Abstract
Both rat derived vascular smooth muscle cells (SMC) and human myofibroblasts contain α smooth muscle actin (SMA), but they utilize different mechanisms to contract populated collagen lattices (PCLs). The difference is in how the cells generate the force that contracts the lattices. Human dermal fibroblasts transform into myofibroblasts, expressing α-SMA within stress fibers, when cultured in lattices that remain attached to the surface of a tissue culture dish. When attached lattices are populated with rat derived vascular SMC, the cells retain their vascular SMC phenotype. Comparing the contraction of attached PCLs when they are released from the culture dish on day 4 shows that lattices populated with rat vascular SMC contract less than those populated with human myofibroblast. PCL contraction was evaluated in the presence of vanadate and genistein, which modify protein tyrosine phosphorylation, and ML-7 and Y-27632, which modify myosin ATPase activity. Genistein and ML-7 had no affect upon either myofibroblast or vascular SMC-PCL contraction, demonstrating that neither protein tyrosine kinase nor myosin light chain kinase was involved. Vanadate inhibited myofibroblast-PCL contraction, consistent with a role for protein tyrosine phosphatase activity with myofibroblast-generated forces. Y-27632 inhibited both SMC and myofibroblast PCL contraction, consistent with a central role of myosin light chain phosphatase.
Keywords: Collagen lattice, myofibroblasts, smooth muscle cells, lattice contraction
Introduction
The contraction of open wounds is due to forces generated within granulation tissue that pull the surrounding skin into the wound (Abercrombie et. al., 1956). There are 2 proposed mechanisms for generating the forces for wound contraction. The first is mechano-tension through contractile forces from a specialized cell within granulation tissue, the myofibroblast (Gabbiani et. al. 1972). The other mechanism is through fibroblast generated tractional forces, where the packing of fine collagen fibrils into longer, thicker collagen fiber bundles, compacts granulation tissue (Ehrlich & Rajaratnam, 1990). While both require Myosin ATPase activity, it is generated differently in each mechanism. Myofibroblasts employ “sustained” myosin ATPase activity (Parizi et al., 2000) and fibroblasts employ “rapid” myosin ATPase activity (Ehrlich et al., 1991).
Myosin ATPase produces the energy for the force that generates the sliding action of myosin-actin filaments within the cell’s cytoskeleton. Myosin ATPase is involved in numerous cell activities, including generating the forces for both cell contraction and cell locomotion. The phosphorylation of the regulatory peptide at serine 19 of myosin light chain (MLC), optimizes myosin ATPase activity (Adelstein, 1982). MLC devoid of phosphorylated serine 19 has minimal myosin ATPase activity. There are “sustained” and “rapid” myosin ATPase activities, which both require phosphorylated MLC at serine 19. Rapid myosin ATPase activity is dependent upon myosin light chain kinase (MLCK), which is a calcium-calmodulin dependent kinase that phosphorylates MLC at serine 19. The dephosphorylation of MLC by MLC phosphatase immediately follows the myosin relocation on the actin filament (Adelstein, 1982). A calmodulin inhibitor or a MLCK inhibitor blocks rapid myosin ATPase activity. The mechanism for sustained myosin ATPase is through the inhibition of MLC phosphatase, resulting in the retention of phosphate within MLC at serine 19 and the preservation of myosin ATPase activity. MLC phosphatase can be inhibited by Rho-Rho kinase phosphorylating a tyrosine residue in one of 4 subunits composing the MLC phosphatase enzyme complex (Kimura et. al., 1996). The myofibroblast utilizes the inhibition of MLC phosphatase activity for generating sustained myosin ATPase activity. Smooth muscle cells can utilize a pathway, involving CPI-17 for regulating MCL phosphatase activity (Niiro et al. 2003).
Contractile forces by blood vessel wall smooth muscle cell (SMC) are responsible for maintaining blood vessel tone, whereas contractile force generated by myofibroblasts, the icon of fibrotic tissues, is the proposed mechanism responsible for producing open wound contraction. Both smooth muscle cells and myofibroblasts are associated with collagen, during the generation of contractile forces. Studying these cell types in a 3-dimensional matrix better mimics the in vivo situation as compared to these cells in monolayer. Bell et al., (1979) introduced fibroblast populated collagen lattices (PCL) and studied their contraction. The Bell system uses a free floating fibroblast PCL, where cell-collagen interactions lead to the compaction of a 3 dimensional collagen matrix. The compaction of free floating collagen lattices is though rapid myosin ATPase activity (Ehrlich et al., 1991). With the free floating fibroblast PCL model, fibroblasts remain elongated and do not express α smooth muscle actin (SMA) in cytoplasmic stress fibers (Ehrlich & Rittenberg, 2000). Tomasek and coworkers introduced the attached-delayed-released (ADR) cell PCL contraction model, where lattices remain attached to their underlying surface for days before they are released (Tomasek et al., 1992). By the time of release the fibroblast population has transformed into myofibroblasts, expressing α-SMA within stress fibers. Myofibroblasts create tension via sustained myosin ATPase activity. When the attached lattice is released, there is a rapid contraction of the lattice associated with the contraction of the resident myofibroblasts (Parizi et al., 2000). Another difference between these lattice contraction models is the end result of collagen organization. The collagen fibrils in free floating PCL contraction are compacted into thicker fibrils as lattice contraction proceeds. In the ADR-PCL model minimal collagen reorganization occurs (Ehrlich, 1988). The mechanism for lattice contraction in the two models is different, where ADR-PCL contraction is mediated by cell contraction and sustained myosin ATPase activity; with free floating PCL contraction there is no fibroblast contraction, the collagen fibrils are reorganized and rapid myosin ATPase generates the tractional forces.
Integrins are critical for lattice contraction (Gullberg et al., 1990; Schiro et al., 1991; Orlandi et al., 2005). The α2β1 integrin is a major collagen receptor for type I collagen, as well as for other fibrillar collagens (Tulla et al., 2001). The data suggests that in human dermal fibroblasts the α2β1 integrin plays an important role in the contraction of collagen lattices (Langholz et. al., 1995), although the α11β1 integrin may also play a role as indicated in studies with cells from mice (Povova et. al., 2007). When the α2β1 integrin is not present, other integrins may be expressed, which can cause collagen lattice contraction (Cooke et. al., 2000). The capacity to contract free floating collagen lattices among SMC populations is related to the expression of α2β1 integrin (Orlandi et. al., 2005). Monoclonal antibody to α2 only partially blocked contraction, whereas antibodies to β1 completely inhibited contraction (Lee et. al., 1995) indicating another integrin also contributes to SMC contraction of collagen lattices.
Human myofibroblasts and rat derived smooth muscle cells generate contractile forces using sustained myosin ATPase activity. However, the generation of sustained myosin ATPase activity differs with the 2 cell types. The differences in generating sustained myosin ATPase activity with these cell types is shown by comparing the actions of 4 agents in the contraction of ADR PCLs. The agents are: ML-7, which blocks MLCK (Saitoh et al., 1987); Y-27632, a Rho-associated kinase (ROCK) inhibitor, which blocks the phosphorylation of a specific peptide subunit in the MLC phosphatase complex (Somlyo & Somlyo, 1998); genistein, a tyrosine kinases inhibitor, which prevents the phosphorylation of protein tyrosine residues that play a role in cell signaling (Akiyama & Ogawara, 1991); and vanadate, which inhibits protein tyrosine phosphatase, preventing the turnover of protein tyrosine phosphate groups (Swarup et al., 1982). It is established that the state of protein tyrosine phosphorylation affects smooth muscle contraction (Todorov et al., 2005; Srivastava & St-Louis, 1997), the formation of actin stress fibers and the size of focal adhesions (Maher et al., 1985).
Methods
Materials
Dulbecco’s modification of Eagle’s medium (DMEM) purchased from Life Technologies (Rockville, MD), fetal bovine serum (FBS) from HyClon (Logan, UT), Y-27632 from EMD Bioscience (San Diego, CA), ML-7, vanadate, genistein, monoclonal anti-α Smooth Muscle Actin (α-SMA) all from Sigma Chemical Co. (St. Louis, MO), donkey anti mouse antibody from Jackson Immuno Research Laboratories (West Grove, PA), and Alexa 488 phalloidin from Invitrogen (Carlsbad, CA).
The two cell lines studied were primary human derived dermal fibroblasts from neonatal foreskin and rat derived SMC from the intimal thickening of the thoracic aorta 15 days after ballooning, which were a gift form Dr. Augusto Orlandi (Orlandi et al 2005). Both cell types were maintained in DMEM with10% FBS and 15 μg/ml of gentamicin, which is referred to as complete DMEM.
Casting Populated Collagen Lattices
Each milliliter of fibroblast and SMC-PCL contained 50,000 cells, 1.25 mg of acid soluble rat tail tendon collagen in 1 mM HCl and complete DMEM. In a 100 mm tissue culture dish (Falcon BD Labware, Franklin Lakes, NJ) 0.2 ml drops of cell-collagen-medium mixture were pipetted onto the surface of the dish and allowed to polymerize before adding 8 ml of complete DMEM. Between 4 and 5 PCLs in each dish were maintained at 37°, with 5% CO2 in a water saturated atmosphere incubator for 3 days without a change of medium. Agents were added 24 hrs before their release on day 4. Cell PCLs were photographed 10–15 minutes and 5 hours after their release at a fixed distance, with a ruler in place and their areas determined by the imaging software ImageJ program (Rasband, 1997–2007). To compare fibroblasts to myofibroblasts capacity to contract ADR PCL, attached fibroblast PCLs were released at 24 hrs, a time point, when myofibroblasts are not present, and at 4 days, a time point, when most of the cell population had converted into myofibroblasts. Lattice contraction for treated lattices was compared to the contraction of control untreated lattice areas, using a two-tail, unequal variance t-test. Significance was P≤ 0.05.
Immuno-histology
Cell PCLs were fixed in buffered 4 % paraformaldehyde in cytoskeletal buffer (137mM NaCl; 5mM KCI; 4mM NaHCO2; 2mM MgCl2; 3.5 mM glucose; 2mM EGTA, 1.5 mM K2HPO4 and 5 mM PIPES pH 6.1) for 5 minutes then transferred to cytoskeletal buffer alone. Cells were permeablized by treating lattices with 0.1% Triton X-100 in cytoskeletal buffer for 1 minute. The lattices were incubated with monoclonal antibody directed to α-SMA, washed 4 times before a second incubation with rhodamine tagged donkey-anti mouse IgG, Alexa phalloidin to identify filamentous actin and DAPI to identify nuclei. The lattices were viewed with a Zeiss Axiovert 135 Microscope with florescence and a 40 × objective water lens. Digital photographs were taken with a Photometrics CoolSnapFX digital camera.
Results
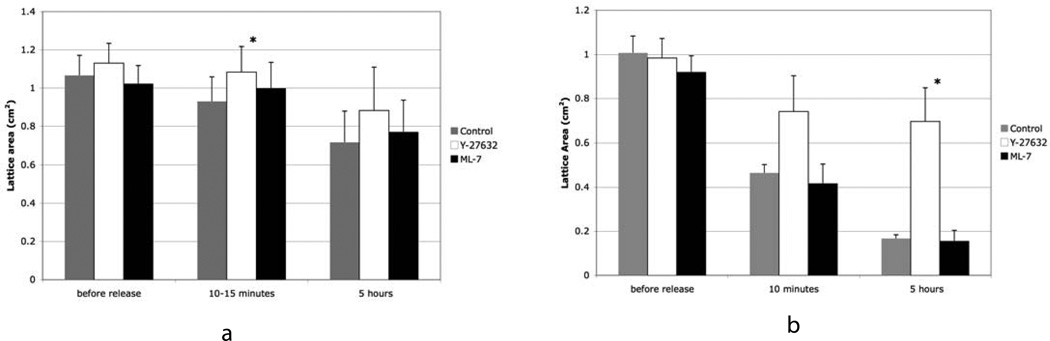
At both day 1 and 4 attached SMC PCLs exclusively contained SMCs. In contrast, at day 1 attached fibroblast PCLs contained over 95% fibroblasts, which did not express α-SMA staining stress fibers and less than 5% myofibroblasts, cells that do expressed α-SMA. At 4 days attached fibroblast PCLs contained 95% myofibroblast (based on counting 117 cells in 20 different images). The release of initially fibroblast PCL at 1 day or 4 days with a spatula initiated lattice contraction, which was rapid, during the first 10 minutes and proceeded at a slower rate over the next 5 hours. Fibroblast PCL released at 1 day showed minimal lattice contraction compared to 4 days (Table I). Differences at 4 days with lattice contraction were noted between vascular SMC PCLs and myofibroblast PCLs. Myofibroblasts were more effective at lattice contraction compared to vascular SMCs (Table I, figures 1 and 2). At 5 hours, the SMC PCLs had contracted by 35% and myofibroblast PCLs had contracted to about 85%. The rate and degree of either myofibroblast or SMC PCL contraction was not affected by the inclusion of 500 nM ML-7, a MLCK inhibitor, 24 hours before release (figure 1). However, at 4 days the rate and degree of myofibroblast PCL contraction was strongly inhibited by 500 nM Y-27632 a ROCK inhibitor (figure 1b), while SMC PCL contraction was strongly inhibited at 10–15 minutes and less strongly inhibited at 5 hours (figure 1a). At 10 to 15 minute after release, Y-27632 treated SMC PCLs had contracted to about 5% (controls 15%) and myofibroblast PCLs had contracted to 25% (controls 55%). At 5 hours, Y-27632 treated smooth muscle cell PCLs had contracted 20% (controls 35%) and the myofibroblast PCLs had contracted by 30% (controls 85%). To test that this was not a result of toxicity we grew myofibroblasts and SMCs with 25 μM and at 250 μM Y-27632 for 2 days. The higher concentration was somewhat toxic for the cells, where some cells rounded up. At the lower concentration there was no evidence of altered cell behavior and no rounded up cells were seen.
Table I.
Attached-Released Collagen Lattice contraction at 1 and 4 Days
| Day 1 time 0 | Day 1 10 min | Day1 | Day 4 time 0 | Day 4 10 min | Day 4 | |
|---|---|---|---|---|---|---|
| Fibroblasts | 194 ±8 mm2 | 173 ±7 | *11% | 133±4 | 65 ± 4 | **52% |
| SMC | 201±14 | 208±16 | 0% NS | 136±19 | 128± 6 | *6% |
p=0.01
p=.0001
Figure 1.
Bar graphs showing changes in area of ADR cell PCLs at 3 time points for lattices populated with SMC in a) and myofibroblasts in b). Data is presented for untreated lattices, lattices treated with 500 nM Y-27632, and lattices treated with 500 nM ML-7. Note that treatment with Y-27632 inhibits lattice contraction for both cell types. The data in a) is the average of two experiments with quadruplicate lattices and in b) the data is from one experiment and is the average of three and four lattices. The results from b) were duplicated in 2 other experiments. It needs to be noted that in a number of experiments, some PCLs freed themselves from the surface of the dish before day 4 and therefore could not be included in the study. The * indicates statistical significance of p<0.05 as compared to controls. The error bars indicate standard deviation.
Figure 2.
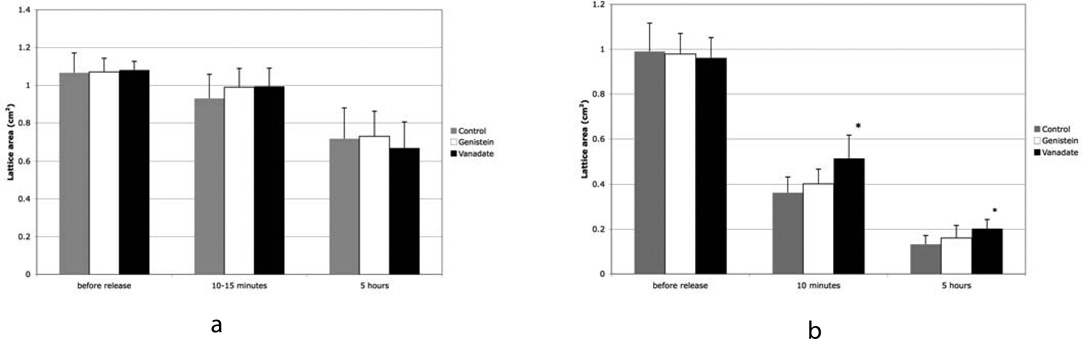
Bar graphs showing changes in area of ADR cell PCLs at 3 time points for lattices populated with SMC in a) and myofibroblasts in b). Data is shown for untreated lattices, lattices treated with 30 μM vanadate, and lattices treated with 10 μM genistein. Note that treatment with vanadate significantly inhibits lattice contraction in myofibroblast PCL. Data in a) was the average of 2 experiments with 4 lattices per experiment. Data in b) was the average of 4 experiments with 4 lattices per experiment. The * indicates statistical significance as compared to control where p<0.05. The error bars indicate standard deviation.
Vanadate and Genistein
Vanadate was added to attached SMC and myofibroblast PCLs at a concentration of 30 μM 24 hours before their release. When protein tyrosine phosphatases were inhibited by vanadate, myofibroblast PCL contraction was inhibited at both 10 minutes and 5 hours (figure 2b). Vanadate significantly inhibited myofibroblast PCL contraction (figure 2b), although the effect was not strong the results are consistent with previous experiments, where a more dramatic effect was seen with higher dosages of vanadate (Ehrlich et al., 2006). The differences between treated and untreated lattices were more pronounced at the 10 minutes period compared to the 5 hour period. SMC PCL contraction was not altered by added vanadate (figure 2a). Genistein at 10 μM inhibits protein tyrosine kinases, and was ineffective at inhibiting lattice contraction of either myofibroblast PCLs or SMC PCLs (figure 2). Comparing untreated myofibroblast PCLs to genistein treated lattices, showed no change in the rate or final size 5 hours after release. Inhibiting protein tyrosine kinases did not affect the contraction of released attached PCLs. Again to test that the effect was not a result of toxicity, myofibroblasts and SMCs were cultured with 30 μM and 100 μM vanadate for 2 days. At 100 μM vanadate the cells died and came off the dish. At 30μM vanadate the cells did not round up and remained attached and elongated.
Cell morphology
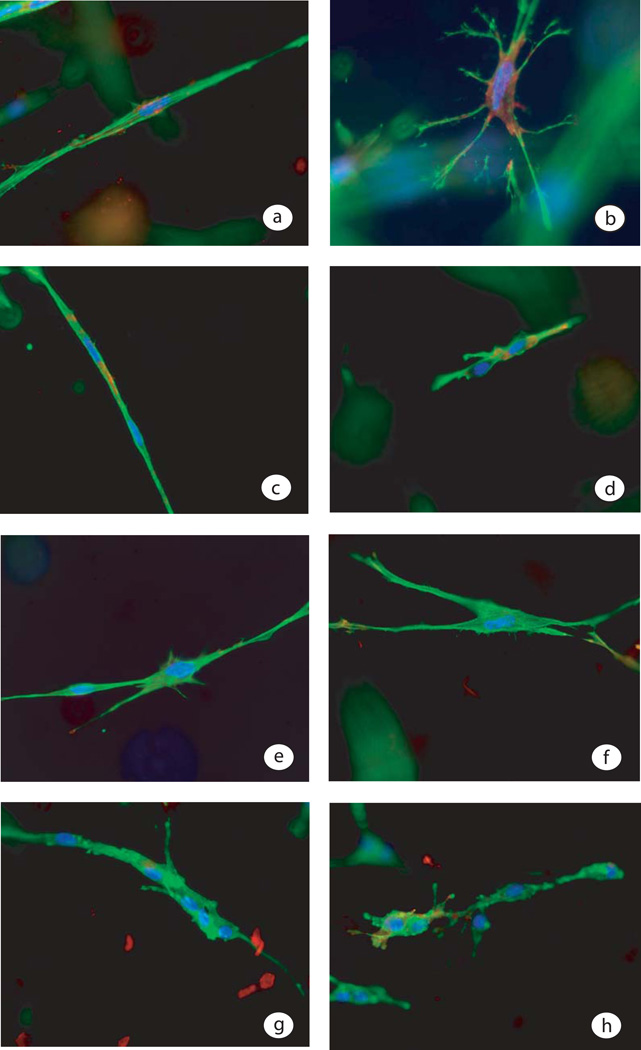
When fibroblasts were cast in a collagen lattice that remained attached to the surface of the tissue culture dish, at 4 days the cells had transformed into a myofibroblast phenotype, which was identified by the expression of α-SMA in stress fibers. A single myofibroblast shown in figure 3a had prominent cytoplasmic stress fibers running in the long axis of the cell and α-SMA in a perinuclear location. Ten minutes after the release of a 4 day myofibroblast PLC, contracted lattices were processed for fluorescent immuno-histology. A released myofibroblast PCL contained contracted myofibroblasts (figure 3b). The morphology of myofibroblasts was different from that of fibroblasts or SMC. The myofibroblasts had a stellate shape with several arms reaching out into the collagen matrix. Unlike the many attached arms found with myofibroblasts in 4 day released lattices, in the one day released lattices there were few fibroblast arms protruding out into the collagen matrix. In figure 3c a fibroblast in an attached collagen lattice at 1 day, showed less prominent actin rich stress fibers and minimal α-SMA staining. A pair of contracted fibroblasts, which is typical of released 1 day fibroblast PCLs, are presented in figure 3. Fibroblasts at 1 day had less capacity to contract PCLs as compared to myofibroblasts in 4 day PCLs. The cells in an untreated control myofibroblast PCL had thick α-SMA cytoplasmic stress fibers (figure 3a), but adding vanadate to a myofibroblast PCL on day 3, 24 hours before release, the stress fibers were thinner and less prominent on day 4 (figure 3e). When vanadate treated myofibroblast PCLs were released at 4 days the cells contracted less than controls and they had fewer arms associated with the collagen fibrils (figure 3f). The vanadate treated cells had thinner cytoplasmic microfilaments and were less effective at lattice contraction. Vascular smooth muscle cells incorporated in attached collagen lattices aggregated into multi-cellular complexes. When compared to SMC PCLs, fibroblasts in PCL were more independent (compare figure 3a and 3g). Vascular smooth muscle cells show some degree of contraction immediately after the lattice was released (see figure 3h), but did not generate much additional contraction beyond 10 minutes.
Figure 3.
Fluorescent staining of cells in PCL is presented, where α-SMA is stained red, phalloidin-stained microfilaments stained green and the nuclei stained blue. Panel a) is a myofibroblast in an attached PCL on day 4 just before release. Panel b) shows a contracted myofibroblasts 10 minutes after release of an attached 4 day myofibroblast PCL. Panel c) shows two attached fibroblast PCL 1 day after casting and panel d) shows two fibroblasts within a 1 day old fibroblast PCL 10 minutes after release. Panel 3e shows two cells in a 4 day old fibroblast PCL treated with vanadate for 24 hrs and panel 3f shows a fibroblast 10 minutes after release in a vanadate treated PCL. Panel 3g shows a vascular SMC in a PCL for 4 days and panel 3h shows vascular SMCs 10 min after release of an attached SMC-PCL.
Discussion
Differences in free floating PCLs containing vascular SMC derived from the intimal thickening of a balloon damaged rat aorta blood vessel has previously been reported (Orlandi et al., 2005). These differences are associated with cell morphology, where greater free floating SMC PCL contraction is seen with more elongated vascular SMC derived from young rat aorta (Orlandi et al., 2005). SMC derived from intimal thickenings on the rat aorta wall are more compact cells that produce less free floating lattice contraction (Orlandi et al., 2005). The compaction of free floating human fibroblast PCL requires the translocation of collagen fibrils and their reorganization into thicker collagen fiber bundles, which occurs in the absence of any observed cell contraction (Ehrlich and Rajaratnam, 1990). Intestinal derived human SMCs cast in free floating collagen lattices are equal to human dermis derived fibroblasts at contracting PCLs (Graham et al., 1984; Ehrlich et al., 1986). Like fibroblast PCL, free floating intestinal derived SMC PCL contraction proceeds in the absence of established cell contraction. When residing in a contracting free floating collagen matrix, both fibroblasts and intestinal SMCs retain an elongated morphology (Graham et al., 1984). The mechanism responsible for SMC contraction of free floating PCLs requires the reorganization of collagen fibrils, which is unrelated to cell contraction (Graham et al., 1987). Free floating vascular SMC PCL contraction starts within 4 to 6 hours after casting the lattice a time, when α-SMA expression is minimal (Orlandi et al., 2005). As a consequence, factors other than the presence of α-SMA in cytoskeletal stress fibers promote lattice contraction. Other factors include integrins that are critical for lattice contraction (Gullberg et al., 1990; Schiro et al., 1991; Orlandi et al., 2005). The α2β1 integrin is a major collagen receptor for type I collagen as well as for other fibrillar collagens (Tulla et al., 2001). The data suggests that with human dermal fibroblasts the α2β1 integrin plays an important role in the contraction of collagen lattices (Langholz et. al., 1995). The capacity to contract free floating PCLs among SMC populations is related to the expression of α2β1 integrin (Orlandi et al., 2005). The mechanism for the contraction of free floating fibroblast PCL changes when lattices are cast with a high density of fibroblasts. Instead of the reorganization of collagen fibrils generating lattice contraction, with high density populations of fibroblasts incorporated in collagen lattices, cell elongation of the spherical shaped cells initially incorporated into PCL, is the mechanism for lattice contraction (Ehrlich & Rittenberg, 2000). In the high density fibroblast PCL, lattice contraction is completed by 6 hrs, a time when cell elongation is completed. If this were the situation here, then lattice contraction would be expected to be completed in the initial 6 hours after casting not at 4 days.
Free floating human fibroblast and rat smooth muscle cell PCLs cast at a moderate cell density produce lattice contraction linking the reorganization of collagen fibrils with PCL compaction. Myofibroblasts, the cell phenotype in 4 day attached fibroblast PCL, show greater cell contraction as well as lattice contraction compared to attached fibroblast PCL released at 1 day. As compared to 1 day fibroblast PCLs, by 4 days fibroblasts have transformed into myofibroblasts and generate more attachments to their surrounding collagen matrix. When the 4 day myofibroblast PCLs are released, the contracting cells have more attachment sites within their surrounding collagen matrix, which results in more collagen fibrils displaced by each contracting myofibroblast. At 1 day fewer collagen fibrils are translocated, because cells have fewer attachments to collagen fibrils. PCLs with vascular SMCs show the least lattice contraction. The possible reason appears related to reduced cell elongation and more cell-cell contacts, generating less cell-collagen contacts.
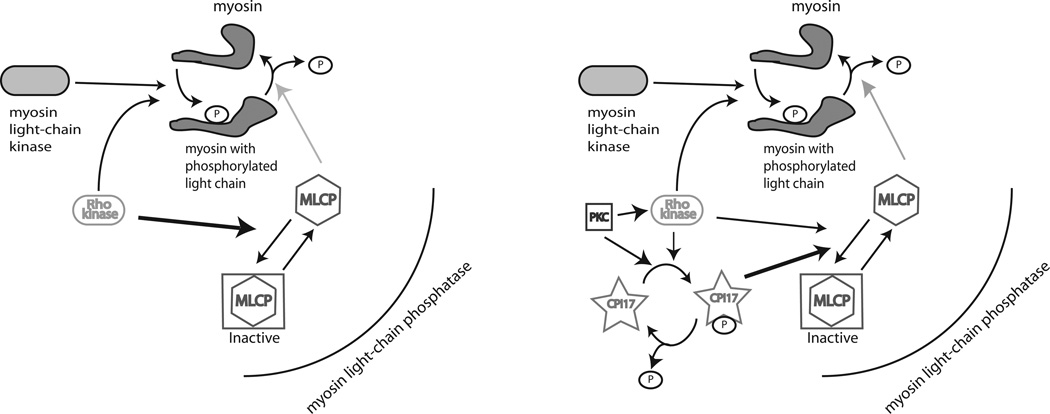
Another possible reason that vascular SMCs contract PCLs less effectively is a difference in the mechanism for generating myosin ATPase activity. In attached cell PCLs the developing cell tension results from sustained myosin ATPase activity, requiring MLC-serine-19 to be retained in a chronic phosphorylated state. Evidence supports the notion that the regulation of MLC phosphatase in SMCs is primarily mediated via CPI-17 (see figure 5), whereas in non-muscle cell lines like fibroblasts another pathway involving Rho-Rho kinase phosphorylation of MLC phosphatase, involving the myosin-binding subunit is more important (Niiro et al., 2003).
Y-27632 inhibits ROCK kinase, which phosphorylates a specific tyrosine residue in one of the 4 peptide subunits of MLC phosphatase and inactivates the enzyme (Totsukawa et al., 2000). In the absence of MLC phosphatase activity, MLC-serine-19 remains in a phosphorylated state, generating sustained myosin ATPase activity. Treating attached myofibroblast PCL with Y-27632 inhibits lattice contraction. It appears Y-27632 restores MLC phosphatase activity resulting in the loss of sustained myosin ATPase activity. In smooth muscle cells CPI-17, not found in fibroblasts, is a more important regulator of the MLC phosphatase activity. CPI-17 is phosphorylated by protein kinase C and Rho kinase at Thr-38, which causes the inhibition of MLC phosphatase (Niiro et al., 2003). Y-27632 inhibited contraction in the SMC by 33% initially and by 57% at 5 hours. This is consistent with the finding that Y-27632 inhibits phosphorylation of CPI-17 at Thr-38 and reduces forces generated by smooth muscle cells (Niiro et al., 2003).
Vanadate limits tyrosine phosphatase activity and has a more subtle effect at restoring MLC phosphatase activity in fibroblast derived PCLs. It is ineffective at blocking sustained myosin ATPase activity in vascular SMC compared to fibroblasts and myofibroblasts. Two possible explanations are that vanadate does not affect the CPI-17 pathway, or it reinforces the pathway. The Tyr42 domain in CPI-17 is necessary to protect the dephosphorylation of MLC phosphatase regulatory peptide at Thr-38 (Ito et al., 2004, Hayashi et al., 2001). Phosphorylation of CPI-17 at Thr-38 activates its inhibitory affect on MLC phosphatase. Vanadate may alter MLC phosphatase activity, utilizing the same pathway.
Genistein and ML-7 do not affect ADR lattice contraction populated with either myofibroblasts or SMCs. The MLCK inhibitor ML-7 has no affect upon ADR cell PCL contraction, because MLCK phosphorylation of MLC plays a minor role in sustained myosin ATPase activity (Totsukawa et al., 2000). ROCK inactivation of MLC phosphatase maintains MLC in a phosphorylated state preserving cell tension. Genistein, a protein tyrosine kinase inhibitor, failed to alter ADR lattice contraction, which suggests unphosphorylated tyrosine protein residues promotes sustained myosin ATPase activity. In contrast to ADR lattice contraction by sustained myosin ATPase, genistein inhibits rapid myosin ATPase activity, which is responsible for free floating fibroblast PCL contraction (Ehrlich et al., 2006). Vanadate has no inhibitory action on smooth muscle cell PCL contraction, but inhibits myofibroblast PCL contraction. Again it demonstrates differences in the sustained myosin ATPase activity between SMCs and myofibroblasts. Vanadate inhibits sustained myosin ATPase activity and the development of cell tension. In the absence of cell tension the transformation of fibroblasts into myofibroblasts is retarded. Vanadate treated SMC PCL contraction may be unaffected, because their dependence upon CPI-17 may preserve sustained myosin ATPase activity.
In conclusion, although both human myofibroblasts and rat SMCs can contract ADR collagen lattices by sustained myosin ATPase activity, the pathway generating the sustained myosin ATPase is different. That difference in generating myosin ATPase is evident by the differences in response to vanadate and Y-27632. In addition, SMC are not effective at contracting ADR lattices, perhaps due to what appears to be less interaction between cells and collagen fibrils.
Figure 4.
Part of the pathway for myosin regulation is shown for myofibroblasts (left) and smooth muscle cells (right). CPI-17 found in smooth muscle cells is the regulator for the deactivation of MLC phosphatase, resulting in sustained myosin ATPase. In contrast, Rho kinase regulates MLC phosphatase activity within myofibroblasts.
Table 2.
Summary of results for ADR collagen lattice contraction
| Released on Day 4 |
Genistein | Vanadate | ML-7 | Y-27632 |
|---|---|---|---|---|
| Myofibroblasts 10–15 minutes |
No effect 1.1 4E-4L |
Less contraction 1.5 4E-4L |
No effect 1.0 1E-4L |
No effect 1.7 1E-3L |
| Myofibroblasts 5 hours |
No effect 1.2 4E-4L |
Less contraction 1.6 4E-4L |
No effect 1.0 1E-4L |
Much less contraction 4.2 1E-3L |
| SMC 10 –15 minutes |
No effect 1.1 2E-4L |
No effect 1.1 2E-4L |
No effect 1.2 2E-4L |
Less contraction 1.2 2E-4L |
| SMC 5 hours | No effect 1.1 2E-4L |
No effect 0.94 2E-4L |
No effect 1.2 2E-4L |
No effect 1.2 2E-4L |
| No Treatment Release Day 1 | No Treatment Release Day 4 | |||
| Fibroblasts | contracted 11% | contracted 52% | ||
| SMC | contracted −0.03% NS | contracted 6% | ||
No effect or NS indicates not statistically significant. The single number in the top part of the table is the ratio of the average treated lattice area over the control. The 4E-4L indicates 4 experiments with 4 lattices in each experiment. The results given for 1 experiment are consistent with results when the experiment was repeated two more times.
Acknowledgements
Funded by:
This work was supported in part by NSF Grant No. DMS-0622971 and NIH grant GM056851.
References
- Abercrombie M, Flint MH, James DH. Wound contraction in relation to collagen formation in scorbutic guinea pigs. J. Embryol. Exp. Morph. 1956:4.167–4.175. [Google Scholar]
- Adelstein R. Calmodulin and the regualtion of the actin-myosin interaction in smooth muscle and nonmuscle cells. Cell. 1982;30(2):349–350. doi: 10.1016/0092-8674(82)90232-x. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Ogawara H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods of Enzymology. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- Bell E, Ivarsson B, Charlotte M. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proce. Natl. Acad. Sci. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke ME, Sakai T, Mosher DF. Contraction of collagen matrices mediated by alpha2beta1A and alpha(v)beta3 integrins. J Cell Sci. 2000;113:2375–2383. doi: 10.1242/jcs.113.13.2375. [DOI] [PubMed] [Google Scholar]
- Ehrlich HP, Griswold TR, Rajaratnam JB. Studies on vascular smooth muscle cells and dermal fibroblasts in collagen matrices. Effects of heparin. Exp. Cell Res. 1986;164:154–162. doi: 10.1016/0014-4827(86)90462-3. [DOI] [PubMed] [Google Scholar]
- Ehrlich HP, Rajaratnam JBM. Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue and Cell. 1990;22(4):407–417. doi: 10.1016/0040-8166(90)90070-p. [DOI] [PubMed] [Google Scholar]
- Ehrlich HP, Rockwell WB, Cornwell TL, Rajaratnam JBM. Demonstration of a direct role for myosin light chain kinase in fibroblast-populated collagen lattice contraction. Journal of Cellular Physiology. 1991;146:1–7. doi: 10.1002/jcp.1041460102. [DOI] [PubMed] [Google Scholar]
- Ehrlich HP, Rittenberg T. Differences In The Mechanism For High Versus Moderate Density Fibroblast Populated Collagen Lattice Contraction. J Cell Physiol. 2000;185:432–439. doi: 10.1002/1097-4652(200012)185:3<432::AID-JCP14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Ehrlich HP, Sun B, Kainth KS, Kromah F. Elucidating the mechanism of wound contraction: Rapid versus sustained myosin ATPase activity in attached-delayed-released compared with free floating fibroblast-populated collagen lattices. Wound Repair Regen. 2006;14:625–632. doi: 10.1111/j.1743-6109.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR, Majno G. Granulation tissue as a contractile organ. a study of structure and function. J. Exp. Med. 1972;135:719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MF, Diegelmann RF, Eslon C, Bitar KN, Ehrlich HP. Isolation and culture of human intestinal smooth muscle cells. Proc. Soc. Exp. Biol. Med. 1984;174(4):503–507. doi: 10.3181/00379727-176-4-rc1. [DOI] [PubMed] [Google Scholar]
- Graham MF, Drucker DE, Perr HA, Diegelmann RF, Ehrlich HP. Heparin modulates human intestinal smooth muscle cell proliferation, protein synthesis, and lattice contraction. Gastroenterology. 1987;93:801–809. doi: 10.1016/0016-5085(87)90443-4. [DOI] [PubMed] [Google Scholar]
- Gullberg D, Tingström A, Thuresson AC, Olsson L, Terracio L, Borg TK, Rubin K. Beta 1 integrin-mediated collagen gel contraction is stimulated by PDGF. Exp. Cell Res. 1990;186:264–272. doi: 10.1016/0014-4827(90)90305-t. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Senba S, Yazawa M, Brautigan D, Eto M. Defining the Structural Determinants and a Potential Mechanism for Inhibition of Myosin Phosphatase by the Protein Kinase C-potentiated Inhibitor Protein of 17 kDa. J. Biol. Chem. 2001;276(43):39855–39863. doi: 10.1074/jbc.M107302200. [DOI] [PubMed] [Google Scholar]
- Ito M, Nakano T, Erdodi F, Hartshorne DJ. Myosin phosphatase: Structure, regulation and function. Mole Cell Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Langholz O, Rockel D, Mauch C, Kozlowska E, Bank I, Krieg T, Eckes B. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by alpha1beta1 and alpha2beta1 integrins. J Cell Biol. 1995;131:1903–1915. doi: 10.1083/jcb.131.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RT, Berditchevski F, Chneg GC, Hemler ME. Integrin-Mediated Collagen Matrix Reorganization by Cultured Human Vascular Smooth Muscle Cells. Circulation Research. 1995;76:209–214. doi: 10.1161/01.res.76.2.209. [DOI] [PubMed] [Google Scholar]
- Maher PA, Pasquale EB, Wang JYJ, Singer SJ. Phosphotyrosine-containing proteins are concentrated in focal adhesions and intercellular junctions in normal cells. PNAS. 1985;82:6576–6580. doi: 10.1073/pnas.82.19.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niiro N, Koga Y, Ikebe M. Agonist-induced changes in the phosphorylation of the myosin binding subunit of myosin light chain phosphatase and CPI17, two regulatory factors of myosin light chain phosphatase, in smooth muscle. Biochem. J. 2003;369:117–128. doi: 10.1042/BJ20021040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi A, Ferlosio A, Gabbiani G, Spagnoli LG, Ehrlich HP. Phenotypic heterogeneity influences the behaviour of rat aortic smooth muscle cells in collagen lattice. Exp Cell Res. 2005;311:317–327. doi: 10.1016/j.yexcr.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Parizi M, Howard EW, Tomasek JJ. Regulation of LPA-promoted myofibroblast contraction: Role of rho myosin light chain kinase, and myosin light chain phosphatase. Exp Cell Res. 2000;254:210–220. doi: 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- Pham C, Greenwood J, Cleland H, Woodruff P. Bioengineered skin substitutes for the management of burns: a systematic review. Burns. 2007;33:946–957. doi: 10.1016/j.burns.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Povova SN, Lundgren-Akerlund E, Wiig H, Gullberg D. Physiology and pathology of collagen receptors. Acta Physiologica. 2007;190:179–187. doi: 10.1111/j.1748-1716.2007.01718.x. [DOI] [PubMed] [Google Scholar]
- Rasband WS. Imagej. Bethesda, MD: NIH; 1997–2007. http://rsb.info.nih.gov/ij/ [Google Scholar]
- Saitoh M, Ishikawa T, Matsushima S, Naka M, Hidaka H. Selective inhibition of catalytic activity f smooth muscle myosin light chain kinase. J Biol Chem. 1987;262:7796–7801. [PubMed] [Google Scholar]
- Schiro JA, Chan BMC, Roswit WT, Kassner PD, Pentland AP, Hemler ME, Eisen AZ, Kupper TS. Integrin α2β1 (vla-2) mediates reorganization and contraction of collagen matrices by human cells. Cell. 1991;67:403–410. doi: 10.1016/0092-8674(91)90191-z. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. From pharmacomechanical coupling to g-proteins and myosin phosphatase. Acta Physiol. Scand. 1998;164:437–448. doi: 10.1046/j.1365-201X.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, St-Louis J. Smooth muscle contractility and protein tyrosine phosphorylation. Mol Cell Biochem. 1997;176:47–51. [PubMed] [Google Scholar]
- Swarup G, Cohen S, Garbers DL. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Comm. 1982;107:1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- Todorov LD, Mihaylova-Todorova ST, Choe SM, P WD. Facilitation of the purinergic contractile response of the guinea pig vas deferens by sodium orthovanadate. JPET. 2005;312(1):407–416. doi: 10.1124/jpet.104.072991. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Haaksma CJ, Eddy RJ, Vaughan MB. Fibroblast contraction occurs on release of tension in attached collagen lattices: dependency on an organized actin cytoskeleton and serum. Anat. Rec. 1992;232:359–368. doi: 10.1002/ar.1092320305. [DOI] [PubMed] [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of rock (rho-kinase) and mlck in spatial regulation of mlc phosphorylation for assembly of stress fibers and focal adhesions in 3t3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulla M, Pentikainen OT, Viitasalo T, Kapyla J, Impola U, Nykvist P, Nissinen L, Johnson MS, Heino J. Selective binding of collagen subtypes by integrin alpha 1i, alpha 2i, and alpha 10i domains. J Biol Chem. 2001;276(51):48206–48212. doi: 10.1074/jbc.M104058200. [DOI] [PubMed] [Google Scholar]