Abstract
Ever since clozapine was first synthesized and tested, it showed the unique property of having antipsychotic action but no Parkinson-like motor side effects. The antipsychotic basis of clozapine is to transiently occupy dopamine D2 receptors in the human striatum, in contrast to haloperidol and chlorpromazine, which have a prolonged occupation of D2 receptors. The chemical structure of clozapine facilitates a relatively rapid dissociation from D2 receptors. After short-term occupation of D2 receptors, peak neural activity raises synaptic dopamine, which then displaces clozapine. While clozapine also occupies other types of receptors, they may not have a significant role in preventing parkinsonism. Clozapine’s transient occupation of D2 receptors permits patients to move easily and comfortably.
Keywords: Dopamine, psychosis, 5HT2A receptor, dopamine D2 receptor
1. Background
The synthesis of clozapine by Schmutz and colleagues1,2 in the 1960s led to a vast number of related compounds.3 This intense interest in preparing many clozapine-related drugs was based on the striking observation that clozapine was an effective antipsychotic medication but did not cause the Parkinson-like signs of tremor, akinesia, and rigidity, as did the traditional antipsychotics chlorpromazine and haloperidol.4
Since those early days, additional advantages and disadvantages of clozapine have become widely known (as reviewed by Wenthur and Lindsley5). In particular, the major disadvantages include a risk of a low number of blood neutrophil granulocytes, the nuisance of blood testing for agranulocytosis, major weight gain6 (10 pounds or more), metabolic syndrome and diabetes, severe constipation, and bowel obstruction.
Nevertheless, despite these clinical risks of clozapine, the absence of Parkinson-like motor side effects is a major advantage of clozapine. As pointed out by Meltzer,7 “the near complete absence of motor side effects contributes to the low rate of discontinuation with clozapine”. In addition, clozapine is effective in treating treatment-resistant schizophrenia8 and lowering the risk of suicide.7
The objective of this overview is to review briefly the basis of clozapine’s unique feature of exerting antipsychotic action while avoiding Parkinson-like side effects.
2. Antipsychotic Basis of Clozapine Action
Ever since the discovery of the antipsychotic/dopamine receptor,9−11 now known as the dopamine D2 receptor, it continues to hold that all antipsychotics act on this receptor to alleviate psychosis. It has been noted that “no drug has yet been identified with antipsychotic action without a significant affinity for the D2 receptor”,12,13 and “all approved antipsychotics share an affinity for the D2 receptor”.14 New drugs designed to produce antipsychotic action without D2 blockade have not succeeded. This includes drugs that are selective for 5HT2A receptors (MDL100907 and pimavanserin [Acadia 103],14 D4 receptors (L745,850), 5HT2A and D4 receptors (fananserin), and D1 receptors (SCH23390).15
For example, the data in Table 1 for a set of seven antipsychotics, including clozapine, show a clear relation between the antipsychotic dissociation constants at the D2 receptor and the therapeutic free antipsychotic concentrations in the treated patients’ plasma (extensive data are provided in Seeman16,17). This relation is not found for the serotonin 5HT2A receptor (Table 1) or for any other receptor. These data support the principle that clozapine acts on D2 receptors, which appears to be a necessary minimum for clinical antipsychotic action. However, it still needs to be explained why clozapine has a unique property of avoiding Parkinson-like motor side effects.
Table 1. Antipsychotic Dissociation Constants, Ki, at Dopamine Receptorsa.
|
Ki (nM) at receptor ([3H]ligand, Kd (nM))b |
|||||
|---|---|---|---|---|---|
| antipsychotic | Cclin (nM)c | D2L (Raclo., 1.9) | D2L (Spip., 0.065) | D2L (Nem., 0.068) | 5HT2A (Ket., −) |
| haloperidol | 1–3 | 0.74 | 2.7 | 8.4 | 74 |
| risperidone | 2–3 | 1.09 | 4 | 19 | 0.2 |
| chlorpromazine | 4–8 | 1.2 | 4.6 | 14 | 2 |
| raclopride | 2–5 | 1.7 | 7.1 | 22 | 4400 |
| amisulpride-S | – | 1.8 | 4.6 | 8 | – |
| isoclozapine | – | 15 | 60 | 130 | 2.2 |
| remoxipride | ∼198 | 67 | 800 | 900 | 6600 |
| clozapine | 40–126 | 75 | 180 | 385 | 4 |
| quetiapine | 130–154 | 140 | 680 | 1400 | 135 |
Ki values (3–14 replicates) were measured at ligand concentrations equal to 2 × Kd. Receptor abbreviations: D2L = D2Long; 5HT2A = serotonin 2A receptor. Ligand abbreviations: Raclo. = raclopride; Spip. = spiperone; Nem. = nemonapride; Ket. = ketanserin.
Clinical free concentration in patient plasma.
3. Clozapine Dissociation Constant
It is essential to recognize the precise value for the clozapine dissociation constant in order to consider clozapine’s unique mechanism of clinical action. Table 1 clearly shows that the inhibition dissociation constant (Ki) for each antipsychotic depends on the dissociation constant (Kd) of the radioligand used.17−20 For example, clozapine has a Ki of 75 nM at the D2 receptor when the loosely bound ligand [3H]raclopride (Kd = 1.9 nM) is used. However, when the tightly bound ligand [3H]nemonapride (Kd = 0.068 nM) is used, the clozapine Ki is much higher (385 nM).
Which of these several Ki values is clinically relevant? Because the antipsychotic clinically normally competes for the water-soluble dopamine neurotransmitter, it is reasonable to use the lowest Ki value for clozapine, 75 nM, which was obtained using the most water-soluble radioligand ([3H]raclopride). It should also be noted that the Ki value of a drug artifactually increases when the in vitro tissue concentration is higher than 0.1 mg of protein/mL.21
4. Clozapine and the Serotonin 5HT2A Receptor
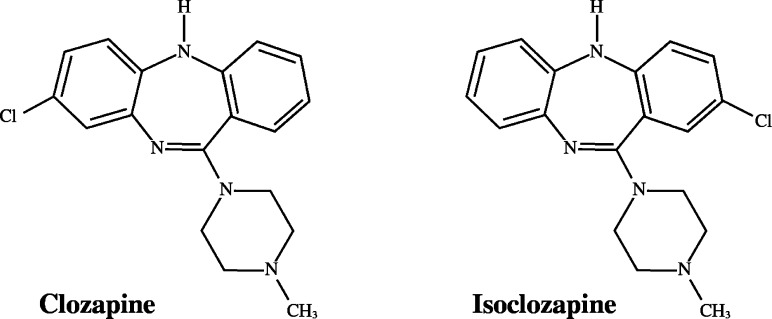
It is often mentioned that clozapine simultaneously blocks the serotonin 5HT2A receptor or other serotonin receptors as well as dopamine D2 receptors, suggesting that the block of serotonin receptors may prevent the Parkinson-like motor side effects of antipsychotics.22−24 While this principle may apply to a few antipsychotics that induce low levels of Parkinson-like motor side effects (so-called atypical antipsychotics), there are a number of exceptions to this principle. Moreover, in the report by Meltzer et al.,22 the greater difference between the serotonin 5HT2A and D2 affinities in atypical antipsychotics was not due to higher 5HT2A affinities but to lower D2 affinities (analysis elsewhere).15 For example, clozapine and isoclozapine (Figure 1) have similar affinities for all receptors that have been tested, including the 5HT2A receptor, except that isoclozapine has a higher affinity than clozapine for D2. In other words, an increase in the affinity of clozapine for D2 (from a Ki of 75 nM for clozapine down to a Ki of 15 nM for isoclozapine) is associated with catalepsy, indicating that the Ki value for the 5HT2A receptor is not critical for the unique action of clozapine.25
Figure 1.
Structures of clozapine and isoclozapine, which have similar inhibition constants Ki at serotonin 5HT2A receptors (Table 1). Clozapine has Ki = 75 nM at the D2 receptor and does not elicit parkinsonism. Isoclozapine has Ki = 15 nM but causes catalepsy, indicating that the absolute value of Ki of D2 (rather than that for the 5HT2A receptor) determines whether or not parkinsonism occurs.
More generally, Table 2A shows that antipsychotics with high selectivity for serotonin 5HT2A receptors (relative to D2 receptors), including amoxapine, isoclozapine, risperidone, can elicit significant parkinsonian signs in patients or catalepsy in animals. Moreover, Table 2B shows that quetiapine, thioridazine, and remoxipride cause little or no parkinsonan signs despite having no selectivity for serotonin 5HT2A receptors. A similar analysis for serotonin 1A receptors showed a similar lack of benefit in preventing Parkinson-like side effects.20
Table 2. Antipsychotics with High or Low Selectivity for 5HT2A Receptors Relative to Ki Values at the D2 Receptora.
| antipsychotic | KiD2/Ki | parkinsonism |
|---|---|---|
| (A) High Selectivity | ||
| amoxapine | 33 | low |
| clozapine | 20 | none |
| isoclozapine | 6.7 | catalepsy |
| risperidone | 5.6 | moderate |
| (B) Low Selectivity | ||
| olanzapine | 2.2 | moderate |
| quetiapine | 1 | none |
| thioridazine | 0.8 | low |
| chlorpromazine | 0.6 | yes |
| fluphenazine | 0.14 | yes |
| remoxipride | 0.01 | none |
| haloperidol | 0.01 | yes |
| raclopride | 0.0004 | yes |
In patients, high occupancy of serotonin 5HT2A receptors is not generally associated with an absence of motor side effects. Clinically, clozapine occupies almost all (actually 96%) of the serotonin 5HT2A receptors at daily clinical doses of 300–600 mg/day.27 Nevertheless, while risperidone also occupies ∼100% of the 5HT2A receptors at 6 mg/day,15,27 risperidone elicits Parkinsonian side effects in at least half of the patients.
It should be noted that Parkinson-like motor side effects occur only when more than ∼72% to 80% of the D2 receptors are occupied by an antipsychotic. Because clozapine only transiently occupies this high level of D2 receptors (see later section),26 the low D2 occupancy by clozapine precludes motor side effects without any need for blockade of other receptors. In contrast, risperidone exceeds this 80% threshold in a dose-dependent manner, thereby resulting in motor side effects.15
A role for serotonin receptors in antipsychotic action is still being examined. For example, pimavanserin added to 2 mg of risperidone/day resulted in antipsychotic action equivalent to 6 mg of risperidone/day.28 However, because risperidone at 2 mg/day occupies 72–88% of the brain serotonin 5HT2A receptors,15,27 the addition of 20 mg of pimavanserin/day (which alone occupies 77% of the 5HT2A receptors29) would not occupy more 5HT2A receptors than risperidone alone. In addition, pimavanserin also occupied a small but detectable number of D2 receptors in human striatum,29 complicating the interpretation of a role for 5HT2A receptors in antipsychotic action.
5. Clozapine and the Cholinergic Muscarinic Receptor
In addition to its potency at 5HT2A receptors, clozapine is very potent at the muscarinic M1 receptor with a dissociation constant of Ki = 9.5 nM.16 However, here too there is no correlation between the risk of parkinsonism and the (Ki at M1)/(Ki at D2) ratio for the atypical antipsychotics, including clozapine. Such data do not make it likely that clozapine prevents parkinsonism by occluding the M1 muscarinic receptor.
Moreover, while clozapine does not cause parkinsonism or catalepsy, isoclozapine (see later section) elicits catalepsy even though it has a strong potency of 14 nM at the muscarinic receptor.16
6. Clozapine and the α2-Adrenoceptor
Because clozapine is also potent at the α2-adrenoceptor with a Ki value of 51 nM,30 blockade of this receptor has been suggested to prevent parkinsonism. There is considerable evidence, however, that antagonism of α2-adrenoceptors does not explain the clozapine prevention or reversal of motor defects caused by D2 blockade.31
7. Clozapine and Glutamate Receptors
A possible action of clozapine on glutamate receptors has also been considered recently.5 There is renewed interest in the hypoglutamate theory of psychosis based on the fact that glutamate antagonists such as phencyclidine and ketamine trigger psychosis. However, it is known clinically that haloperidol (which has a high affinity for D2 but a low affinity for glutamate receptors) effectively alleviates the psychosis secondary to phencyclidine or ketamine.32,33 Moreover, it appears that glutamate receptor agonists such as pomaglumetad methionil and LY404,039 do not have any antipsychotic efficacy. A four-week trial had little or no efficacy against positive symptoms or negative signs when compared with olanzapine.34,35 While there does not at present appear to be a significant role for glutamate receptors in the antipsychotic treatment of schizophrenia, these are only limited studies, and much remains to be learned about the possible benefit of this approach.
8. Clozapine and Fast-Off-D2 Antipsychotics
Upon the discovery of the two main factors that determine a drug’s dissociation constant (Ki) value, namely, the Kd of the [3H]ligand and the tissue concentration,18,21 it became clear that the appropriate Ki for clozapine was 75 nM. Moreover, as shown in Table 1, this high value for clozapine and a similar high value for quetiapine immediately indicated that these two drugs had Ki values higher than any of the other antipsychotics and likely had a unique mechanism of interaction with the D2 receptor, namely, “loose binding to the D2 receptor”.35,37
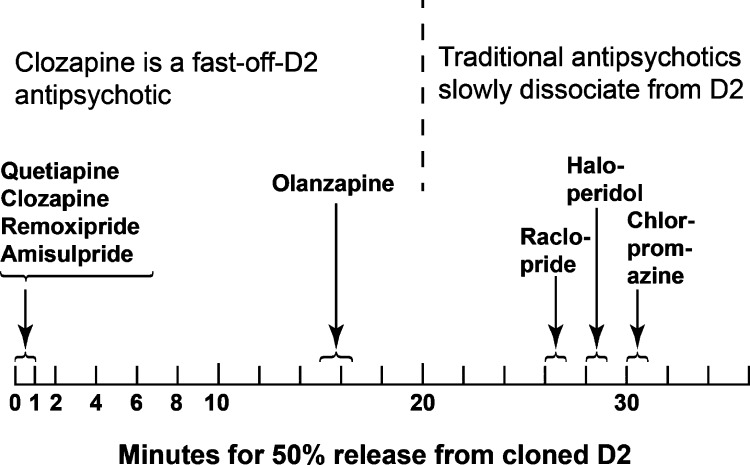
Therefore, in order to determine directly whether clozapine is indeed loosely bound to D2, the dissociation rates of [3H]clozapine and other tritiated antipsychotic drugs were directly measured on human-cloned D2Long receptors.26,37,38 A qualitative summary of these data (Figure 2) shows that [3H]clozapine was 50% displaced at 15 s by 100 μM raclopride.
Figure 2.
[3H]Clozapine, [3H]quetiapine, [3H]remoxipride, and [3H]amisulpride quickly dissociated or were quickly displaced from human-cloned D2 receptors upon the rapid addition of 100 μM raclopride in vitro. In contrast, [3H]olanzapine, [3H]haloperidol, [3H]raclopride, and [3H]chlorpromazine were displaced much more slowly from the D2 receptors. (Adapted from ref (26).)
The rapid displacement or dissociation of clozapine was clearly associated with its low affinity or high Ki constant of 75 nM. This relation between the Ki value and the displacement rate was supported by the fact that isoclozapine, with its low Ki value of 15 nM, was 50% displaced in 216 s (Seeman, unpublished observation).
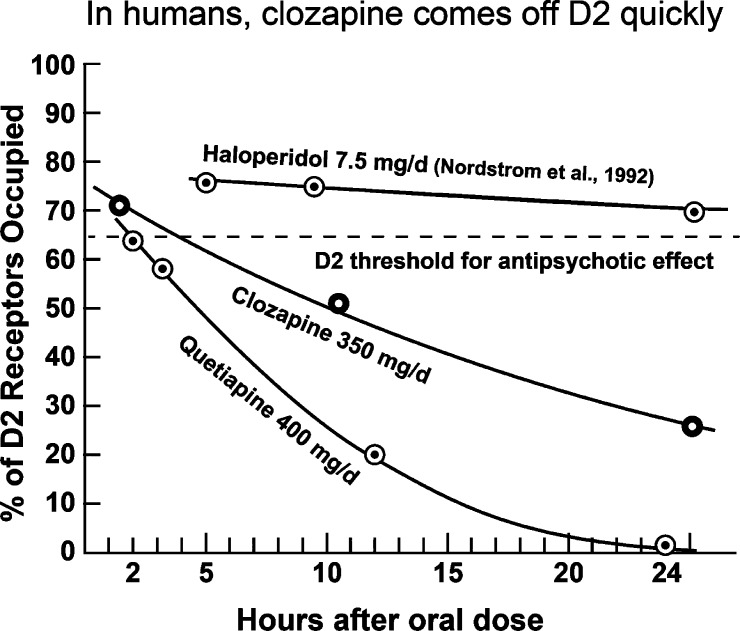
However, are such in vitro data (Figure 2) relevant to humans clinically? The data in Figure 3 show that they are. In fact, clozapine occupies about 72% of the D2 receptors in the human striatum 2 h after administration, followed by a quick reduction down to less than 30% by 24 h (see refs elsewhere26). The time course is even faster with quetiapine, as shown by Kapur et al.39 These findings are in contrast to those for haloperidol, where an oral dose of 7.5 mg occupied more than 65% of human brain D2 receptors for at least 27 h (Figure 3).40 In addition, the time-course data in Figure 3 are consistent with the Ki data mentioned in Table 1. That is, the slowest time course occurred with haloperidol and its very low Ki value of 0.74 nM, followed by the faster time course of clozapine with its Ki of 75 nM, and followed by the fastest time course of quetiapine with its high Ki of 140 nM.
Figure 3.
An oral dose of 350 mg occupied 72% of human striatal D2 receptors at 2 h, with the occupation falling to less than 30% at 24 h. The occupation of human D2 dropped even faster with oral quetiapine (400 mg). Oral haloperidol (7.5 mg) occupied high levels of D2 for at least 27 h.26,40
Could this principle of a fast-off-D2 antipsychotic lead to the development of a new fast-off-D2 antipsychotic with no parkinsonism and no metabolic side effects?
In fact, Tresadern et al.41 have analyzed the physicochemical properties, including MW, lipophilicity, charge (pKa), and surface area, of 1800 D2 antagonists in order to determine which properties determine the rate of dissociation from the D2 receptor. They concluded that increased hydrophilicity, increased number of nitrogen atoms, lower MW, fewer rings, less chiral centers, and increased partial positive charge all contribute to a faster dissociation from the D2 receptor.
Following the work of Tresadern et al.41 and using the radioligand dissociation method37 in a search for a fast-off-D2 antagonist, Langlois et al.42 identified N-[1-(3,4-difluorobenzyl)piperidin-4-yl]-6-(trifluoromethyl)pyridazin-3-amine (JNJ-37822681) as a fast-dissociating D2 ligand. This compound has a high Ki of 158 nM using [3H]spiperone,42 and a Ki of 25–30 nM using [3H]raclopride.43 The compound has a low affinity for 5HT2A receptors (Ki = 2896 nM) and for histamine H1 receptors (Ki = 4931 nM). In accordance with these Ki values, the compound was found to be effective in treating schizophrenia without parkinsonian motor side effects, elevated prolactin, or weight gain.44 The compound transiently occupies 60–74% of human D2 receptors after an oral dose of 20 mg.45
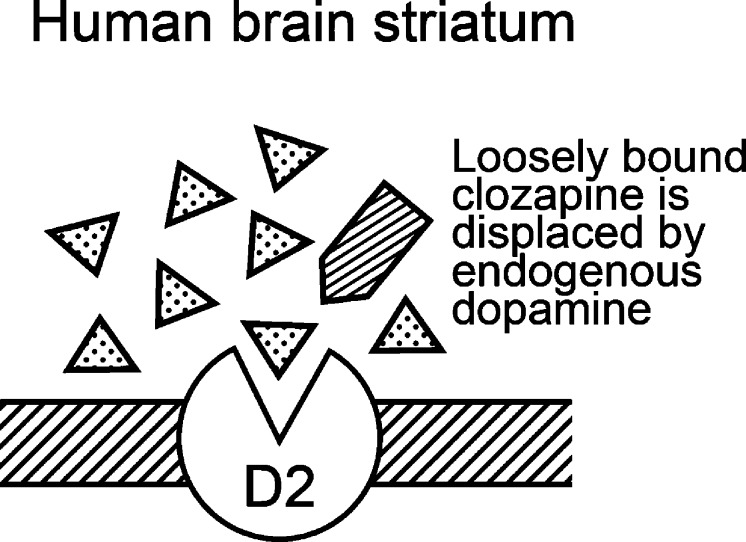
9. Clozapine and Endogenous Dopamine
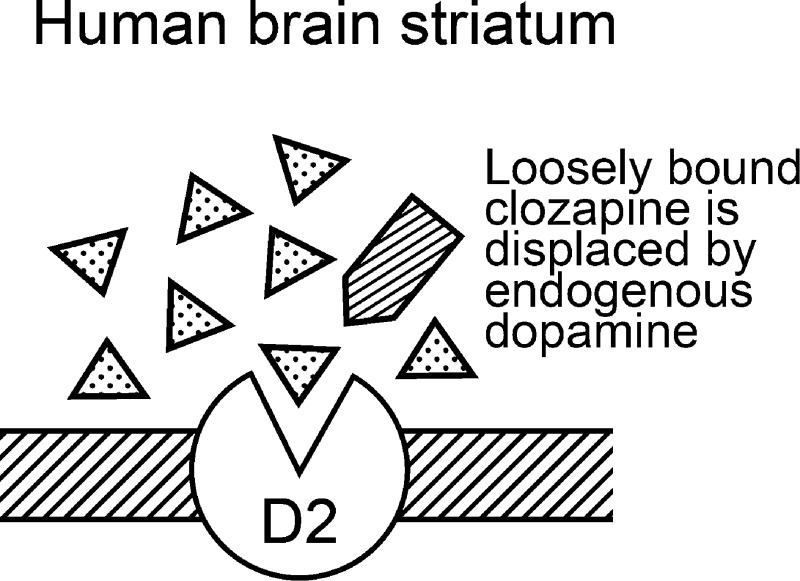
The main benefit of clozapine and other fast-off-D2 antipsychotics is that they bind briefly to the D2 receptor, triggering a suppression of psychotic symptoms, while allowing endogenous dopamine to displace the loosely bound antipsychotic drug from the motor-controlling brain regions.15,19,37,46 Upon release from the nerve terminal of a dopamine neuron, the concentration of dopamine in the synapse at peak firing frequency is between 100 and 500 nM, which is sufficient to compete and replace weakly D2-bound drugs such as clozapine (Figure 4) but less effective in replacing tightly bound haloperidol.
Figure 4.
The high concentration (∼100 nM to 500 nM) of dopamine in the synapse during repetitive neural activity can readily displace loosely D2-bound clozapine in the human striatum of patients.
The surprising take-home lesson that clozapine teaches us is that a short-term transiently high occupation of D2 receptors is sufficient to maintain an antipsychotic action without D2 receptors being occupied.37 Clozapine readily occupies the D2 receptors at rest when the resting level of synaptic dopamine is about 2–10 nM, but the clozapine is replaced during peak neural activity when dopamine in the synapse rises to 100–500 nM.19,37 This principle of transient occupation of D2 has been used to develop “extended” antipsychotic dosing, where a traditional drug such as haloperidol can be administered every second day with safe and effective clinical results.47
These basic physicochemical considerations may explain why patients with schizophrenia feel more comfortable, more mobile, and more agile with clozapine than with any tightly D2-bound antipsychotic drug.
Author Contributions
The author is science advisor to Clera Inc.
The authors declare no competing financial interest.
References
- Hunziker F.; Fischer E.; Schmutz J. (1967) 11-Amino-5H-dibenzo[b,e]-1,4-diazepine. 10. Mitteilung über siebengliedrige Heterocyclen. Helv. Chim. Acta 50(6), 1588–1599. [Google Scholar]
- Schmutz J. (1975) Neuroleptic piperazinyl-dibenzo-azepines. Chemistry and structure–activity relationships. Arzneimittelforschung 25(5), 712–720. [PubMed] [Google Scholar]
- de Paulis T.; Betts C. R.; Smith H. E.; Mobley P. L.; Manier D. H.; Sulser F. (1981) Synthesis of clozapine analogues and their affinity for clozapine and spiroperidol binding sites in rat brain. J. Med. Chem. 24(9), 1021–1026. [DOI] [PubMed] [Google Scholar]
- Bürki H. R.; Sayers A. C.; Ruch W.; Asper H. (1977) Effects of clozapine and other dibenzo-epines on central dopaminergic and cholinergic systems. Structure–activity relationships. Arzneimittelforschung 27(8), 1561–1565. [PubMed] [Google Scholar]
- Wenthur C. J.; Lindsley C. W. (2013) Classics in chemical neuroscience: Clozapine. ACS Chem. Neurosci. 4, 1018–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison D. B.; Mentore J. L.; Heo M.; Chandler L. P.; Cappelleri J. C.; Infante M. C.; Weiden P. J. (1999) Antipsychotic-induced weight gain: A comprehensive research synthesis. Am. J. Psychiatry 156(11), 1686–1696. [DOI] [PubMed] [Google Scholar]
- Meltzer H. Y. (2012) Clozapine: Balancing safety with superior antipsychotic efficacy. Clin. Schizophr. Relat. Psychoses 6(3), 134–144. [PubMed] [Google Scholar]
- Kane J.; Honigfeld G.; Singer J.; Meltzer H. (1988) Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatry 45(9), 789–796. [DOI] [PubMed] [Google Scholar]
- Seeman P.; Chau-Wong M.; Tedesco J.; Wong K. (1975) Brain receptors for antipsychotic drugs and dopamine: Direct binding assays. Proc. Natl. Acad. Sci. U.S.A. 72, 4376–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P.; Lee T.; Chau-Wong M.; Wong K. (1976) Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 261, 717–719. [DOI] [PubMed] [Google Scholar]
- Madras B. K. (2013) History of the discovery of the antipsychotic dopamine D2 receptor: A basis for the dopamine hypothesis of schizophrenia. J. Hist. Neurosci. 22(1), 62–78. [DOI] [PubMed] [Google Scholar]
- Su T. P.; Malhotra A. K.; Hadd K.; Breier A.; Pickar D. (1997) D2 dopamine receptor occupancy: A crossover comparison of risperidone with clozapine therapy in schizophrenic patients. Arch. Gen. Psychiatry 54(10), 972–973. [DOI] [PubMed] [Google Scholar]
- Pickar D. (1995) Prospects for pharmacotherapy of schizophrenia. Lancet 345(8949), 557–562. [DOI] [PubMed] [Google Scholar]
- Ebdrup B. H.; Rasmussen H.; Arnt J.; Glenthøj B. (2011) Serotonin 2A receptor antagonists for treatment of schizophrenia. Expert Opin. Invest. Drugs 20(9), 1211–1223. [DOI] [PubMed] [Google Scholar]
- Kapur S.; Seeman P. (2001) Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics? A new hypothesis. Am. J. Psychiatry 158, 360–369. [DOI] [PubMed] [Google Scholar]
- Seeman P. (2006) Targeting the dopamine D2 receptor in schizophrenia. Expert Opin. Ther. Targets 10(4), 515–531. [DOI] [PubMed] [Google Scholar]
- Seeman P. (2011) All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2High receptors. CNS Neurosci. Ther. 17(2), 118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P.; Van Tol H. H. M. (1995) Deriving the therapeutic concentrations for clozapine and haloperidol: The apparent dissociation constant of a neuroleptic at the dopamine D2 or D4 receptor varies with the affinity of the competing radioligand. Eur. J. Pharmacol., Mol. Pharmacol. Sect. 291, 59–66. [DOI] [PubMed] [Google Scholar]
- Seeman P.; Corbett R.; Nam D.; Van Tol H. H. M. (1996) Dopamine and serotonin receptors: Amino acid sequences and clinical role in neuroleptic parkinsonism. Jpn. J. Pharmacol. 71, 187–204. [DOI] [PubMed] [Google Scholar]
- Seeman P. (2010) Dopamine D2 receptors as treatment targets in schizophrenia. Clin. Schizophr. Relat. Psychoses 4(1), 56–73. [PubMed] [Google Scholar]
- Seeman P.; Ulpian C.; Wreggett K. A.; Wells J. (1984) Dopamine receptor parameters detected by 3H-spiperone depend on tissue concentration: Analysis and examples. J. Neurochem. 43, 221–235. [DOI] [PubMed] [Google Scholar]
- Meltzer H. Y.; Matsubara S.; Lee J. C. (1989) Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J. Pharmacol. Exp. Ther. 251(1), 238–246. [PubMed] [Google Scholar]
- Meltzer H. Y.; Li Z.; Kaneda Y.; Ichikawa J. (2003) Serotonin receptors: Their key role in drugs to treat schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 27, 1159–1172. [DOI] [PubMed] [Google Scholar]
- Meltzer H. Y. (2012) Serotonergic mechanisms as targets for existing and novel antipsychotics. Handb. Exp. Pharmacol. 212, 87–124. [DOI] [PubMed] [Google Scholar]
- Kapur S.; McClelland R. A.; Vanderspeck S. C.; Wadenberg M.-L. G.; Baker G.; Nobrega J.; Zipursky R.; Seeman P. (2002) Increasing D2 affinity results in the loss of clozapine’s atypical antipsychotic action. Neuroreport 13(6), 831–835. [DOI] [PubMed] [Google Scholar]
- Seeman P. (2002) Atypical antipsychotics: Mechanism of action. Can. J. Psychiatry 47, 27–38. [PubMed] [Google Scholar]
- Kapur S.; Zipursky R. B.; Remington G. (1999) Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am. J. Psychiatry 156(2), 286–293. [DOI] [PubMed] [Google Scholar]
- Meltzer H. Y.; Elkis H.; Vanover K.; Weiner D. M.; van Kammen D. P.; Peters P.; Hacksell U. (2012) Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2 mg/day, but does not enhance efficacy of haloperidol, 2 mg/day: Comparison with reference dose risperidone, 6 mg/day. Schizophr. Res. 141(2–3), 144–152. [DOI] [PubMed] [Google Scholar]
- Nordstrom A. L.; Mansson M.; Jovanovic H.; Karlsson P.; Halldin C.; Farde L.; Vanover K. E.; Hacksell U.; Brann M. R.; Davis R. E.; Weiner D. M. (2008) PET analysis of the 5-HT2A receptor inverse agonist ACP-103 in human brain. Int. J. Neuropsychopharmacol. 11(2), 163–171. [DOI] [PubMed] [Google Scholar]
- Boyajian C. L.; Leslie F. M. (1987) Pharmacological evidence for α2 adrenoceptor heterogeneity: Differential binding properties of [3H]rauwolscine and [3H]idazoxan in rat brain. J. Pharmacol. Exp. Ther. 241(3), 1092–1098. [PubMed] [Google Scholar]
- McAllister K. H.; Rey B. (1999) Clozapine reversal of the deficits in coordinated movement induced by D2 receptor blockade does not depend upon antagonism of α2 adrenoceptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 360(6), 603–608. [DOI] [PubMed] [Google Scholar]
- Giannini A. J.; Nageotte C.; Loiselle R. H.; Malone D. A.; Price W. A. (1984) Comparison of chlorpromazine, haloperidol and pimozide in the treatment of phencyclidine psychosis: Da-2 receptor specificity. J. Toxicol., Clin. Toxicol. 22(6), 573–579. [DOI] [PubMed] [Google Scholar]
- Giannini A. J.; Underwood N. A.; Condon M. (2000) Acute ketamine intoxication treated by haloperidol: A preliminary study. Am. J. Ther. 7, 389–391. [DOI] [PubMed] [Google Scholar]
- Kinon B. J.; Zhang L.; Millen B. A.; Osuntokun O. O.; Williams J. E.; Kollack-Walker S.; Jackson K.; Kryzhanovskaya L.; Jarkova N. (2011) A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J. Clin. Psychopharmacol. 31, 349–355. [DOI] [PubMed] [Google Scholar]
- Seeman P. (2012) Comment on “A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia” by Kinon et al. J. Clin. Psychopharmacol. 32, 291–292. [DOI] [PubMed] [Google Scholar]
- Seeman P.; Tallerico T. (1998) Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol. Psychiatry 3, 123–134. [DOI] [PubMed] [Google Scholar]
- Seeman P.; Tallerico T. (1999) Rapid release of antipsychotic drugs from dopamine D2 receptors: An explanation for low receptor occupancy and early clinical relapse upon drug withdrawal of clozapine or quetiapine. Am. J. Psychiatry 156, 876–884. [DOI] [PubMed] [Google Scholar]
- Seeman P. (2005) An update on fast-off-D2 atypical antipsychotics. Am. J. Psychiatry 162, 1984–1985. [DOI] [PubMed] [Google Scholar]
- Kapur S.; Zipursky R.; Jones C.; Shammi C. S.; Remington G.; Seeman P. (2000) A positron emission tomography study of quetiapine in schizophrenia—A preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch. Gen. Psychiatry 57, 553–559. [DOI] [PubMed] [Google Scholar]
- Nordström A. L.; Farde L.; Halldin C. (1992) Time course of D2-dopamine receptor occupancy examined by PET after single oral doses of haloperidol. Psychopharmacology (Berlin) 106(4), 433–438. [DOI] [PubMed] [Google Scholar]
- Tresadern G.; Bartolome J. M.; Macdonald G. J.; Langlois X. (2011) Molecular properties affecting fast dissociation from the D2 receptor. Bioorg. Med. Chem. 19(7), 2231–2241. [DOI] [PubMed] [Google Scholar]
- Langlois X.; Megens A.; Lavreysen H.; Atack J.; Cik M.; te Riele P.; Peeters L.; Wouters R.; Vermeire J.; Hendrickx H.; Macdonald G.; De Bruyn M. (2012) Pharmacology of JNJ-37822681, a specific and fast-dissociating D2 antagonist for the treatment of schizophrenia. J. Pharmacol. Exp. Ther. 342(1), 91–105. [DOI] [PubMed] [Google Scholar]
- Petersson K. J.; Vermeulen A. M.; Friberg L. E. (2013) Predictions of in vivo prolactin levels from in vitroKi values of D2 receptor antagonists using an agonist–antagonist interaction model. AAPS J. 15(2), 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. E.; Kent J. M.; Daly E.; Janssens L.; Van Osselaer N.; Hüsken G.; Anghelescu I.-G.; Van Nueten L. (2012) A double-blind, randomized, placebo-controlled study with JNJ-37822681, a novel, highly selective, fast dissociating D2 receptor antagonist in the treatment of acute exacerbation of schizophrenia. Eur. Neuropsychopharmacol. 22(10), 721–733. [DOI] [PubMed] [Google Scholar]
- te Beek E. T.; de Boer P.; Moerland M.; Schmidt M. E.; Hoetjes N. J.; Windhorst A. D.; van Berckel B. N.; Cohen A. F.; van Gerven J. M.; Lammertsma A. A. (2012) In vivo quantification of striatal dopamine D2 receptor occupancy by JNJ-37822681 using [11C]raclopride and positron emission tomography. J. Psychopharmacol. 26(8), 1128–1135. [DOI] [PubMed] [Google Scholar]
- Seeman P.; Guan H.-C.; Niznik H. B. (1989) Endogenous dopamine lowers the dopamine D2 receptor density as measured by [3H]raclopride: Implications for positron emission tomography of the human brain. Synapse 3, 96–97. [DOI] [PubMed] [Google Scholar]
- Remington G.; Seeman P.; Feingold A.; Mann S.; Shammi C.; Kapur S. (2011) “Extended” antipsychotic dosing in the maintenance treatment of schizophrenia: A double-blind, placebo-controlled trial. J. Clin. Psychiatry 72(8), 1042–1048. [DOI] [PubMed] [Google Scholar]