in blood and organs. Survival rates were determined over an 8-day period, with assessment every 12 h. Pre- and post-operatively, all mice had unlimited access to food and water.
CO administration
Air containing CO (250 ppm) was administrated to animals for 24 h before CLP (pre-administration). After CLP, the animals were exposed to CO again in exposure chambers as described (23). For post-administration, the animals were exposed to CO (250 ppm) immediately after CLP surgery until the end of the observation period. For in vitro experiments, cells were exposed to CO (250 ppm) in air containing 5% CO2 in exposure chambers as previously described (24).
FITC labeling of E. coli and E. faecalis and phagocytosis assay
E. coli and E. faecalis were heat inactivated and incubated at a concentration of 6×109–8×109 CFU/ml with 0.2 mg FITC/ml (Sigma-Aldrich) in phosphate-buffered saline (PBS) for 1 h at room temperature in the dark, as described (15). The bacteria were then washed five times with 1 ml PBS to remove free FITC, resuspended in 1 ml PBS, and stored at −80°C. The 6×108–8×108-CFU nonlabeled or FITC-labeled E. coli or E. faecalis were injected into the peritoneum of Becn1+/+ and Becn1+/− mice. After 24 h, mouse peritoneal polymorphonuclear cells were isolated using 15 ml of DMEM cell culture media with 10% fetal bovine serum (FBS), 10,000 U/ml penicillin, 10,000 mg/ml streptomycin, 29.2 mg/ml l-glutamine, and 10 U/ml heparin and then incubated for 1 h at 37°C. Attached cells were washed thrice with warm PBS and then treated with 0.2% Trypan blue for 1 min at room temperature to quench extracellular fluorescence. The cells were washed twice, treated with a 5-mM EDTA PBS solution, scraped, and then centrifuged for 5 min at 2147 g. The pellet was resuspended with 400 μl of 4% FBS and 0.009% sodium azide containing PBS. Total cells were counted, and 1×106 cells were scanned by flow cytometry (15).
In vitro phagocytosis assay
In vitro phagocytosis assays were performed using the Vybrant phagocytosis assay kit from Invitrogen Life Technologies, according to the manufacturer's instructions. BMDM, seeded into a 96-well ELISA plate at a concentration of 1–2×105 cells/well, were allowed to adhere for 4 h to the bottom of the well, and then treated with LPS for the indicated times. FITC-conjugated E. coli bioparticles were added, and phagocytosis was allowed to proceed for 1 h. The bioparticles were removed, and Trypan blue was added to the wells for 2 min to quench extracellular bioparticles. The Trypan blue was then removed, and the amount of bioparticles engulfed by the cells was quantitatively measured using SpectraMax Gemini EM ELISA plate reader (Molecular Devices), at 480 nm excitation and 520 nm emission.
Bacteria culture from blood and tissues
Serial log 10 dilutions of whole blood (from the right atrium of heart) and homogenized tissues (left lung, liver left medial lobe, and whole spleen) were made, and aliquots were cultured on LB agar plates overnight at 37°C. CFUs of bacteria were counted and calculated.
Transmission electron microscopy
For EM, tissue sections were fixed in 2.5% glutaraldehyde in PBS after experimental manipulations. These tissues were photographed using a JEOL JEM 1210 transmission electron microscope (JEOL) at 80 or 60 kV onto EM film (ESTAR thick base; Kodak) and printed onto photographic paper.
GFP-LC3 assay
mLEC or BMDM cells were seeded at 1×105 cells/well in 12-well dishes. After 24 h, each well was ∼80%–90% confluent, and refed with complete media. GFP-LC3B expression plasmid (2 μg) was incubated with Lipofectamine™ LTX (Invitrogen) for 1 h and then added to each well 3 h after media change. After 6–8 h, the media was aspirated and complete growth media was replaced in each well. Exposure to CO was initiated 24 h post-transfection. To examine the distribution of GFP-LC3B, cells were observed under a fluorescence microscope, and digital images were acquired for analysis (SPOT; Diagnostic Instruments, Inc.).
Western immunoblot analysis
Western blotting was performed as previously described (21). Protein concentrations of cell lysates and frozen tissue homogenates were determined using Coomassie plus protein assay (Thermo Fischer Scientific). An equal volume of protein was boiled for 5 min in sample buffer and resolved by SDS/PAGE using NuPage Novex Bis-Tris 4%–12% polyacrylamide gels. Proteins were transferred to polyvinylidene difluoride membranes by electroblotting, and then probed with the indicated antibodies. Blots were developed with enhanced chemiluminescence reagents (Thermo Fischer Scientific).
Cytokine measurements
Mouse IL-1β cytokine in plasma were measured with ELISA kit (R&D Systems). Mouse IL-18 in plasma was measured by ELISA (MBL International).
Real-time PCR
Total RNA was extracted from tissues using TRIZOL reagent (Invitrogen), and converted to cDNA using high-capacity cDNA archive kit (Applied Biosystems). Quantitative real-time PCR was performed as described (23). Primers for SLAM and TaqMan Master Mix for gene expression assays were purchased from Applied Biosystems. Conditions were as follows: hold 2 min at 50°C and 10 min at 95°C, followed by two-step PCR for 40 cycles of 95°C for 15 s followed by 60°C for 1 min. Gene expression was analyzed by the comparative threshold cycle method, using GUS-β rRNA as the internal standards.
Statistics
Data are expressed as mean±SEM. For comparisons between two groups, we used two-tailed unpaired Student's t-test. For comparison among more than two groups and multiple comparisons, we used an ANOVA test. Analysis of bacterial cultures was made by nonparametric Mann–Whitney U analysis. Comparisons of mortality were made by analyzing Kaplan–Meier survival curves, and then, the log-rank test was used to assess for differences in survival.
Supplementary Material
Abbreviations Used
- Atg
autophagy-related gene
- BMDM
bone marrow-derived macrophage
- CLP
cecal ligation and puncture
- CO
carbon monoxide
- CORM
CO-releasing molecule
- DMEM
Dulbecco's modified Eagle's medium
- EM
electron microscopy
- FBS
fetal bovine serum
- GFP
green fluorescence protein
- HO
heme oxygenase
- I/R
ischemia/reperfusion
- LC3B
microtubule associated protein-1 light chain 3B
- LPS
lipopolysaccharide
- mLEC
mouse lung endothelial cell
- NO
nitric oxide
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PI3KC3
class III phosphatidylinositol 3-kinase
- SLAM/CD150
signaling lymphocyte-activation molecule
Acknowledgments
This work was supported by NIH grants P01 HL108801, R01-HL60234, R01-HL55330, R01-HL079904, and an FAMRI Clinical Innovator Award to A.M.K.C. S.W.C. was supported by the Bio and Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean Government (MEST) (2012M3A9C3048687). S.W.R. received salary support from the Lovelace Respiratory Research Institute. The authors thank Emeka Ifedigbo for coordination of animal work. They also thank Marlene Rabinovitch (Stanford University, Stanford, CA) for providing LC3B heterozygous mice, and Beth Levine (UT Southwestern Medical Center, Dallas, TX) for providing the Becn1 heterozyous mice.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aita VM, Liang XH, Murty VV, Princus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, and Lecine B. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics 59: 59–65, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Anaya DA. and Nathens AB. Risk factors for severe sepsis in secondary peritonitis. Surg Infect (Larchmt) 4: 355–362, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Berger SB, Romero X, Ma C, Wang G, Faubion WA, Liao G, Compeer E, Keszei M, Rameh L, Wang N, Boes M, Regueiro JR, Reinecher HC, and Terhorst C. SLAM is a microbial sensor that regulates bacterial phagosome functions in macrophages. Nat Immunol 11: 920–927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, and Fisher CJ. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344: 699–709, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bone RC, Grodzin CJ, and Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest 112: 235–243, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Carchman EH, Rao J, Loughran PA, Rosengart MR, and Zuckerbraun BS. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology 53: 2053–2062, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen ZH, Kin HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB, Dhir R, Landreneau RJ, Schuchert MJ, Yousem SA, Nakahira K, Ryter SW, Morita K, and Choi AM. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One 3: e3313, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chhikara M, Wang S, Kern SJ, Ferreyra GA, Barb JJ, Munson PJ, and Danner RL. Carbon monoxide blocks lipopolysaccharide-induced gene expression by interfering with proximal TLR4 to NF-κB signal transduction in human monocytes. PLoS One 12: e8139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang N, Shinohara M, Dalli J, Mirakaj V, Kibi M, Choi AMK, and Serhan CN. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J Immunol 190: 6378–6388, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung SW, Liu X, Macias AA, Baron RM, and Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest 118: 239–247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crotzer VL. and Blum JS. Autophagy and its role on MHC-mediated antigen presentation. J Immunol 182: 3335–3341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding Y, Kim JK, Kim SI, Na HJ, Jun SY, Lee SJ, and Choi ME. TGF-{beta}-1 protects against mesangial cell apoptosis via induction of autophagy. J Biol Chem 285: 37909–37919, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolinay T, Szilasi M, Liu M, and Choi AM. Inhaled carbon monoxide confers anti-inflammatory effects against ventilator-induced lung injury. Am J Respir Crit Care Med 170: 613–620, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Dupont N, Jiang S, Pilli M, Ornatowski W, Bhattacharya D, and Deretic V. Autophagy-based unconventional secretory pathway for extracellular delivery if IL-1β. EMBO J 30: 4701–4711, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredenburgh LE, Suárez Velandia MM, Ma J, Olszak T, Cernadas M, Englert JA, Chung SW, Liu X, Begay C, Padera RF, Blumberg RS, Walsh SR, Baron RM, and Perrella MA. Cyclooxygenase-2 deficiency leads to intestinal barrier dysfunction and increased mortality during polymicrobial sepsis. J Immunol 187: 5255–5267, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He C. and Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43: 67–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoetzel A, Dolinay T, Schmidt R, Choi AM, and Ryter SW. Carbon monoxide in sepsis. Antioxid Redox Signal 9: 2013–2026, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Hoetzel A, Vagts DA, Loop T, Humar M, Bauer M, Pahl HL, Geiger KK, and Pannen BJ. Effect of nitric oxide on shock-induced hepatic heme oxygenase-1 expression in the rat. Hepatology 33: 925–937, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss RS. and Karl IE. The pathology and treatment of sepsis. N Engl J Med 348: 138–150, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Keyse SM. and Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA 86: 99–103, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HP, Ryter SW, and Choi AM. CO as a cellular signaling molecule. Annu Rev Phamacol Toxicol 46: 411–449, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Kim HP, Wang X, Nakao A, Kim SI, Murase N, Choi ME, Ryter SW, and Choi AM. Caveolin-1 expression by mean of p38beta mitogen activated protein kinase mediates the antiptoliferative effect of carbon monoxide. Proc Natl Acad Sci USA 102: 11319–11324, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SJ, Kim HP, Jin Y, Choi AM, and Ryter SW. Beclin 1 deficiency is associated with increased hypoxia-induced angiogenesis. Autophagy 7: 829–839, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SJ, Ryter SW, Xu JF, Nakahira K, Kim HP, Choi AM, and Kim YS. Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. Am J Respir Cell Mol Biol 45: 867–873, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine B. and Kroemer G. Autophagy in the pathogenesis of disease. Cell 132: 27–42, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402: 672–676, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, Hengartner MO, and Green DR. Microtubule-associated protein 1 light chain 3 alpha (LC3B)-associated phagocytosis is required for the efficient clearance of death cells. Proc Natl Acad Sci USA 108: 17306–17401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Mizushima N. and Komatsu M. Autophagy: renovation of cells and tissues. Cell 147: 728–741, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Nakahira K, Haspel JA, Rathinam VK, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, and Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP inflammasome. Nat Immunol 3: 222–230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otterbein LE, Bach FH, Alam J, Soares M, Tao LH, Wysk M, Davis RJ, Flavell RA, and Choi AM. Carbon monoxide has anti inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6: 422–428, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Otterbein LE, Mantell LL, and Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol 276: L68 8–L694, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Otterbein LE, May A, and Chin BY. Carbon monoxide increases macrophagy bacterial clearance through Toll-like receptor (TLR) 4 expression. Cell Mol Biol (Noisy-le-grand) 51: 433–440, 2005 [PubMed] [Google Scholar]
- 33.Pinsky MR. Sepsis: a pro- and anti- inflammatory disequilibrium syndrome. Conrib Nephrol 132: 354–366, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, and Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345: 1368–1377, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Ryter SW, Alam J, and Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86: 583–650, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Saitoh T, Fujima N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, and Akira S. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature 456: 264–268, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Sammut IA, Foresti R, Clark JE, Exon DJ, Vesely MJ, Sarathchandra P, Green CJ, and Motterlini R. Carbon monoxide is a major contributor to the regulation of vascular tone in aortas expressing high level of hemeoxygenase-1. Br J Phamacol 7: 1437–1444, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanjuan MA, Dillon CP, Tait SWG, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, and Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450: 1253–1257, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Tenhunen R, Marver HS, and Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA 61: 748–755, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Underhill DM. and Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol 12: 492–502, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vernon PJ. and Tang D. Eat-me: autophagy, phagocytosis, and reactive oxygen species signaling. Antioxid Redox Signal 6: 677–691, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virgin HW. and Levine B, Autophagy genes in immunity. Nat Immunol 10: 461–470, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, and Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem 282: 1718–1726, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Zhou Y, Kim HP, Song R, Zarnegar R, Ryter SW, and Choi AM. Hepatocyte growth factor protects against hypoxia/reoxygenation-induced apoptosis in endothelial cells. J Biol Chem 279: 5237–5243, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Watson RO, Manzanillo PS, and Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 150: 803–805, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wegiel B, Gallo DJ, Raman KG, Karlsson JH, Ozanich B, Chin BY, Tzeng E, Admad S, Ahmed A, Baty CJ, and Otterbein LE. Nitric oxide-dependent bone marrow progenitor mobilization by carbon monoxide enhances endothelial repair after vascular injury. Circulation 121: 537–548, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wegiel B, Hanto DW, and Otterbein LE. The social network of carbon monoxide in medicine. Trends Mol Med 19: 3–11, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yue Z, Horton A, Bravin M, DeJager PL, Selimi F, and Heintz N. A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron 35: 921–933, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Yue Z, Jin S, Yang C, Levine AJ, and Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA 100: 15077–15082, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, and Lee PJ. Carbon monoxide inhibition of apoptotic during ischemia-reperfusion injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase-3. J Biol Chem 278: 1248–1258, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.