Abstract
Background
A comparative effectiveness intervention by this team improved initial fecal occult blood testing (FOBT) rates from 3% to 53% among community clinic patients. The purpose of this study was to evaluate the effectiveness and costs associated with a literacy-informed intervention on repeat FOBT testing.
Methods
Between 2008 and 2011, a three-arm quasi-experiential comparative effectiveness evaluation was conducted in 8 community clinics in Louisiana. Clinics were randomly assigned to receive: enhanced care, a screening recommendation and FOBT kit annually; a brief educational intervention where patients additionally received a literacy appropriate pamphlet and simplified FOBT instructions; or nurse support where a nurse manager provided the education and followed up with phone support. In year 2 all materials were mailed. The study consisted of 461 patients, ages 50–85, with a negative initial FOBT.
Results
Repeat FOBT rates were 38% enhanced care, 33% education, and 59% with nurse support (p=0.017). After adjusting for age, race, gender, and literacy, patients receiving nurse support were 1.46 times more likely to complete repeat FOBT screening than those receiving education (95% CI 1.14–1.06, p=0.002) and 1.45 times more likely than those in enhanced care but this was not significant (95% CI 0.93–2.26 p=0.10). The incremental cost per additional person screened was $2,450 for nurse over enhanced care.
Conclusion
A mailed pamphlet and FOBT with simplified instructions did not improve annual screening.
Impact
Telephone outreach by a nurse manager was effective in improving rates of repeat FOBT yet this may be too costly for community clinics.
Keywords: Health Literacy, Colon Cancer Screening, Annual screening, Cost effectiveness, Federally Qualified Health Centers
INTRODUCTION
Colorectal cancer screening (CRC) rates remain persistently lower among adults who have low socioeconomic status, limited health literacy skills, minority race/ethnicity, and/or live in rural locations.(1–6) Improving CRC screening in these populations is a public health objective (7). Fecal Occult Blood Tests (FOBTs) offer an effective, more acceptable, lower cost screening option for priority populations (8–12). FOBTs are the primary mode of CRC screening throughout the world and in areas of the U.S. where access to gastroenterologists and colonoscopy is limited (13–14). U.S. clinical guidelines recommend annual FOBT screening as an acceptable screening modality (1, 15–20).
The National Colorectal Cancer Roundtable has highlighted the potential for Federally Qualified Health Centers (FQHCs) to improve CRC screening among the 20 million individuals who receive care at these centers (21). Recent studies have shown promising results in increasing initial CRC screening rates using FOBTs in urban community clinics, state wide family practice networks, and healthcare delivery systems (8, 9, 11, 22–33). Strategies to improve initial rates of CRC screening have included patient-directed interventions (written materials, DVDs, mailed FOBT kits and reminders, telephone counseling, use of clinic-based nurses, medical assistants, and health educators) and physician-directed interventions (chart stickers, electronic reminders, academic detailing) (11, 22–32).
Encouraging annual FOBT is difficult (19, 33–36). In one state health plan, less than half (44%) of patients completing an initial FOBT completed a repeat test the following year (19). Medicare claims data indicate very low rates for annual (repeat) screening and only slightly higher rates of biennial FOBT use (37). Only 18% of Medicare beneficiaries in Kansas completed at least one FOBT in a two year period and 4% completed a test annually (37). In a recent study assessing screening in a private healthcare system, rates of eligible patients being current with CRC screening by any means over two years ranged from 26% with usual care to 65% with nurse navigation (30). A national V.A. retrospective analysis of 5 year adherence to FOBT found 42% of eligible patients received one FOBT, 26% received two tests, 17% three tests, and 14% four tests (36). In a recent retrospective analysis in a community health network, Liss and colleagues (35) recently reported that only 25% of patients, who completed an initial FOBT, completed a repeat FOBT within 18 months. Of importance none of the patients (0%) with 0 clinic visits completed a repeat FOBT, indicating a need for interventions that do not involve face to face interactions between patients and providers.
The authors recently reported on a literacy-informed comparative effectiveness intervention targeting low income and uninsured populations who received care at one of eight FQHCs. First year (initial) FOBT completion rates improved from 3% to 39% with enhanced care, 57% with an intervention that included literacy-appropriate educational tools and 61% with the educational tools and additional nurse support (p<0.012) (31).
The objective in this report was to compare the effectiveness of interventions designed to increase adherence without requiring face-to-face encounters with a provider on repeat FOBT testing one year after initial testing. Our secondary objective was to evaluate if these interventions were cost-effective and hence potentially sustainable by the FQHCs.
MATERIALS AND METHODS
Study Design and Sample
A three-arm, quasi-experimental (i.e. based on randomization of sites, but not patients within sites) comparative effectiveness evaluation was conducted among three FQHC networks in predominately rural areas of Louisiana between May 2008 and August 2011. The study team determined that randomizing patients within a FQHC network was not an optimal study design, because of the diffuse nature of interventions and concern of contamination among patients who belonged in a network FQHC that shared providers and staff. The target population was the five FQHC networks in predominantly rural north Louisiana. Three FQHC parent networks participated in this study and were assigned to one of the three study arms by simple randomization (the other two network FQHCs were involved in cancer screening programs at the time). The study statistician (AR) generated the allocation of parent network to arm by using computer generated random numbers. At the time of randomization, each participating FQHC parent network was affiliated with multiple clinics which were assigned to the same study arm as their parent network. This resulted in two clinics in the enhanced care arm, two in the educational strategy arm, and three in the nurse support arm. After the first year of the study, one additional clinic was enrolled in the enhanced care arm due to limited patient recruitment in this arm. The eight clinics were located in eight towns in seven parishes across the state. Baseline rates of CRC screening at each clinic ranged from 1 to 3 percent.
The three study arms included: 1) An enhanced version of usual care where patients waiting for a schedule appointment with their provider received a recommendation for CRC screening and an FOBT kit with a stamped envelope addressed to the clinic. 2) A literacy-informed educational intervention where, beyond enhanced care, a clinic-based research assistant (RA) gave patients brief structured education that included risk factors of colorectal cancer and the benefits of screening annually, using a pamphlet written on a 5th grade level and short video to illustrate key points. The RA also demonstrated how to do the test using the simplified written instructions and used teach back to confirm patient understanding. 3) A nurse manager strategy where patients received enhanced care and a designated clinic nurse provided the literacy informed education and followed-up by telephone using motivational interviewing (38).
Participants
In year 1, patients were recruited through a multi-step process. First, while taking patients’ vital signs, a medical assistant at each clinic identified potentially eligible participants by the age listed in their chart. Medical assistants were trained to ask patients age 50 or older if they would be willing to talk to an onsite RA about participating in a CRC screening study prior to their physician encounter. Patients aged 76–85 were included per the request of clinic directors. Further eligibility included: 1) English-speaking, 2) enrolled as a patient in the clinic, 3) not requiring screening at an earlier age according to American Cancer Society (ACS) guidelines, (1) 4) not up-to-date with United States Preventive Services Task Force (USPSTF) (20) CRC screening recommendations (i.e., an FOBT annually, flexible sigmoidoscopy every 5 years or colonoscopy every 10 years), 5) not having an acute medical concern (i.e. medical staff believing patients too ill to be interviewed). All participants were consented prior to data collection.
At enrollment, 1055 patients were identified as meeting age criteria, of these 33 (3.1%) refused to participate and 61 (5.8%) were ineligible because they were up to date on CRC screening. A total of 961 patients were consented and enrolled, with a determined cooperation rate of 91.1%. A total of 512 patients completed the initial FOBT within one year. Of these, 51 had a positive FOBT and received a provider referral for a colonoscopy and were therefore not eligible for a repeat annual FOBT. This paper focuses on the 461 patients who had an FOBT in year 1 with a negative result and would therefore qualify as being eligible for a repeat annual FOBT. For the overall study, the primary outcomes were initial and repeat screening, and power calculations were originally done for each outcome. The Louisiana State University Health Sciences Center – Shreveport Institutional Review Board approved the study.
Instruments
A structured survey which included patient demographics and literacy assessment were administered orally in year 1 at enrollment. A more detailed description of the survey has been reported previously (39). Literacy was assessed using the Rapid Estimate of Adult Literacy in Medicine (REALM) (40). Raw REALM scores (0–66) can be converted into reading grade levels (<61, below ninth grade reading level, an indicator of limited literacy) and (≥61, ninth grade reading level or above, an indicator of adequate literacy).
Theoretical Framework
The intervention and its components were designed following health literacy best practices and the health learning capacity framework for understanding the cognitive and psychosocial skills patients need to effectively manage their health (41–43). The Health Belief Model and Social Cognitive theories guided the framing of intervention content to address the salience of CRC screening and the need to take action (44–46). The health learning capacity framework guided the intervention design and development. This framework deconstructs patient roles and minimizing the cognitive demands and perceived difficulty associated with obtaining and completing an FOBT. This can be achieved through understandable information that explains the importance and benefits of annual screening, coupled with explicit instructions that simplify the tasks necessary to properly complete and return the test. All arms were designed to overcome key patient CRC screening barriers such as access to tests, lack of recommendation, and not receiving prompting annually. The educational strategy and nurse support arms were designed to additionally address limited knowledge, negative beliefs, poor self-efficacy, complexity of independently completing the test, and lack of motivation. The education materials were developed in collaboration with patients, providers, and our community advisory boards to help insure they were useful, understandable, appealing, and cultural appropriate (31). The nurse arm was included as an intervention strategy to determine the added benefit of more in-depth counseling and telephone follow up support to encourage FOBT completion.
Interventions
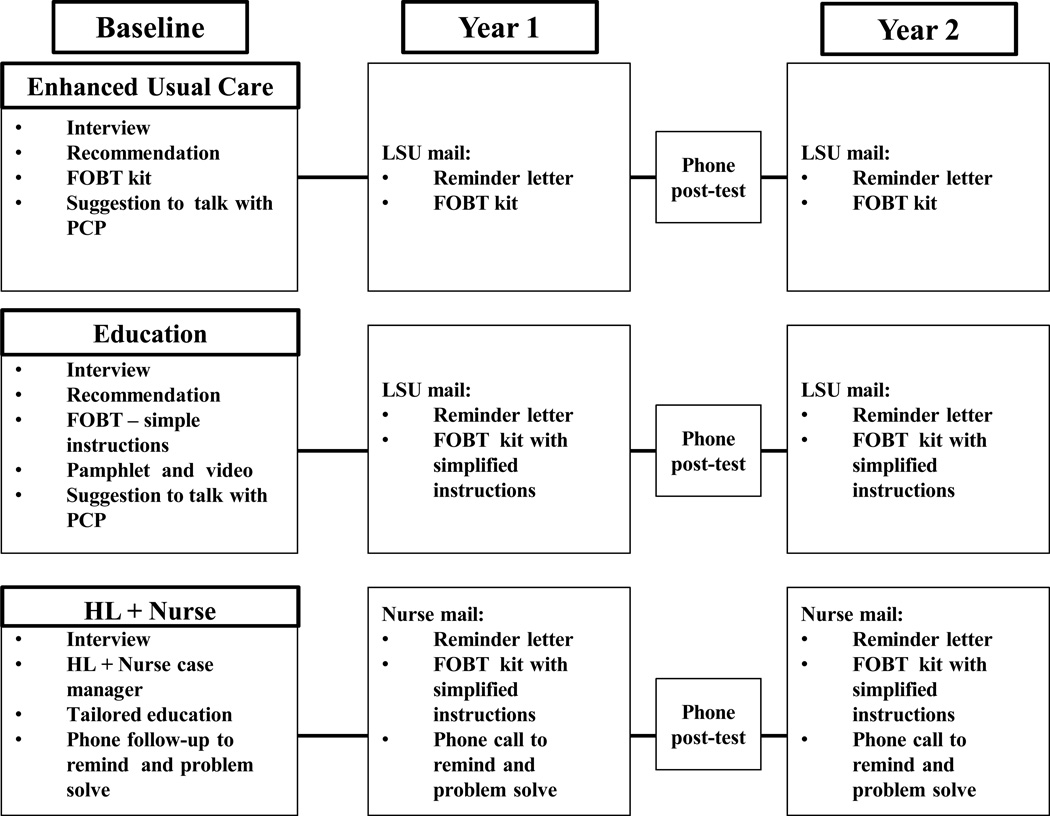
Year 1 and 2 interventions, both stepped intensive strategies, are illustrated in Fig 1. Ethically to ensure all patients received an FOBT annually, there was no pure usual care control arm either year. All year 2 interventions were designed to increase repeat FOBT completion without face to face contact with a provider. The clinic in-services and training of the clinic-based RAs in year 1 have been reported previously (31).
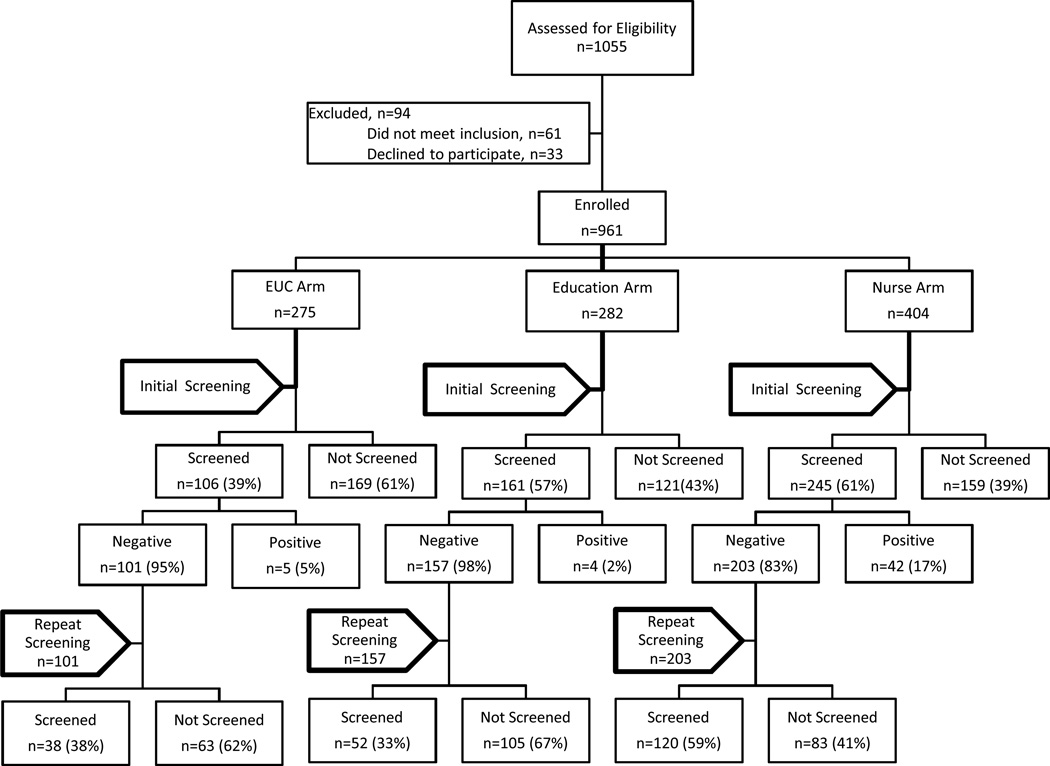
Figure 1.
Flowchart of Initial and Repeat Screening
Enhanced Care Arm (Using Mailing)
Twelve months after the initial FOBT was returned, the central RA located at the academic medical center mailed a letter from the participant’s clinic to remind those who had a negative initial test that it was time for their annual FOBT test and that a kit would be mailed the following week. The central RA then mailed the FOBT kit with a return stamped envelope addressed to the clinic. Patients returned the FOBT to the clinic by mail using a pre-addressed stamped envelope. Regular clinic protocol was followed for positive test results and if diagnostic testing was needed. In year 2 the central RA was hired and trained to mail patients the study materials.
Literacy-Informed Education Arm (Using Mailing)
Twelve months after initial FOBT was returned, a central RA mailed a reminder letter from the patient’s clinic and the following week mailed the FOBT kit with the same simplified FOBT instructions and pamphlet the patient had received at enrollment the previous year. Patients returned FOBT kits to the clinic by mail using a pre-addressed stamped envelope. Tracking and follow-up were done in the same manner as the enhanced care arm. This RA training was the same as in the Enhanced Care Arm.
Nurse Support Arm (Using Mailing and telephone follow-up support)
Twelve months after the initial FOBT was returned, the nurse mailed patients the same materials as those received the previous year. If patients did not return their FOBT results, the nurses followed up with a supportive phone call within two weeks and again in one month to identify and problem-solve barriers, as well as motivate them to complete the test. Patients returned FOBT results to the clinic by mail using a pre-addressed stamped envelope. The nurse recorded and tracked all results. If results were positive, the nurse manager called patients to discuss results, facilitate appointments with their primary care provider and if indicated, schedule patients for a diagnostic colonoscopy at the appropriate treatment center. The nurse manager training included health literacy communication and motivational interviewing techniques, use of a tracking system, and a protocol for mailing materials to patients, contacting them by phone and assisting them with navigation if a test was positive.
Outcomes
The primary outcome was completion of a repeat FOBT within 12–18 months of the initial negative FOBT. Repeat screening was documented by the clinic nurse (enhanced care and education arms) or study nurse (nurse case manager arm).
Statistical Analysis
Repeat FOBT completion rates were defined as the percentage of patients who returned a second FOBT to the clinic within 12–18 months after returning their initial FOBT. The denominator for all analyses is 512 patients completing the initial FOBT. To examine whether patients in study arms differed on baseline characteristics, analysis of variance was used for age and chi-square tests were used for categorical factors (age categories, gender, education, race, marital status and literacy level). Screening ratios were defined as the ratio of repeat FOBT completion rates between two arms. Both screening ratios and pairwise tests for FOBT completion were calculated using generalized estimating equations which accounted for clustering by clinic. Multivariate analyses adjusted for age, race, gender, and literacy level.
Cost Effectiveness Analysis
Incremental costs for the repeat FOBT for the nurse case manager arm over the enhanced care arm were 40% of two nurses ($106,280) and cost of pamphlets ($600). Costs and number screened were normalized to the enhanced care arm to account for differences in sample size. The incremental cost effectiveness measured as incremental costs per additional person screened, was calculated as the total difference in cost of the nurse arm and enhanced care arm divided by the total number of additional persons screened in the nurse arm. We have used this approach to determine costs and cost effectiveness previously for CRC screening promotion studies (31, 47).
RESULTS
Baseline characteristics of the sample of participants who completed initial FOBT screening are compared among arms in Table 1. Participants ranged in age from 50 to 85 years. 75% were female and 31% lacked a high school diploma. The majority (63%) were African American, over half (51%) had limited literacy (i.e. read < 9th grade level). There were significant differences across arms for race/ethnicity, marital status and literacy.
Table 1.
Characteristics of Study Sample at Baseline, Stratified by Study Arm
| All Patients (n=461) |
Study Arm |
p-value | |||
|---|---|---|---|---|---|
| Characteristic | Enhanced care (n=101) |
Education (n=157) |
Nurse (n=203) |
||
| Age, Mean (sd) | 58.4 (7.2) | 57.7 (7.6) | 58.3 (6.6) | 58.8 (7.4) | 0.50 |
| N (%) | N (%) | N (%) | N (%) | ||
| Age Categories | |||||
| 50–59 | 290 (63) | 72 (71) | 92 (59) | 126 (62) | 0.20 |
| 60–69 | 132 (29) | 20 (20) | 53 (34) | 59 (29) | |
| 70–85 | 39 (8) | 9 (9) | 12 (8) | 18 (9) | |
| Female | 347 (75) | 83 (82) | 114 (73) | 150 (74) | 0.18 |
| Years of Education | |||||
| Less than high school | 144 (31) | 34 (34) | 49 (31) | 61 (30) | 0.88 |
| High school grad | 211 (46) | 43 (43) | 76 (48) | 92 (45) | |
| Some College | 77 (17) | 16 (16) | 25 (16) | 36 (18) | |
| ≥ College Graduate | 29 (6) | 8 (8) | 7 (4) | 14 (7) | |
| Race | |||||
| African-American | 290 (63) | 68 (67) | 62 (39) | 160 (79) | <0.0001 |
| Caucasian/Hispanic | 171 (37) | 33 (33) | 95 (61) | 43 (21) | |
| Marital Status | |||||
| Single | 127 (28) | 21 (21) | 24 (15) | 82 (40) | <0.0001 |
| Married | 179 (39) | 44 (44) | 88 (56) | 47 (23) | |
| Separated | 26 (6) | 6 (6) | 6 (4) | 14 (7) | |
| Divorced | 76 (16) | 18 (18) | 21 (13) | 37 (18) | |
| Widowed | 53 (12) | 12 (12) | 18 (11) | 23 (11) | |
| Literacy Level | |||||
| Limited (0–60) | 235 (51) | 68 (67) | 51 (32) | 116 (57) | <0.0001 |
| Adequate (61–66) | 226 (49) | 33 (33) | 106 (68) | 87 (43) | |
In year 1, 53% of all eligible patients enrolled in the study completed FOBT screening. Of the 461 patients who completed an initial negative FOBT, 46% completed repeat FOBTs (see Figure 1). Repeat FOBT screening rates for the 461 patients with an initial negative test were 37.6% in the mailed enhanced care arm, 33.1% in the mailed education arm and 59.1% in the nurse support arm (p=0.01). (Table 2) When adjusting for age, race, gender and literacy, patients receiving nurse support were 1.46 times more likely to complete annual repeat FOBT (95% CI 1.14–1.86, p=0.002) compared to those receiving education. They were 1.45 times more likely to complete screening than those in enhanced care but this was not significant (95% CI 0.93–2.26 p=0.10). When screening ratios among arms were investigated by literacy level, the nurse arm was significantly different from the education arm both in the limited (p=0.0003) and adequate literacy groups (p=0.0001) (Table 3). In comparison to the enhanced care arm the incremental annual program cost per additional person screened for the nurse arm was $2,450 (Table 4). Of the 83 people in the nurse arm who did not return their FOBT, 46 (55%) were reached after one call. For the remaining 37 people, no contact was made after three calls.
Table 2.
Return rates of initial and repeat FOBT
| Study Arm |
p-value | ||||
|---|---|---|---|---|---|
| All Patients (n=461) |
Enhanced care (n=101) |
Education (n=157) |
Nurse (n=203) |
||
| N (%) | N (%) | N (%) | N (%) | ||
| Return of BOTH initial + repeat FOBT | 210 (46) | 38 (37.6) | 52 (33.1) | 120 (59.1) | 0.010 |
| Return of initial FOBT Repeat FOBT not returned Year 2 | 251 (54) | 63 (62.4) | 105 (66.9) | 83 (40.9) | |
| Screening Ratio | 1.00 | 0.99 | 1.45 | ||
| 95% Confidence Interval | (0.68– 1.46) | (0.93 – 2.26) | |||
| p-value | 0.98 | 0.10 | |||
| Screening Ratio | 1.00 | 1.46 | |||
| 95% Confidence Interval | (1.14 – 1.86) | ||||
| p-value | 0.002 | ||||
Screening ratios and p-values control for age (in years), race (African American vs Caucasian and Hispanic), and gender.
Table 3.
Return rates of initial and repeat FOBT by Literacy level
| Limited Literacy | |||||
|---|---|---|---|---|---|
| Study Arm |
p-value | ||||
| All Patients (n=235) |
Enhanced care (n=68) |
Education (n=51) |
Nurse (n=116) |
||
| N (%) | N (%) | N (%) | N (%) | ||
| Return of BOTH initial + repeat FOBT | 108 (46) | 23 (33.8) | 13 (25.5) | 72 (62.1) | 0.0013 |
| Return of initial FOBT Repeat FOBT not returned Year 2 | 127 (54) | 45 (66.2) | 38 (74.5) | 44 (37.9) | |
| Screening Ratio 95% Confidence Interval p-value |
1.00 | 0.83 (0.39– 1.76) 0.63 |
1.72 (0.77 – 3.86) 0.19 |
||
| Screening Ratio 95% Confidence Interval p-value |
1.00 | 2.07 (1.40 – 3.05) 0.0003 |
|||
| Adequate Literacy | |||||
|---|---|---|---|---|---|
| Study Arm |
p-value | ||||
| All Patients (n=226) |
Enhanced care (n=33) |
Education (n=106) |
Nurse (n=87) |
||
| N (%) | N (%) | N (%) | N (%) | ||
| Return of BOTH initial + repeat FOBT | 102 (45) | 15 (45.5) | 39 (36.8) | 48 (55.2) | 0.0003 |
| Return of initial FOBT Repeat FOBT not returned Year 2 | 124 (55) | 18 (54.5) | 67 (63.2) | 39 (44.8) | |
| Screening Ratio 95% Confidence Interval p-value |
1.00 | 0.89 (0.63– 1.26) 0.51 |
1.24 (0.90 – 1.70) 0.19 |
||
| Screening Ratio 95% Confidence Interval p-value |
1.00 | 1.39 (1.18 – 1.64) <0.0001 |
|||
Screening ratios and p-values control for age (in years), race (African American vs Caucasian and Hispanic), gender, and literacy (2 categories).
Table 4.
Cost-effectiveness analysis
| A | Sample size in EUC arm | 101 |
| B | Number screened in EUC arm | 38 |
| C | Sample size in NCM arm | 203 |
| D | Number screened in NCM arm | 120 |
| E | Number screened in NCM arm normalized to size of EUC arm (row D × row A)/row C | 59.7 |
| F | Additional number screened in NCM arm normalized to size of EUC arm = row E - row B | 21.7 |
| Incremental costs of NCM arm | ||
| G | Mailed brochures | $600 |
| H | Personnel | $106,280 |
| I | Non-personnel | $0 |
| J | Total Incremental Costs of NCM arm | $106,880 |
| K | Total NCM incremental costs normalized to size of EUC arm (row J × row A)/row C | $53,177 |
| Incremental cost-effectiveness ratio = row K/row F | $2,450 |
DISCUSSION
To the authors’ knowledge, this study is the first evaluation of the comparative effectiveness and costs associated with health literacy directed interventions designed to improve repeat annual FOBT screening among predominately rural community clinic patients. The repeat interventions were designed without the need for a clinic visit. Slightly over half (53%) of study participants underwent an initial FOBT test within three months of enrollment. Among those with a negative test, 46% completed repeat screening the following year. Repeat screening rates were highest among patients who received the educational intervention with telephone support and follow-up by the nurse manager, and were significantly higher than rates in the education alone arm. Due to a smaller sample size in the enhanced care arm, similar differences between the nurse arm and the enhanced care arm only trended toward significance.
The educational intervention followed health literacy best practices for imparting understandable and actionable information to promote CRC screening, yet in the second year, the illustrated, literacy and culturally appropriate pamphlet and simplified FOBT instructions mailed with a reminder and FOBT kit did not improve repeat screening over enhanced care (mailed reminder, kit and standard instructions) overall or for participants in the low literacy group. This is important, as the strategy closely resembled several other interventions mindful of cost and sustainability that were effective at improving initial screening rates (8, 11, 23–29). Several community clinic-based studies have mailed FOBTs to patients directly. Gupta (8) found mailed FOBTs with instructions and stamped return envelope and automated telephone reminder call coupled with “live” phone reminders for patients who had not completed screening within three weeks resulted in significantly higher FOBT completion (41%) than usual care clinic visit-based offers to complete screening (12%). Walsh found self -reported FOBT screening rates of ethnic minority patients improved from 8% in usual care to 15% with mailed FOBT and brochure to 24 %with additional telephone counseling by a community health advisor (48). In a stepped up intervention, Coronado found FOBT rates improved from 2% for patients in usual care to 26% for those receiving a letter, mailed FOBT kit with literacy and culturally appropriate pamphlet with instructions to 31% among those who additionally received telephone reminders and a home visit from the clinic medical assistant (49). However these studies focused on initial screening and not repeat screening.
Few studies have focused on increasing CRC screening completion in community clinics over multiple years. A recently initiated randomized clinical trial by Baker (33) is evaluating the comparative effectiveness of a multifaceted intervention to improve repeat FOBT screening among Latino patients in a FQHC network in a single urban location. Eligible patients will be randomized to a high standard usual care (team-based care for promoting CRC, point-of-care alerts for clinicians, and performance measurement and feedback to providers) versus augmented care (mailed FOBT kits, low literacy materials, automated phone and text message reminder, in-person follow-up calls from a CRC Screening Coordinator and communication of results to patients along with a reminder card highlighting when the patient is next due for screening). The results of this study have yet to be reported. Our finding that repeat FOBT screening rates were highest among community clinic patients who received on-going telephone support by their nurse manager are similar to those recently reported with insured patients. These studies found greatest improvement in regular CRC screening adherence (by any recommended means) among patients who received a mailed reminder, pamphlet, and FOBT kit with pictorial instructions coupled with telephone outreach by a nurse providing motivational counseling (30,50). In light of all of these findings, one conclusion might be that more intensive resources, such as dedicated time by a nurse or care coordinator will likely be needed for a CRC screening promotion program to be successful over time. Our findings further indicate that personal supportive follow-up, though costly, may be particularly important for community clinic patients. Pending the findings of the Baker study, leveraging available electronic health records and clinician decision support, as well as consumer mobile technologies (i.e. SMS text reminders) to automate many of the tracking and follow-up functions may also be an effective means to impart interventions at low cost.
There also may be lessons to be learned from public health CRC screening projects abroad where FOBT kits are mailed from a centralized health service to eligible individuals. These programs have been successful at initiating and sustaining FOBT screening, however, the onset of these initiatives are still relatively recent (15, 34, 51–52) and the mechanisms explaining successful repeat screening strategies are not entirely clear. Of note in these population-based programs, which in essence reduce geographic and cost barriers (in many countries there is no cost to patients), inequities were still found among those from rural areas and those of lower socioeconomic status (50). This may indicate a need for a more personal follow-up to sustain screening among these groups.
While the cost for implementing the nurse arm is not affordable for community health centers that operate with limited financial resources, it is possible that modifications of the intervention could be clinically effective and also affordable. If a less expensive clinic staff such as a medical assistant was used, estimates could fall to $495 per additional patient completing repeat screening; an 80% reduction in cost. This may be a reasonable approach since the main reason patients gave the nurses during the phone calls for not returning their FOBTs were not fear of finding cancer, but that they had lost the kit or they forgot to complete the test or mail it to the clinic. These FOBT barriers are supported by other studies in safety net settings which found that patients reported similar barriers as the primary reason patients failed to complete an FOBT (53). However this approach still might not be affordable among these clinics.
Since FQHCs have recently added CRC screening as a quality indicator (21) there may be greater incentives to take on an organized regional approach to CRC screening (4, 15) by structuring a collaborative outreach program using a designated medical assistant to mail FOBT kits and provide follow-up reminder calls for patients from multiple clinics.
Limitation
This study has several limitations. Differences were noted between arms in sociodemographic characteristics and adjustments were therefore made in the statistical analyses. Other limitations relate to the generalizability of the results, which included predominantly African American and female patients receiving care from FQHCs in one state. However, this is representative of FQHC populations in the southern region of the United States. Half of the sample had low literacy, which is more common in older, lower-income populations. No information was gathered on patients who refused to participate at enrollment, although this was a very small proportion of approached patients (3%). No process measures were collected to evaluate whether the nurses delivered motivational interviewing during the phone calls, however information was collected on the number of patients reached and the number of calls made to each patient. Another limitation was that there was no true usual care arm. Due to ethical concerns and to ensure that every patient in the study had access to a screening kit, the central RA mailed out the FOBT kits to every patient who qualified in the enhanced care arm. Also, it is possible that there may be unmeasured patient factors such as social support, number of clinic visits, and changes in comorbidity that could be attributed to repeat screening completion. Further studies might explore such barriers. Our cost-effectiveness analysis is based on evaluation of the incremental effectiveness and costs of FOBT testing via different interventions. Finally, the study design was quasi-experimental. However, Glasgow et al. (54) indicated that quasi-experimental approaches (e.g. modeling, convenience sampling, limited number of settings) rather than randomized clinical trials are used commonly in comparative effectiveness research for pragmatic reasons.
Conclusions
For FOBT screening to be an effective measure for CRC detection it must be an annual event – and hence strategies for improving repeat screening must be developed, particularly in resource challenged settings. This study’s findings illustrate a need to provide more than a mailed reminder, FOBT kit, and simplified instructions to sustain FOBT screening rates annually in community clinics. In fact written material alone – no matter how literacy appropriate, may not be effective with patients with low literacy. Helping disadvantaged populations will likely require more personal outreach and ongoing support, although these approaches will be difficult to support in economically challenged environments. Telephone follow-up and support by a dedicated clinic nurse is more effective, however it is too costly to implement in resource limited settings. Future research should explore leveraging less expensive clinic staff, distributing the workload over multiple clinics, or possibly using electronic health record technology or automated phone calls to offset some of the costs that may likely be necessary to maintain the success of a screening program that requires annual follow-up.
Figure 2.
Health Literacy Intervention Flow Chart
ACKNOWLEDGEMENTS
Funded by the National Cancer Institute (R01CA115869) T. Davis
We would like to thank Ivory Davis, MSN and Cara Pugh, BSN, for their commitment to helping patients completed screening.
Footnotes
No conflicts are noted by the authors related to the work described.
References
- 1.American Cancer Society. Cancer Facts & Figures 2011. 2012. [Google Scholar]
- 2.Coughlin S, Thompson T. Colorectal cancer screening practices among men and women in rural and nonrural areas of the United States, 1999. J Rural Health. 2004;20(2):118–124. doi: 10.1111/j.1748-0361.2004.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention: Vital signs: Colorectal cancer screening, incidence, and mortality--United States, 2002–2010. MMWR. 2011;60(884) [PubMed] [Google Scholar]
- 4.Taplin SH, Haggstrom D, Jacobs T, Determan A, Granger J, Montalvo W, et al. Implementing colorectal cancer screening in community health centers: addressing cancer health disparities through a regional cancer collaborative. Med Care. 2008;46(9):S74. doi: 10.1097/MLR.0b013e31817fdf68. [DOI] [PubMed] [Google Scholar]
- 5.United States Dept of Health: Healthy People 2020: US Dept. of Health and Human Services. 2010. [Google Scholar]
- 6.Bandi P, Cokkinides V, Smith R, Jemal A. Trends in Colorectal Cancer Screening With Home-Based Fecal Occult Blood Tests in Adults Ages 50 to 64 Years, 2000–2008. Cancer. 2012;118(20):5092–5099. doi: 10.1002/cncr.27529. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. [Accessed December 15, 2011];CDC Health Disparities and Inequalities Report — United States, 2011 MMWR. 2011 http://www.cdc.gov/mmwr/pdf/other/su6001.pdf. [PubMed]
- 8.Gupta S, Haim E, Rockey D, Hammons M, Koch M, Carter E, et al. Comparative Effectiveness of Fecal Immunochemical Test Outreach, Colonoscopy Outreach, and Usual Care for Boosting Colorectal Cancer Screening Among the Underserved: A Randomized Clinical trial. JAMA Intern Med. 2013 Aug 5; doi: 10.1001/jamainternmed.2013.9294. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inadomi J, Vijan S, Janz N, Fagerlin A, Thomas JP, Lin YV, et al. Adherence to Colorectal Cancer Screening: A Randomized Clinical Trial of Competing Strategies. Arch Intern Med. 2012;172(7):575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris R, Kinsinger L. Less is More: Not “Going the Distance” and Why. J Natl Cancer Inst. 2011;103(23):1726–1728. doi: 10.1093/jnci/djr446. [DOI] [PubMed] [Google Scholar]
- 11.Potter MB, Walsh JM, Yu TM, Gildengorin G, Green L, McPhee S. The effectiveness of the FLU-FOBT program in primary care a randomized trial. Am J Prev Med. 2011;41(1):9–16. doi: 10.1016/j.amepre.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Fisher J, Fikry C, Troxel A. Cutting Cost and Increasing Access to Colorectal Cancer Screening: Another Approach to Following the Guidelines. Cancer Epidemiol Biomarkers Prev. 2006;15(1):108–113. doi: 10.1158/1055-9965.EPI-05-0198. [DOI] [PubMed] [Google Scholar]
- 13.Heitman S, Hilsden R, Au F, Downden S, Manns B. Colorectal Cancer Screening for Average-Risk North Americans: An Economic Evaluation. PLOS Medicine. 2010;7(11):e1000370. doi: 10.1371/journal.pmed.1000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilschut JA, Habbema JDF, Van Leerdam ME, Hol L, Lansdorp-Vogelaar I, Kuipers EJ, et al. Fecal occult blood testing when colonoscopy capacity is limited. J Natl Cancer Inst. 2011;103(23):1741–1751. doi: 10.1093/jnci/djr385. [DOI] [PubMed] [Google Scholar]
- 15.Levin T, Jamieson L, Burley D, Reyes J, Oehrli M, Caldwell C. Organized Colorectal Cancer Screening in Integrated Health Care Systems. Epidemiol Rev. 2011;33:101–110. doi: 10.1093/epirev/mxr007. [DOI] [PubMed] [Google Scholar]
- 16.Niv Y, Lev-El M, Fraser G, Abuksis G, Tamir A. Protective effect of faecal occult blood test screening for colorectal cancer: worse prognosis for screening refusers. Gut. 2002;50:33–37. doi: 10.1136/gut.50.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo D, Cambon A, Wu D. Evaluating the long-term effect of FOBT in colorectal cancer screening. Cancer Epidemiology. 2012;36:e54–e60. doi: 10.1016/j.canep.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Wender R. Barriers to screening for colorectal cancer. Gastrointest Endosc Clin N Am. 2002;12(1):145–170. doi: 10.1016/s1052-5157(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 19.Fenton J, Elmore J, Buist D, Reid R, Tancredi D, Baldwin L. Longitudinal Adherence With Fecal Occult Blood Test Screening in Community Practice. Ann Fam Med. 2012;8(5):397–401. doi: 10.1370/afm.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Preventive Services Task Force: Screening for Colorectal Cancer. Ann Intern Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 21.Sarfaty M, Doroshenk M, Hotz J, Brooks D, Hayashi S, Davis TC, et al. Strategies for Expanding Colorectal Cancer Screening at Community Health Centers. CA Cancer J Clin. 2013 doi: 10.1002/caac.21191. In press. [DOI] [PubMed] [Google Scholar]
- 22.Levy B, Daly J, Xu Y, Ely J. Mailed Fecal Immunochemical Tests Plus Educational Materials to Improve Colon Cancer Screening Rates in Iowa Research Network (IRENE) Practices. Journal of the American Board of Family Medicine. 2012;25(1):73–82. doi: 10.3122/jabfm.2012.01.110055. [DOI] [PubMed] [Google Scholar]
- 23.Tu SP, Taylor V, Yasui Y, Chun A, Yip MP, Acorda E, et al. Promoting culturally appropriate colorectal cancer screening through a health educator: A randomized controlled trial. Cancer. 2006;107(5):959–966. doi: 10.1002/cncr.22091. [DOI] [PubMed] [Google Scholar]
- 24.Thompson N, Boyko E, Dominitz J, Belcher D, Chesebro B, Stephens L, et al. A Randomized Controlled Trial of a Clinic -Based Support Staff Intervention to Increase the Rate of Fecal Occult Blood Test Ordering. Prev Med. 2000;30:244–251. doi: 10.1006/pmed.1999.0624. [DOI] [PubMed] [Google Scholar]
- 25.Lane D, Messina C, Cavanagh M, Chen J. A Provider Intervention to Improve Colorectal Cancer Screening in County Health Cancers. Med Care. 2008;46:S109–S116. doi: 10.1097/MLR.0b013e31817d3fcf. [DOI] [PubMed] [Google Scholar]
- 26.Roetzheim R, Christman L, Jacobsen P, Schroeder J, Abdulla R, Hunter S. A randomized controlled trial to increase cancer screening among attendees of community health centers. Ann Fam Med. 2004;2(4):294. doi: 10.1370/afm.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sequist TD, Zaslavsky AM, Marshall R, Fletcher RH, Ayanian JZ. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2009;169(4):364–371. doi: 10.1001/archinternmed.2008.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Church TR. Offering patients colorectal cancer screening. J Natl Cancer Inst. 2005;97:328–329. doi: 10.1093/jnci/dji078. [DOI] [PubMed] [Google Scholar]
- 29.Dietrich AJ, Tobin JN, Cassells A, Robinson C, Greene M, Sox C, et al. Telephone care management to improve cancer screening among low-income women: a randomized, controlled trial. Ann Intern Med. 2006 Apr 18;144(8):563–571. doi: 10.7326/0003-4819-144-8-200604180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green B, Wang C, Anderson M. An Automated intervention With Stepped Increases in Support to Increase uptake of Colorectal Cancer Screening: A Randomized Trial. Ann Intern Med. 2013;158:301–311. doi: 10.7326/0003-4819-158-5-201303050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis T, Arnold C, Rademaker A, Bennett C, Bailey S, Platt D, et al. Improving Colorectal Cancer Screening in Community Clinics. Cancer. 2013 Aug 20; doi: 10.1002/cncr.28272. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Jk, Reis V, Liu S, Conn L, Groessl E, Ganiats T, et al. Improving fecal occult blood testing compliance using a mailed educational reminder. J Gen Intern Med. 2009 Nov;24(11):1192–1197. doi: 10.1007/s11606-009-1087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker D, Brown T, Buchanan D, Weil J, Cameron K, Ranalli L, et al. Design of a randomized controlled trial to assess the comparative effectiveness of a multifaceted intervention to improve adherence to colorectal cancer screening among patients cared for in a community health center. BMC Health Services Research. 2013;13:153. doi: 10.1186/1472-6963-13-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia M, Borras J, Binefa G, Mila N, Espinas J, Moreno V. Repeated screening for colorectal cancer with fecal occult blood test in Catalonia, Spain. European Journal of Cancer Prevention. 2012;21:42–45. doi: 10.1097/CEJ.0b013e32834a7e9b. [DOI] [PubMed] [Google Scholar]
- 35.Liss D, Petit-Homme A, Feinglass J, Buchanan D, Baker D. Adherence to Repeat Fecal Occult Blood Testing in an Urban Community Health Center Network. J Community Health. 2013 Apr 2; doi: 10.1007/s10900-013-9685-x. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Gellad ZF, Stechuchak KM, Fisher DA, Olsen MK, McDuffie JR, Ostbye T, et al. Longitudinal Adherence to Fecal Occult Blood Testing Impacts Colorectal Cancer Screening Quality. Am J Gastroenterol. 2011;106:1125–1134. doi: 10.1038/ajg.2011.11. [DOI] [PubMed] [Google Scholar]
- 37.Engelman K, Ellerbeck E, Ahluwalia J, Nazir N, Velasco A. Fecal Occult Blood Test Use by Kansas Medicare Beneficiaries. Prev Med. 2001;33:622–626. doi: 10.1006/pmed.2001.0936. [DOI] [PubMed] [Google Scholar]
- 38.Hecht J, Borrelli B, Breger R, Defrancesco C, Ernst D, Resnicow K. Motivational interviewing in community-based research: Experiences from the field. Ann Behav Med. 2005;9(2):29–34. doi: 10.1207/s15324796abm2902s_6. [DOI] [PubMed] [Google Scholar]
- 39.Davis T, Arnold CL, Rademaker A. FOBT Completion in FQHCs. Impact of Physician Recommendation, FOBT Information or Receipt of the FOBT Kit. J Rural Health. 2012;28:306–311. doi: 10.1111/j.1748-0361.2011.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis T, Long S, Jackson R, Mayeaux E, George R, Murphy P, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25(6):391. [PubMed] [Google Scholar]
- 41.Weiss B, Schwartzberg J, Davis T, Parker R, Sokol P, Williams M. Health literacy and patient safety: Help patients understand: manual for clinicians. AMA Foundation. 2007 [Google Scholar]
- 42.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion: National Action Plan to Improve Health Literacy. Washington, D.C.: U.S. Department of Health and Human Services; [Google Scholar]
- 43.Wolf MS, Wilson EA, Rapp DN, Waite KR, Bocchini MV, Davis TC, et al. Literacy and learning in health care. Pediatrics. 2009 Nov;124(Sup 3):S275–S281. doi: 10.1542/peds.2009-1162C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 45.Rosenstock I, Strecher V, Becker M. Social learning theory and the health belief model. Health Educ Q. 1988;15(2):175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 46.Janz NK, Champion VL, Strecher VJ. The health belief model. In: Glanz K, Lewis F, Rimer B, editors. Health Educ Behav. San Francisco: Jossey-Bass; 2008. pp. 31–44. [Google Scholar]
- 47.Wolf MS, Fitzner KA, Powell EF, McCaffrey KR, Pickard AS, McKoy JM, et al. Costs and Cost Effectiveness of a Health Care Provider-Directed Intervention to Promote Colorectal Cancer Screening Among Veterans. J Clin Oncol. 2005;23(34):8877. doi: 10.1200/JCO.2005.02.6278. [DOI] [PubMed] [Google Scholar]
- 48.Walsh JM, Salazar R, Nguyen TT, Kaplan C, Nguyen LK, Hwang J, et al. Healthy colon, healthy life: a novel colorectal cancer screening intervention. Am J Prev Med. 2010;39(1):1–14. doi: 10.1016/j.amepre.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coronado GD, Golovaty I, Longton G, Levy L, Jimenez R. Effectiveness of a clinic-based screening promotion program for underserved Hispanics. Cancer. 2011;117(8):1745–1754. doi: 10.1002/cncr.25730. [DOI] [PubMed] [Google Scholar]
- 50.Green B, Wang C, Horner K, Catz S, Meenan RT, Vernon SW, et al. Systems of Support to Increase Colorectal Cancer Screening and Follow-up Rates (SOS): design, challenges, and baseline characteristics of trial participants. Contemp Clin Trials. 2010 Nov;31(6):589–603. doi: 10.1016/j.cct.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janda M, Hughes K, Auster J, Leggett B, Newman B. Repeat participation in colorectal cancer screening utilizing fecal occult blood testing: A community-based project in a rural setting. Journal of Gastroenterology and Hepatology. 2010;25:1661–1667. doi: 10.1111/j.1440-1746.2010.06405.x. [DOI] [PubMed] [Google Scholar]
- 52.Hahm MI, Park EC, Choi KS, Lee HY, Park JH, Park S. Inequalities in adoption of cancer screening from a diffusion of innovation perspective: identification of late adopters. Cancer Epidemiol. 2011;35(1):90–96. doi: 10.1016/j.canep.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Quick BW, Hester CM, Young KL, Greiner KA. Self-Reported Barriers to Colorectal Cancer Screening in a Racially Diverse, Low-Income Study Population. J Community Health. 2013;38:285–292. doi: 10.1007/s10900-012-9612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glasgow RE, Doria-Rose VP, Khoury MJ, Elzarrad M, Brown ML, Stange KC. Comparative effectiveness research in cancer: what has been funded and what knowledge gaps remain? J Natl Cancer Inst. 2013 Jun 5;105(11):766–773. doi: 10.1093/jnci/djt066. PMID: 2357885. [DOI] [PMC free article] [PubMed] [Google Scholar]