Abstract
Stress and chronically elevated glucocorticoid levels have been shown to disrupt parental behavior in mothers; however, almost no studies have investigated corresponding effects in fathers. The present experiment tested the hypothesis that chronic variable stress inhibits paternal behavior and consequently alters pup development in the monogamous, biparental California mouse (Peromyscus californicus). First-time fathers were assigned to one of three experimental groups: chronic variable stress (CVS, n=8), separation control (SC, n=7), or unmanipulated control (UC, n=8). The CVS paradigm (3 stressors per day for 7 days) successfully stressed mice, as evidenced by increased baseline plasma corticosterone concentrations, increased adrenal mass, decreased thymus mass, and a decrease in body mass over time. CVS altered paternal and social behavior of fathers, but major differences were observed only on day 6 of the 7-day paradigm. At that time point, CVS fathers spent less time with their pairmate and pups, and more time autogrooming, as compared to UC fathers; SC fathers spent more time behaving paternally and grooming the female mate than CVS and UC fathers. Thus, CVS blocked the separation-induced increase in social behaviors observed in the SC fathers. Nonetheless, chronic stress in fathers did not appear to alter survival or development of their offspring: pups from the three experimental conditions did not differ in body mass gain over time, in day of eye opening, or in basal or post-stress corticosterone levels. These results demonstrate that chronic stress can transiently disrupt paternal and social behavior in P. californicus fathers, but does not alter pup development or survival under controlled, nonchallenging laboratory conditions.
Keywords: chronic stress, paternal behavior, parental care, California mouse, corticosterone, pup development, biparental
Introduction
Vertebrate animals respond to challenging situations by mounting a highly conserved stress response that includes activation of the hypothalamic-pituitary-adrenal (HPA) axis (Armario, 2006; Sapolsky, 2002). This response promotes survival under stressful or demanding organismal or environmental conditions (Charmandari et al., 2005; McEwen 2005; Sapolsky, 2002) by inhibiting non-essential activities and processes (e.g., digestion, anabolism, reproduction) and promoting functions that are immediately necessary (e.g., glucose mobilization, vigilance, catabolism; Sapolsky, 2002; Sapolsky et al., 2000). Consequently, stress has been implicated in mediating a trade-off between current and future reproductive effort, including both sexual and parental behavior (see Breuner et al., 2008; Moore and Hopkins, 2009; Ricklefs and Wikelski, 2002). Accordingly, stress, especially pronounced and/or prolonged stress, is predicted to result in decreased parental care and reduced survival of current offspring (Wasser and Barash, 1983; Wingfield and Sapolsky, 2003; Wingfield et al., 1998). Glucocorticoids increase rapidly in response to stressors and affect numerous physiological, neural, cognitive, sensory, and behavioral functions; therefore, they are thought to play a major role in the behavioral changes that occur in response to stress (McEwen 2005; Sapolsky et al., 2000; Wingfield and Sapolsky, 2003).
Consistent with these predictions, both acute and chronic stress have been shown to disrupt maternal behavior in females, and this disruption seems to be mediated at least in part by glucocorticoids. For example, house mouse (Mus musculus) mothers showed a decrease in pup retrievals following 30 min of restraint stress when compared to non-stressed mothers (Yamada et al., 2002). Similarly, Norway rat (Rattus norvegicus) mothers exposed to predator odor retrieved fewer pups and took longer to engage in nursing than did mothers under control conditions (Sukikara et al., 2010). Additionally, rat mothers exposed to the stressor of a novel cage with limited bedding for 5 min were more abusive toward foster pups than were mothers who were given ample bedding and allowed to habituate to the new cage (Roth and Sullivan, 2005). Comparable effects have also been noted in the face of chronic stress. Chronic social stress in rat mothers, implemented by a daily 1-h introduction of an unfamiliar male to the mothers’ cage, resulted in decreased maternal care (pup grooming and nursing) and increased latency to initiate nursing as compared to control mothers (Nephew and Bridges, 2011). Similarly, rat mothers exposed to wet bedding and a forced foraging paradigm did not nurse pups as often as control mothers (Leonhardt et al., 2007), and rat mothers given inadequate nesting materials exhibited more fragmented maternal care and spent more time away from pups than did control mothers (Ivy et al., 2008).
These effects of chronic stress may be mediated by glucocorticoids, as chronic corticosterone treatment, in the absence of experimentally induced stress, decreased the time spent nursing and time in contact with pups in rat mothers, as compared to vehicle-injected mothers (Brummelte et al., 2006). Similarly, marmoset mothers (Callithrix jacchus) that were chronically injected with cortisol spent less time carrying infants when compared to vehicle-injected mothers (Saltzman and Abbott, 2009). Taken together, these results support the hypothesis that glucocorticoid elevation alone, as well as stress, can disrupt parental behavior in mothers.
Few studies, however, have addressed the relationship between stress and parental behavior performed by fathers. In the majority of mammalian species the mother is the sole provider of parental care, but in 6–10% of mammals, including humans, males also invest heavily in offspring (Kleiman and Malcolm, 1971). In addition, 90% of bird species are biparental (Lack, 1968), and in the taxonomic families in which parental care is observed, ∼46% of amphibians and ∼60% of fish species practice paternal care (Gross and Shine, 1981). In species in which it occurs, male parental care greatly enhances offspring survival and development (e.g., Galilaea tilapia, Sarotherodon galilaeus, Balshine-Earn, 1997; house mice, Barnett and Dickson, 1985; deer mice, Peromyscus spp., Bester-Meredith and Marler, 2001; California mice, P. californicus, Bredy et al., 2004; humans, Homo sapiens, Lamb, 2010; degu, Octodon degus, Ovtscharoff et al., 2006; Mongolian gerbils, Meriones unguiculatus, Piovanatti and Vieira, 2004; striped mice, Rhabdomys pumilio, Schradin and Pillay, 2004; termites, Zootermopsis nevadensis, Shellman-Reeve, 1997); however, almost nothing is known about how stress can alter paternal investment in offspring.
Only two studies have specifically investigated the effects of stress on paternal or male alloparental care, while a handful have looked at the relationship between glucocorticoid levels and fatherhood. In virgin male prairie voles (Microtus ochrogaster), males’ corticosterone levels following a forced-swim stressor were negatively correlated with licking and grooming of pups, but positively correlated with pup retrievals (Bales et al., 2006), suggesting that an acute stressor and elevated corticosterone levels may alter some aspects of pup care by males. Correlational studies of fathers’ prepartum baseline corticosterone levels have also produced varying results: studies of humans and hamsters (Phodopus spp.) suggest that fathers’ baseline salivary (human) or plasma (hamster) glucocorticoid levels naturally increase prior to the birth of offspring (Berg and Wynne-Edwards, 2001; Reburn and Wynne-Edwards, 1999, respectively), but a study in biparental Oldfield mice (Peromyscus polionotus) indicated that increased fecal corticosterone concentrations in fathers leading up to the birth of pups were associated with decreased pup survival (Good et al., 2005). Similarly, higher baseline cortisol concentrations have been associated with lower rates of infant carrying in marmoset fathers (Calithrix kuhlii; Nunes et al., 2001), but with increased male alloparental effort in meerkats (Suricata suricatta; Carlson et al., 2006). Thus, no consensus has emerged about how acute stress or baseline levels of glucocorticoids affect paternal care in general.
The California mouse is a monogamous and biparental rodent species (Ribble, 1991, 1992a,b; Ribble and Salvoni, 1990) in which infant care by both parents maximizes offspring survival rates, accelerates offspring development, and increases parents’ reproductive success, both in the lab and in the field, especially under energetically challenging conditions (Bredy et al., 2004; Cantoni and Brown, 1997a,b; Dudley, 1974; Gubernick and Teferi, 2000; Gubernick et al., 1993; Wright and Brown, 2002). Neither baseline nor stress-induced corticosterone levels differed among male reproductive groups (virgin, paired but not breeding, and first-time fathers) in this species (Chauke et al., 2011; Harris and Saltzman, 2013), suggesting that males’ corticosterone levels are not directly influenced by fatherhood. Additionally, it appears that neither acute stress nor acute corticosterone elevation alters paternal behavior. A study by our lab showed that fathers’ licking/grooming of pups and proximity to pups did not differ during a 5-min exposure to predator odor, as compared to the 5 min prior to odor exposure (Chauke et al., 2011), suggesting that a very brief stressor was not sufficient to alter paternal care. Moreover, when compared to vehicle-injected controls, first-time fathers acutely (once) injected with corticosterone did not show altered paternal behavior (Harris et al., 2011). Nonetheless, it is not known how chronic stress affects paternal behavior in these animals. Furthermore, to our knowledge, no study to date has experimentally investigated the effects of chronic stress on paternal care in any mammalian father. In this study, therefore, we tested the hypothesis that chronic stress in fathers can disrupt paternal behavior, leading to decreased rates of growth, development and/or survival in offspring.
We exposed first-time California mouse fathers to chronic variable stress for 7 days and characterized the effects on paternal care and pup development. We predicted that chronically stressed fathers would exhibit decreased rates of paternal behavior and possibly increased aggression toward their pups, as compared to non-stressed control fathers, and that these behavioral effects of chronic stress would be associated with reduced survival rates, slower growth, delayed development, and/or altered circulating corticosterone levels in pups. Chronic stress refers to stress that occurs on a prolonged or repeated basis, as opposed to an acute or infrequent occurrence (Jankord and Herman, 2009; McEwen, 1998; Torres and Nowson, 2007). Moreover, chronic stress can be operationally defined by the physiological responses it elicits: increased circulating glucocorticoid concentrations, decreased body mass, increased adrenal gland mass, and decreased thymus mass (Marin et al., 2007; Ostrander et al., 2006; Simpkiss and Devine, 2003); therefore, we measured these endpoints as validation of the chronic stress protocol.
Methods
Animals
California mice, descendants of mice purchased from the Peromyscus Genetic Stock Center (University of South Carolina, Columbia, SC) in 2007, were bred and housed in the University of California, Riverside vivarium. Mice were housed in polycarbonate cages (44 × 24 × 20 cm) lined with aspen shavings, provided with ad libitum food (Purina rodent chow 5001) and water, and given cotton for nesting material. Ambient temperature was held at approximately 23°C with humidity of about 65%; lights were on from 0500–1700 (14:10 L:D cycle). Prior to the start of the experiment animal cages were cleaned once per week. Mice were weaned from their natal cage at 27–32 days of age (prior to the birth of any younger siblings), ear-marked for identification, and placed into same-sex groups of four until experimentation. UCR has full AAALAC accreditation, and all procedures were approved by the UCR IACUC and conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
This experiment was part of a larger study that included an additional 29 experimental males (virgin males and non-breeding males [paired with a tubally ligated female]). A small subset of data from the chronically stressed and unmanipulated control fathers used here (fathers’ body mass, organ masses, and plasma corticosterone concentrations) is also presented elsewhere (de Jong et al., 2013) as part of the validation of the chronic variable stress paradigm. The separation control males were not included in the larger experiment, and their physiological parameters in response to the separation paradigm are included in this article for adequate comparison. Any sections of overlapping data are explicitly stated and appropriately referenced.
Experimental design
We used a total of 23 first-time fathers, each housed with a female pairmate and the pair’s first litter of pups. Each father was randomly assigned to one of three experimental groups: chronic variable stress (CVS; n=8), separation control (SC; n=7), and unmanipulated control (UC; n=8). At 100–150 days of age, each male was pair-housed with an unrelated adult female (120–160 days of age). We subsequently weighed all animals twice each week in order to monitor health, determine pregnancy, and gather data for calculation of pre-experimental baseline body mass. When a female had gained substantial body mass (6–10 g) the pair was moved to a double-cage system (two regular, polycarbonate housing cages connected by a clear plastic tube approximately 10 cm in length with openings of approximately 5 cm in diameter) to facilitate the experimental manipulations. Hereafter, the double cage will be referred to as the home cage, and each individual cage within the double-cage setup will be referred to as a cage-half.
Experimentation began 1–3 days postpartum (day of birth = day 0); the first day of chronic stress or control procedures is referred to as day 1. All males were weighed on days 1, 3, 5 and 7 between 0900 and 1000h, and baseline blood samples for analysis of plasma corticosterone concentrations were collected on days 1, 4, and 8 (Table 1). CVS fathers were subjected to 7 days of chronic variable stress. SC fathers followed the same schedule as CVS fathers, except that they were isolated from their mate and pups each time CVS fathers underwent stressor exposure, but were not otherwise stressed, in order to control for the CVS fathers’ separation from their mates and pups. UC fathers were left undisturbed except for data-collection procedures (weighing and blood collection). In order to minimize disturbance to the families, cages were not cleaned during the 7-day experimentation period. Instantaneous behavioral scans were performed on each male and its cagemates 15 times and 10-minute behavioral observations were performed 4 times during the period of data collection (see below). On day 8, all fathers were exposed to a novel stressor for assessment of the acute corticosterone stress response, two post-stress blood samples were collected, and animals were subsequently euthanized and dissected.
Table 1.
Sequence of experimental procedures in first-time California mouse fathers and their pups. Chronically stressed fathers (CVS, n=8) were exposed to 7 different acute stressors in random order (on average 3 times per day). Separation control fathers (SC, n=7) were separated from their mate and pups each time CVS fathers underwent stressor exposures, and unmanipulated control (UC, n=8) fathers were not subjected to experimental stressors or separations. See de Jong et al., 2013 for additional details.
| Manipulation | Data Collection | ||||
|---|---|---|---|---|---|
| Day | UC | SC | CVS | Fathers | Pups |
| −62 to- 34 |
-- | -- | -- | (pairs formed) | -- |
| −2 to 0 | -- | -- | -- | (pups born) | -- |
| 1 | -- | separation x2 | stress x2 | blood sample; body mass | -- |
| 2 | -- | separation x3 | stress x3 | 10-min observation (lights-off, post-stress) | -- |
| 3 | -- | separation x3 | stress x3 | 10-min observation (lights-on, baseline); body mass | body mass |
| 4 | -- | separation x4 | stress x4 | blood sample; 10-min observation (lights-off, baseline) | -- |
| 5 | -- | separation x3 | stress x3 | body mass | body mass |
| 6 | -- | separation x2 | stress x2 | 10 min-observation (lights-on, post-stress) | -- |
| 7 | -- | separation x4 | stress x4 | body mass | body mass |
| 8 | oil injection euthanasia |
oil injection euthanasia |
oil injection euthanasia |
blood samples (basal, 10 min and 40 min post- injection); organ masses |
body mass |
| 9–18 | -- | -- | -- | -- | daily: body mass, checked for eye opening |
| 19 | -- | -- | -- | -- | basal blood sample from all pups |
| 20 | -- | -- | -- | -- | post-stress blood sample from all pups |
Growth, development and plasma corticosterone concentrations of pups were monitored to assess whether pups raised by a stressed father differed from pups raised by a non-stressed father. Pups were weighed and dye-marked for identification on post-natal day (PND) 3 and subsequently weighed on the same schedule as their father until day 8 of the CVS experiment, after which pups were weighed, dye-marked and checked daily. On PND19, a blood sample was collected for analysis of basal plasma corticosterone concentrations, and on PND20, pups were exposed to predator odor for 5 minutes, immediately after which a post-stress blood sample was collected and pups were euthanized.
Chronic variable stress paradigm
Mice in the CVS group were exposed to seven different stressors over a 7-day period, with 2–4 stressor exposures per day (one stressor every 6–10 h; see de Jong et al., 2013 for full details). We exposed CVS fathers to an average of three stressors per day because pilot studies showed that a paradigm utilizing two stressors per day was not sufficient to chronically elevate corticosterone levels in male California mice (unpub. data). Stressors were as follows: 1) wet bedding: the mouse was placed for 1 h in a clean cage containing a mixture of wood shavings and tap water; 2) shaker: the mouse was placed in a small plastic container on a lab shaker rotating at 200 rpm for 15 min; 3) injection of hypertonic saline: the mouse was injected i.p. with 1.5M NaCl solution (1.5 ml/100g body mass); 4) cold exposure: the mouse was placed in a plastic container in a refrigerator (inside temperature: 4°C) for 15 min; 5) restraint: the mouse was placed in a “decapicone” (DC M200, Braintree Scientific, Braintree, MA), which was folded tightly, clipped shut, and hung on a suspended wire so that the male was facing downward for 15 min; 6) forced swim: the mouse was placed in 850 mL tap water (24–26°C) and was forced to swim for 5 min; 7) predator urine: the mouse was placed in a clean cage containing wood shavings and exposed to a stainless steel, wire-mesh tea-ball (diameter: 4.5 cm) containing a cotton ball soaked with 1 mL of predator urine (bobcat, coyote, wolf, or mountain lion; Maine Outdoor Solutions, Herman, MA) for 8 min. Preliminary data indicated that each stressor used in the CVS paradigm resulted in a robust, acute increase in plasma corticosterone concentration (Chauke et al., 2011; Harris et al., 2012; unpub. data).
To control for repeated removal of CVS fathers from their families, SC fathers were isolated from their mate and pups at the same times and for the same durations that CVS fathers were removed from their families for stressor exposures; however, SC fathers were not exposed to experimental stressors. To isolate SC fathers in a manner that involved minimal handling and stress, males were placed alone in one cage-half of the double-cage home cage, and the tube connecting the two halves was plugged for the duration of the CVS males’ stressor. UC fathers were left undisturbed during the stressor duration.
To determine whether chronic variable stress potentiated the corticosterone response to a novel stressor, as previously described in rats (Marin et al., 2007), all fathers were injected s.c. with 0.2 mL of sesame oil on day 8 at 0900h, 10 h after the previous stressor exposure. Blood samples were collected immediately before (baseline), and 10 and 40 min after injection to characterize the corticosterone response to a novel stressor; oil injection has been shown to markedly increase circulating corticosterone levels in male California mice (unpub. data). For the final (40 min post-oil injection) blood sample, mice were decapitated, and trunk blood was collected into a heparinized weigh boat. Brains were then harvested, flash-frozen in dry ice and stored for in situ hybridization (de Jong et al., 2013), and organs were dissected out and weighed.
Indices of chronic stress
Blood sample collection
All blood samples, except for the final one collected from each father, were collected from the retro-orbital sinus using 70 µl heparinized microhematocrit tubes while mice were under isoflurane anesthesia. For retro-orbital samples, blood was always collected within 3 min of disturbance to the animal’s cage (mean ± SEM: 60.2 ± 1.4 s; range: 30–124 s) and baseline samples (days 1, 4, and first sample on day 8) were always collected at approximately 0900h (0841–0930 h). Following decapitation, the final blood sample (trunk blood) was collected in weigh boats primed with 0.1 ml of heparin (1000 USP units/ml). Trunk blood was always collected within 90 s of disturbance to the cage (mean ± SEM: 44.5 ± 3.7 s; range: 26–90 s; n=21; 0936–1005 h). Immediately after collection, all blood samples were centrifuged for 12 min (13,300 rpm, 4°C), and plasma was removed and stored at −80°C until assay.
Plasma corticosterone assay
Plasma was assayed in duplicate for corticosterone using an 125I double-antibody radioimmunoassay kit (#07–120102, MP Biomedicals, Costa Mesa, CA) previously validated for this species (Chauke et al., 2011). Samples from each experimental group were balanced evenly across three assays; however, all samples from an individual mouse were always analyzed in a single assay run. The standard curve ranged from 12.5 ng/ml (91% bound) to 1000 ng/ml (20% bound), and plasma samples were assayed using dilutions ranging from 1:100 to 1:800 depending on anticipated corticosterone concentrations. Inter- and intra-assay coefficients of variation were 11.2% and 4.7%, respectively (n = 45 assays).
Organ masses
Immediately following decapitation of fathers on day 8, organs (adrenal glands, testes, thymus, spleen) were dissected out, placed in sterile saline, blotted dry 3x and weighed to the nearest 0.00001 g.
Behavior
This study was designed to evaluate both the effects of CVS on paternal behavior and pup outcomes (this paper) and the effects of fatherhood on responses to CVS (de Jong et al., 2013). Although this dual use of the study was beneficial in that it allowed us to reduce our use of animals, it placed numerous constraints on the experiment, including necessitating minimization of disturbance to the animals outside of stressor exposures, as well as minimizing the number of procedures that could be performed on fathers but not on the other groups. In light of these logistical constraints, we collected two types of behavioral data: instantaneous scans and 10-minute observations. The instantaneous scans were conducted to provide a snapshot of behavior under baseline conditions over the course of the experiment while limiting disturbance to the animals. The 10-min behavioral observations were spread across the experimental paradigm (see details below) and were used for more in-depth, focused analyses of behavior under different conditions.
Instantaneous scans
An instantaneous scan of each family was performed immediately prior to each stressor exposure occurring between 0700 and 2300h on days 2–7 (15 scans total). The male’s and female’s locations with respect to each other and to the pups (same cage-half or different cagehalf of the home cage) and movement (walking/jumping or not locomoting), and behavior (parental or non-parental), were recorded. Behavior was considered parental if the mother or father was actively caring for a pup (i.e., licking, carrying, manipulating with paws or mouth, sniffing, and, for females, nursing) or was passively interacting with the pup (i.e., family huddle [entire family huddling/sleeping together and visible to the observer], huddling pup, family in nest [all animals in cotton and not visible to observer], or that parent only sleeping on pup). Data from the scans were organized into four bins: days 2–4, lights-on (n=6 scans); days 2–4, lights-off (n=2 scans); days 5–7, lights-on (n=5 scans); and days 5–7, lights-off (n=2 scans). For each bin, the proportion of total scans during which each behavior was observed was tabulated for each mouse and used for analysis.
Ten-minute behavioral observations
In addition to instantaneous scans, each family’s behavior was videotaped for 10 min four times during the experiment (days 2, 3, 4, and 6). Two observations (days 2 and 6) occurred immediately following either exposure to a stressor (CVS fathers) or separation from and reunion with the family (SC fathers) or no disturbance (UC fathers), and two (days 3 and 4) were conducted under baseline conditions (no stressor or separation within the previous 6 h). Within each observation condition (post-stress or baseline), one observation was performed during lights-on (inactive period; day 3 at 0900h; day 6 at 0800h) and the other during lights-off (active period; day 2 at 2000h, day 4 at 2100h; see Table 1). For CVS fathers, post-stress observations began when males were returned to the opposite cage-half from their mate and pups, immediately following stressor exposure; for SC fathers, post-stress observations began when the tube plugs were removed at the end of the separation period, allowing the male to rejoin its mate and pups (UC fathers were time-matched to the other groups).
Videos were scored for paternal and non-paternal behaviors by a single, trained observer using JWatcher software (Blumstein and Daniel, 2007). We recorded durations of several behaviors (autogroom, family huddle, family in nest, male groom female, male sniff female, male huddle pup(s), male lick pup(s), male perform kyphosis, male sniff pup(s), male carry pup(s), and male only not in view). Additionally, the numbers of jumps and rears performed by the male were counted, and measures of males’ activity (walking/jumping or sitting still/sleeping) and males’ and females’ locations (within 10 cm of pup(s), touching one or more pups, not in the same cage-half as any pup, male/female in the same cage-half) were recorded every 30 s. Jumps were recorded as a measure of activity, and rears are thought to be associated with exploratory behavior (Espejo, 1997). Location and activity data are presented as proportion of total 30-s scans (out of 20).
Prior to analysis, related behaviors were combined into composites. Paternal behavior was calculated as the total duration of lick pup(s), huddle pup(s), and kyphosis (de Jong et al., 2009, 2010; Harris et al., 2011). Group huddle was calculated as the total duration of visible family huddling and time the family spent under the nest out of view (nests are small, and animals tend to sit together under the small piece of cotton; unpub. obs.). Carry pup(s) and sniff pup(s) did not occur frequently enough to warrant analysis. Additionally, no fighting or aggressive behavior was observed at any time, and mice were rarely observed eating, drinking or performing any other non-scored behaviors.
Pup development
We collected data from the pups of 8 CVS fathers (14 pups), 7 SC fathers (11 pups) and 7 UC fathers (13 pups), totaling 38 pups in all (due to logistical constraints, data could not be collected from the litter [4 pups] of one UC father). Litters from which data were collected contained one (N=6 litters), two (N=15 litters), or three pups (N=1 litter), as is typical for this species (Harris et al., 2011; McCabe and Blanchard, 1950). All pups were weighed and dyemarked on PND3 (regardless of fathers’ experimental day) between 0830 and 1230 h. Green food color was applied to one limb with a cotton swab for identification.
During the CVS paradigm, pups were weighed on the same schedule as their father to minimize disturbance to the family. Fathers were sacrificed at pup PND8-10 (day 8 of the fathers’ experimental protocol). At this time, each mother and her litter were moved from their double cage to a single cage, and data collection and marking of pups occurred daily thereafter. In addition to body mass, pups were checked for the developmental milestone of eye opening. On PND19, a blood sample was collected from each pup at 1430–1520 h to characterize baseline plasma corticosterone levels; pups were weighed after blood collection on this day to avoid disturbing them prior to sample collection. On the following day (PND20), pups were exposed to predator urine for 5 min (see above for methods) and a blood sample was collected immediately afterwards, time-matched to the previous baseline sample; pups were then weighed. All blood samples were collected from the retro-orbital sinus under isoflurane anesthesia using heparinized microhematocrit tubes; time between cage disturbance or end of predator-urine exposure and sample collection exceeded 3 min in only one instance (mean ± SEM: 90.8 ± 3.9 s; range: 49–210 s; n=76 samples). Samples were processed and stored as described above. Following the post-stress blood sample, pups were weighed, sex was determined, and pups were then sacrificed. Mothers were also sacrificed and dissected to check for evidence of pregnancy (visible embryos).
Analysis
Fathers
All data were checked for normality using the Shapiro-Wilk test and were transformed if necessary to meet normality assumptions. Corticosterone concentrations were log10-transformed and analyzed via a 3×3 repeated-measures ANOVA. For analysis of basal corticosterone data, Pillai’s Trace multivariate output was used due to lack of sphericity (Berger and Selhorst, 1983). Baseline body mass was calculated as the average of the last three body mass values prior to the start of data collection, and for analysis body mass was calculated as percent change from baseline and analyzed via a 3×5 repeated-measures ANOVA with Fisher’s LSD post-hoc tests, as we predicted that CVS fathers would lose body mass over time. Organ masses were analyzed as total organ mass (sum of right and left sides, for testes and adrenals) via ANCOVA with day 1 body mass as a covariate; Fisher’s LSD post-hoc tests were used due to a priori predictions. All starting parameters (father’s age, father’s mass, latency to birth of first litter) were compared among groups via one-way ANOVA. Behavioral data from instantaneous scans and 10-min observations were analyzed via Kruskal-Wallis tests with appropriate nonparametric post-hoc tests (Siegel and Castellan, 1988). Due to logistical and/or dissection issues, some physiological measures (day 7 body mass, day 8 blood sample, and/or organ mass) were missing for one UC father and one CVS father, leaving n=7 for both groups in certain analyses. Effect size estimates are reported as either Cohen’s d (post-hoc t-tests), eta squared (calculated as SSeffect/SStotal for ANOVA and ANCOVA, or χ2/[N-1] for Kruskal-Wallis analyses), or partial eta squared (for corticosterone RM ANOVA).
Pups and females
All data were checked for normality using the Shapiro-Wilk test and were transformed if necessary to meet normality assumptions. All corticosterone concentrations were log10-transformed. Due to sex differences in corticosterone levels and growth in some species (McCormick et al., 1995; Weinstock, 2001; Zambrano et al., 2006), we initially performed paired t-tests using opposite-sex littermates to test for effects of sex on starting (PND3) and ending (PND20) body mass, day of eye opening, basal corticosterone levels and post-stress corticosterone levels. A total of 10 litters (5 CVS, 3 SC, 2 UC) contained one pup of each sex (only litters with exactly two pups, one of each sex, were included) and were therefore included in these analyses. Sex of pups did not significantly affect any of the measures (P>0.168 for all cases) and therefore was not factored into any subsequent analyses.
After paired t-tests, pup body mass, day of eye opening, and log10-transformed corticosterone concentrations were each analyzed via linear mixed model with father’s treatment, time (day), and the time*treatment interaction as fixed factors. Family ID was entered as a random factor to control for maternal and natal-cage effects, and time was included as a repeated factor when appropriate (corticosterone and body mass). One UC pup was excluded from all analyses because it was extremely small and abnormally formed at birth and did not grow normally.
Data on presence of embryos at the time of dissection were not available for all females, and the proportion of females with data was not consistent across experimental conditions (UC: 3/7, SC: 6/7, CVS: 7/8). These data were analyzed via Fisher’s Exact test.
Results
Starting parameters
Males in the CVS, SC, and UC groups did not differ in age on day 1 of the experiment (overall mean ± SEM: 159.4 ± 3.5 days), latency from pairing until birth of pups (41.7 ± 1.7 days), number of pups born (1.9 ± 0.1), or age of pups on day 1 of the experiment (2.0 ± 0.1, range: 1–3; see Table 2). Groups did, however, differ in the length of time that they were housed in the double-cage setup prior to day 1 of the experiment (F2,20=4.12, P=0.032, η2=0.29). Fathers in the chronic variable stress condition spent less time in the double cage than unmanipulated control fathers (4.3 ± 0.8 vs. 8.6 ± 1.6 days, respectively; t=2.59, P=0.044, Cohen’s d =1.30; Tukey’s HSD); neither of these groups differed from separation control fathers (4.6 ± 1.1 days).
Table 2.
Starting variables and organ mass data (mean ± SEM) from first-time California mouse fathers that were unmanipulated controls (UC, n=8) or separation controls (SC, n=7), or underwent a chronic variable stress paradigm (CVS, n=8). P-values less than 0.05 are bolded and if groups share a letter they did not differ significantly following post-hoc analysis.
| Variable | UC | SC | CVS | P-Valueb |
|---|---|---|---|---|
| Age at day1 (days) | 161.5 ± 3.6 | 160.3 ± 7.7 | 156.5 ± 7.0 | 0.835 |
| Latency from pairing to parturition (days) |
42.5 ± 3.0 | 41.3 ± 3.1 | 41.3 ± 3.2 | 0.948 |
| Litter size | 2.3 ± 0.3 | 1.7 ± 0.2 | 1.8 ± 0.2 | 0.216 |
| Pup age at day 1 (days) | 2.0 ± 0.2 | 2.0 ± 0.3 | 2.0 ± 0.3 | 0.999 |
| Thymus mass (g)a | 0.077 ± 0.006c | 0.066 ± 0.006c | 0.038 ± 0.006d | 0.001 |
| Total adrenal mass (g)a | 0.014 ± 0.002 | 0.016 ± 0.002 | 0.019 ± 0.002 | 0.091 |
| Spleen mass (g)a | 0.073 ± 0.007 | 0.072 ± 0.007 | 0.059 ± 0.007 | 0.300 |
| Total testis mass (g)a | 0.371 ± 0.029 | 0.386 ± 0.030 | 0.364 ± 0.027 | 0.859 |
Body-mass-corrected means taken at mass of 40.47g
P-value for main effect of experimental group from one-way ANOVA or ANCOVA
Effects of CVS on body mass, organ masses, and corticosterone levels
Body mass
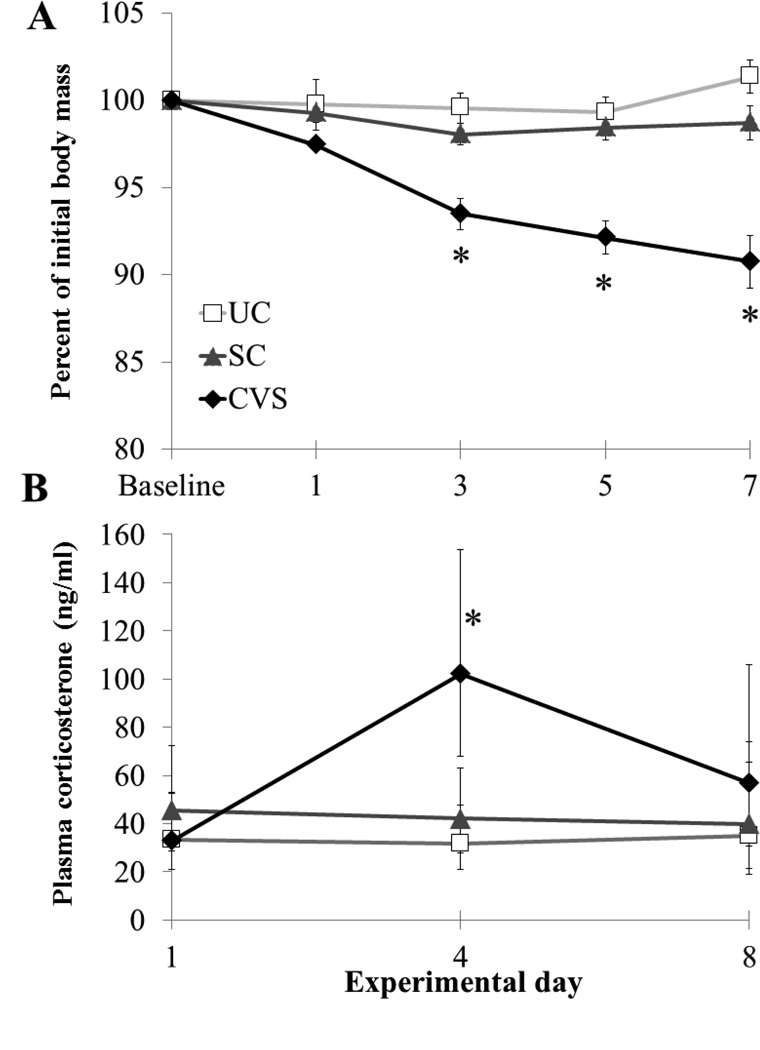
Fathers’ body mass changed significantly over time (main effect of time: F4,76=10.91, P<0.001, η2=0.22), differed by experimental group (main effect of group: F2,19=23.41, P<0.001, η2=0.76), and changed differently across time among groups (time*group interaction: F8,76=9.33, P<0.001, η2=0.38; Fig. 1A). Within time points, groups did not differ from one another at baseline or on day 1. By day 3, however, CVS fathers weighed less than both UC fathers (t=5.80, P<0.001, Cohen’s d= 3.00; Fisher’s LSD) and SC fathers (t=4.02, P=0.001, Cohen’s d =2.08; Fisher’s LSD), whereas UC and SC fathers did not differ from each other. This was also true on day 5 (CVS vs. UC: t=5.78, P<0.001, Cohen’s d=2.99; CVS vs. SC: t=4.98, P<0.001, Cohen’s d=2.58) and day 7 (CVS vs. UC: t=6.19, P<0.001, Cohen’s d=3.20; CVS vs. SC: t=4.65, P<0.001, Cohen’s d= 2.48; all Fisher’s LSD post-hoc comparisons).
Figure 1.
Physiological responses of first-time California mouse fathers exposed to a 7-day chronic variable stress paradigm (CVS, n=8), a separation control paradigm (SC, n=7), or no manipulation (UC, n=7–8). Data from CVS and UC fathers are also presented in de Jong et al., 2013. A) CVS fathers lost body mass over the course of the experiment whereas SC and UC fathers did not. *P<0.05 comparing CVS to SC and UC; SC and UC did not differ at any time point. See results for additional statistical information. Data are presented as mean ± SEM. B) Back-transformed plasma corticosterone concentration measured at 0900h on days 1, 4 and 8 (presented as geometric mean ± 95% confidence interval). CVS fathers had significantly higher corticosterone levels on day 4 when compared to both SC and UC fathers (*P<0.05).
Organ masses
For all fathers combined, right and left organ masses were highly correlated (testes: r=0.87, n=22, P<0.001; adrenal glands: r=0.79, n=22, P<0.001), and therefore each male’s rightand left-side masses were summed to obtain total testis and total adrenal masses. Wet organ masses (total testis, total adrenal, thymus, spleen) were analyzed via ANCOVA with day 1 body mass as a covariate (Table 2). Initially, body mass, group and the body mass* group interaction terms were included in the ANCOVA. The interaction term was not significant for any organ of interest (P>0.3 in all cases) and was removed from the model. Body mass was significant only for wet thymus mass (F1,18=17.96, P<0.001). After we controlled for effects of body mass, wet thymus mass differed among experimental groups (F2,18=13.00, P=0.001, η2=0.07): thymi of CVS fathers weighed less than those of both UC (t=4.93, P<0.001, Cohen’s d =2.54) and SC fathers (t=3.38, P=0.004, Cohen’s d=1.75); UC and SC fathers did not differ (P=0.192, Cohen’s d = 0.72; Fisher’s LSD post-hoc tests). No other organs differed in mass among the three experimental groups. Because we had predicted an increase in adrenal mass in the CVS fathers, we performed a planned comparison between CVS and UC fathers on total adrenal mass (ANCOVA result for mass-corrected effect of experimental condition: F2,18=2.74, P=0.091, η2=0.02 ). This analysis revealed that CVS fathers had heavier body-mass-corrected adrenal glands than UC fathers (0.019 ± 0.002 vs. 0.014 ± 0.002 g at body mass of 39.60 g; t=2.57, P=0.032, Cohen’s d = 1.17; Fisher’s LSD post-hoc test).
Basal corticosterone concentrations
Basal corticosterone concentrations changed over time (main effect of time: F2,17=7.21, P=0.005, partial η2= 0.46), and this effect differed among experimental groups (time*group interaction: F4,36=5.02, P=0.003, partial η2=0.36; Fig. 1B). On day 4, CVS fathers had higher plasma corticosterone concentrations than did UC fathers (t=4.27, P<0.001, Cohen’s d =2.28) and SC fathers (t=3.24, P=0.004, Cohen’s d =1.74; Fisher’s LSD), while UC and SC fathers did not differ (P=0.320, Cohen’s d = 0.55). Groups did not differ in basal corticosterone level at any other time point, and the main effect of experimental group was not significant (F2,18=2.70, P=0.095, partial η2=0.23).
Corticosterone response to a novel stressor
Fathers’ plasma corticosterone concentrations changed acutely following application of a novel stressor (oil injection) on day 8 (main effect of time: F2,36=84.09, P<0.001, η2 =0.80; for all animals combined, basal: 43.03 ng/ml [12.95, 18.54], 10-min post: 754.50 [246.36, 365.80], 40-min post: 341.75 [128.55, 206.05]; data presented as back-transformed means with 95% confidence intervals); see de Jong et al., 2013). This response did not, however, differ among experimental groups (main effect of group: F2,18=0.50, P=0.618, η2 =0.01; time*group interaction: F4,36=1.70, P=0.172, η2 =0.03). Oil injection elevated corticosterone levels above baseline concentrations in all groups at both 10 min (t=15.95, P<0.001, Cohen’s d =8.56; Sidak-corrected post-hoc test) and 40 min post-injection (t=8.11, P<0.001, Cohen’s d=4.34; Sidak-corrected post-hoc test). Plasma corticosterone was also higher at 10 min post-injection when compared to 40 min post-injection (t=3.28, P=0.013, Cohen’s d=1.75; Sidak-corrected post-hoc test).
In addition to plasma corticosterone concentrations at each time point, area under the curve was analyzed using two different equations (Pruessner et al., 2003). The first, AUCg, represents the integrated amount of corticosterone produced over time with respect to a starting value of zero, thus not accounting for baseline (pre-injection) levels of circulating hormone. The second, AUCi, characterizes the response of the HPA axis to oil injection by evaluating the amount of hormone produced above the starting baseline level (thus taking baseline values into consideration). Groups did not differ in either measure of integrated corticosterone production (AUCg, F2,18=0.53, P=0.600, η2 =0.06; AUCi, F2,18 =0.79, P=0.470, η2 =0.08).
Effects of CVS on paternal behavior
Instantaneous scans
Only one difference among groups was found in any of the instantaneous scans performed immediately before stressors (CVS group), separation (SC group), or no manipulation (UC group; Table 3). During the dark (active) phase on days 2–4 (n=2 scans), CVS fathers were less likely to be in the cage-half with at least one pup than were fathers from the UC or SC groups (χ2=1138, P=0.003; Kruskal-Wallis test; CVS vs. UC: P<0.05; CVS vs. SC: P<0.05; post-hoc test for Kruskal-Wallis test; Siegel and Castellan, 1988); UC and SC fathers did not differ (P>0.05). Mothers’ locomotion, location, and behavior did not differ at any time point (see Table 3), and all mothers, regardless of their pairmate’s condition, spent the majority of the observation time with their pups.
Table 3.
Behavioral data (median, range) from instantaneous scans in unmanipulated control (UC, n=8), separation control (SC, n=7), and chronically stressed (CVS, n=8) first-time California mouse fathers and their female mates. Data presented are proportion of scans, within each time slot, that the male or female was moving (walking/jumping), in the same cage-half as at least one pup, or behaving parentally (parental or not). Statistics are from Kruskal-Wallis tests; P-values less than 0.05 are bolded and if groups share a letter they did not differ significantly following post-hoc analysis.
| Behavior | UC | SC | CVS | χ2 | P- value |
η2a |
|---|---|---|---|---|---|---|
| Male in same cage-half as pups | ||||||
| D2-4 Light | 1.00 (1.00–1.00) | 1.00 ( 0.80–1.00) | 1.00 ( 0.80–1.00) | 2.46 | 0.293 | 0.11 |
| D5-7 Light | 1.00 (0.80–1.00) | 1.00 (0.80–1.00) | 1.00 (0.60–1.00) | 1.67 | 0.433 | 0.08 |
| D2-4 Dark | 1.00 (0.00–1.00)a | 1.00 (0.50–1.00)a | 0.50 (0.00–0.50)b | 11.3 8 |
< 0.001 |
0.52 |
| D5-7 Dark | 0.50 (0.00–1.00) | 0.50 (0.00–1.00) | 1.00 (0.50–1.00) | 5.22 | 0.074 | 0.24 |
| Female in same cage-half as pups | ||||||
| D2-4 Light | 1.00 (1.00–1.00) | 1.00 ( 1.00–1.00) | 1.00 ( 1.00–1.00) | 1.28 | 0.526 | 0.06 |
| D5-7 Light | 1.00 (1.00–1.00) | 1.00 (0.80–1.00) | 1.00 (1.00–1.00) | 0.48 | 0.786 | 0.02 |
| D2-4 Dark | 1.00 (0.00–1.00) | 1.00 (0.50–1.00) | 1.00 (0.50–1.00) | 2.29 | 0.319 | 0.10 |
| D5-7 Dark | 1.00 (0.50–1.00) | 1.00 (0.50–1.00) | 1.00 (0.50–1.00) | 0.48 | 0.786 | 0.02 |
| Male behaving paternally | ||||||
| D2-4 Light | 1.00 ( 0.67–1.00) | 1.00 ( 0.67–1.00) | 0.92 ( 0.60–1.00) | 0.57 | 0.752 | 0.03 |
| D5-7 Light | 0.80 (0.40–1.00) | 0.80 (0.60–1.00) | 0.90 (0.40–1.00) | 0.05 | 0.976 | <0.0 1 |
| D2-4 Dark | 0.25 (0.00–1.00) | 0.50 (0.00–1.00) | 0.25 (0.00–0.50) | 2.55 | 0.280 | 0.12 |
| D5-7 Dark | 0.50 (0.00–1.00) | 0.50 (0.00–1.00) | 0.00 (0.00–1.00) | 1.01 | 0.603 | 0.05 |
| Female behaving maternally | ||||||
| D2-4 Light | 1.00 (0.87–1.00) | 1.00 ( 1.00–1.00) | 1.00 ( 1.00–1.00) | 0.65 | 0.721 | 0.03 |
| D5-7 Light | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.13 | 0.568 | 0.05 |
| D2-4 Dark | 0.75 (0.50–1.00) | 0.50 (0.50–0.50) | 0.75 (0.12–1.00) | 1.25 | 0.534 | 0.06 |
| D5-7 Dark | 1.00 (0.50–1.00) | 0.50 (0.50–1.00) | 0.75 (0.50–1.00) | 0.57 | 0.750 | 0.03 |
| Locomoting (male) | ||||||
| D2-4 Light | 0.00 ( 0.00–0.00) | 0.00 ( 0.00–0.17) | 0.00 ( 0.00–0.17) | 1.13 | 0.568 | 0.05 |
| D5-7 Light | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.20) | 3.93 | 0.140 | 0.18 |
| D2-4 Dark | 0.50 (0.00–1.00) | 0.50 (0.00–0.50) | 0.50 (0.00–1.00) | 2.25 | 0.325 | 0.10 |
| D5-7 Dark | 0.50 (0.00–1.00) | 0.50 (0.00–1.00) | 0.50 (0.00–1.00) | 0.37 | 0.832 | 0.02 |
| Locomoting (female) | ||||||
| D2-4 Light | 0.00 ( 0.00–0.00) | 0.00 ( 0.00–0.00) | 0.00 ( 0.00–0.00) | 1.21 | 0.547 | 0.05 |
| D5-7 Light | 0.00 (0.00–0.20) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.73 | 0.694 | 0.03 |
| D2-4 Dark | 0.50 (0.00–1.00) | 0.00 (0.00–1.00) | 0.00 (0.00–0.50) | 1.79 | 0.392 | 0.09 |
| D5-7 Dark | 0.00 (0.00–0.50) | 0.50 (0.00–0.50) | 0.00 (0.00–0.50) | 0.73 | 0.694 | 0.03 |
Effect size estimate, calculated as Χ2/(N-1) (Green and Salkind, 2008)
Ten-minute behavioral observations
Only one behavior, male sniff female (χ2=9.23, P=0.010; Kruskal-Wallis test), differed among experimental groups on day 2 (lights-off, post-stressor or post-separation); post-hoc tests revealed that at this time point, SC males sniffed the female pairmate more than did UC males (P<0.05); neither of these groups differed from CVS males. No other behaviors on day 2, as well as on day 3 (lights-on, baseline) or day 4 (lights-off, baseline), differed among groups (P>0.1 in all cases, Kruskal-Wallis tests; see Table 4 for all behaviors). On day 6 (lights-on, post-stressor or post-separation), however, the groups differed significantly in almost all of the behaviors measured, including duration of paternal behavior (χ2=17.23, P<0.001; Kruskal-Wallis test), male autogroom (χ2=17.684, P<0.001), family huddle (χ 2=11.58, P=0.003), male groom female (χ2=15.19, P=0.001), male sniff female (χ 2=11.17, P=0.003), male rears (χ2=11.40, P=0.003), proportion of time the male spent walking/jumping (χ2=6.74, P=0.034) and resting/still (χ2=14.97, P=0.001). Each measure of male location also differed significantly among groups (P<0.009 in all cases, see Table 4). Female location did not differ (Table 4).
Table 4.
Behavioral data (median, range) from four 10-min observations in unmanipulated control (UC, n=8), separation control (SC, n=7) and chronically stressed (CVS, n=8) first-time California mouse fathers. Conditions during observation were as follows: Day 2 – dark-phase, immediately following no specific manipulation (UC), 10-min separation (SC), or 10-min predator-urine stressor (CVS); Day 3 – light-phase, baseline observation (no stress or separation in the previous 6 h); Day 4 – dark-phase, baseline observation; Day 6 – light-phase, immediately following no specific manipulation (UC), 15-min separation (SC), or 15 min of restraint (CVS). Statistics are from Kruskal-Wallis tests; P-values less than 0.05 are bolded and if groups share a letter they did not differ significantly following post-hoc analysis (day 6 locomotion did not differ following post-hoc tests).
| Behavior | UC | SC | CVS | χ2 | P- value |
η2a |
|---|---|---|---|---|---|---|
| Autogrooming (duration) | ||||||
| Day 2 | 17.1 (0.0–203.8) | 20.0 (5.5–65.2) | 36.2 (0.0 –189.0) | 0.21 | 0.899 | 0.01 |
| Day 3 | 0.0 (0.0–11.2) | 0.0 (0.0–131.8) | 0.0 (0.0–186.2) | 0.67 | 0.717 | 0.03 |
| Day 4 | 101.2 (0.0–117.8) | 38.3 (0.0–115.1) | 36.0 (0.0–116.8) | <0.01 | >0.999 | <0.01 |
| Day 6 | 6.7 (0.0–62.9)a | 71.4 (24.4–221.9)a,b | 271.5 (140.8–547.0)b | 17.68 | <0.01 | 0.80 |
| Paternal behavior composite (duration) | ||||||
| Day 2 | 135.7 (0.0–794.0) | 236.0 (0.0–423.2) | 43.4 (0.0–193.9) | 0.80 | 0.669 | 0.04 |
| Day 3 | 0.0 (0.0–2.4) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 1.88 | 0.392 | 0.09 |
| Day 4 | 0.0 (0.0–50.7) | 3.2 (0.0–516.6) | 10.2 (0.0–561.3) | 1.56 | 0.460 | 0.07 |
| Day 6 | 0.0 (0.0–0.0)a | 5.1 (0.0–85.9)b | 0.0 (0.0–0.0)a | 17.23 | <0.001 | 0.78 |
| Group huddle (duration) | ||||||
| Day 2 | 167.9 (0.0–438.3) | 0.0 (0.0 –256.1) | 144.6 (0.0–435.8) | 2.48 | 0.289 | 0.12 |
| Day 3 | 599.2 (597.6–599.9) | 599.0 (213.2–599.5) | 599.2 (283.7–599.4) | 1.56 | 0.458 | 0.07 |
| Day 4 | 50.5 (0.0–484.8) | 111.9 (0.0–312.6) | 108.3 (4.4–582.9) | 0.72 | 0.697 | 0.03 |
| Day 6 | 598.6 (0.0–599.5)a | 322.4 (69.0–593.8)a,b | 0.0 (0.0–591.2)b | 11.58 | <0.001 | 0.53 |
| Male groom female (duration) | ||||||
| Day 2 | 0.0 (0.0–92.1) | 0.0 (0.0–0.0) | 0.0 (0.0 –21.0) | 2.10 | 0.351 | 0.10 |
| Day 3 | 0.0 (0.0–0.0) | 0.0 (0.0–5.2) | 0.0 (0.0–0.0) | 1.12 | 0.572 | 0.05 |
| Day 4 | 0.0 (0.0–36.4) | 0.0 (0.0–41.6) | 0.0 (0.0–24.3) | 0.13 | 0.936 | <0.01 |
| Day 6 | 0.0 (0.0–30.7)a | 61.0 (15.4–209.8)b | 0.0 (0.0–8.2)a | 15.19 | <0.001 | 0.69 |
| Male sniff female (duration) | ||||||
| Day 2 | 0.0 (0.0–0.0)a | 13.1 (0.0–27.0)b | 6.6 (0.0–14.3)a,b | 9.23 | 0.010 | 0.44 |
| Day 3 | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | <0.01 | >0.999 | <0.01 |
| Day 4 | 0.0 (0.0–9.1) | 0.0 (0.0–3.7) | 0.0 (0.0–15.4) | 0.33 | 0.849 | 0.02 |
| Day 6 | 0.0 (0.0–0.0)a | 7.6 (0.0–42.0)b | 0.0 (0.0–1.5)a,b | 11.56 | <0.001 | 0.53 |
| Jump (count) | ||||||
| Day 2 | 3 (0–125) | 2 (0– 141) | 2 (0– 144) | 0.08 | 0.961 | <0.01 |
| Day 3 | 0 (0–0) | 0 (0–1) | 0 (0–0) | 2.29 | 0.319 | 0.10 |
| Day 4 | 25 (0–269) | 5 (0–258) | 4 (0–241) | 0.83 | 0.660 | 0.04 |
| Day 6 | 0 (0–0) | 0 (0–14) | 0 (0–45) | 4.11 | 0.128 | 0.19 |
| Rear (count) | ||||||
| Day 2 | 11 (0–22) | 16 (4 –46) | 19 (4 –71) | 3.95 | 0.139 | 0.19 |
| Day 3 | 0 (0–0) | 0 (0–13) | 0 (0–0) | 2.29 | 0.319 | 0.10 |
| Day 4 | 17 (0–61) | 15 (0–82) | 32 (0–69) | 0.48 | 0.786 | 0.02 |
| Day 6 | 0 (0–5)a | 8 (1–73)b | 6 (0–29)b | 11.40 | <0.001 | 0.52 |
| Locomoting (proportion) | ||||||
| Day 2 | 0.13 (0.00–0.45) | 0.15 (0.05–0.70) | 0.15 (0.05–0.90) | 1.13 | 0.568 | 0.05 |
| Day 3 | 0.00 (0.00–0.00) | 0.00 (0.00–0.10) | 0.00 (0.00–0.05) | 1.15 | 0.562 | 0.05 |
| Day 4 | 0.15 (0.00–0.80) | 0.10 (0.00–0.70) | 0.20 (0.00–0.65) | 0.60 | 0.741 | 0.03 |
| Day 6 | 0.00 (0.00–0.10) | 0.10 (0.00–0.65) | 0.05 (0.00–0.30) | 6.74 | 0.034 | 0.30 |
| Sleeping/still (proportion) | ||||||
| Day 2 | 0.55 (0.20–0.75) | 0.45 (0.00–0.55) | 0.20 (0.00–0.70) | 3.20 | 0.202 | 0.15 |
| Day 3 | 1.00 (0.95–1.00) | 1.00 (0.65–1.00) | 1.00 (0.40–1.00) | 0.86 | 0.652 | 0.04 |
| Day 4 | 0.15 (0.00–0.90) | 0.30 (0.00–1.00) | 0.45 (0.00–1.00) | 1.99 | 0.371 | 0.09 |
| Day 6 | 0.95 (0.70–1.00)a | 0.20 (0.05–0.70)b | 0.35 (0.00–0.65)b | 14.97 | <0.001 | 0.68 |
| Location | UC | SC | CVS | χ2 | P- value |
η2a |
|---|---|---|---|---|---|---|
| Male in same cage-half as female (proportion) | ||||||
| Day 2 | 0.60 (0.00–1.00) | 0.60 (0.25–0.85) | 0.80 (0.50–0.95) | 1.78 | 0.410 | 0.08 |
| Day 3 | 1.00 (1.00–1.00) | 1.00 (0.85–1.00) | 1.00 (0.90–1.00) | 1.15 | 0.562 | 0.05 |
| Day 4 | 0.50 (0.00–1.00) | 0.65 (0.25–0.90) | 0.85 (0.70–1.00) | 3.31 | 0.191 | 0.16 |
| Day 6 | 1.00 (0.90–1.00)a | 0.90 (0.35–1.00)a,b | 0.25 (0.00–1.00)b | 12.30 | <0.001 | 0.56 |
| Male w/in 10 cm of pups (proportion) | ||||||
| Day 2 | 0.58 (0.50–1.00) | 0.60 (0.00–0.80) | 0.70 (0.00–0.90) | 0.23 | 0.890 | 0.01 |
| Day 3 | 1.00 (1.00–1.00) | 1.00 (0.40–1.00) | 1.00 (0.60–1.00) | 1.15 | 0.562 | 0.05 |
| Day 4 | 0.35 (0.00–0.85) | 0.50 (0.05–1.00) | 0.33 (0.10–1.00) | 0.92 | 0.633 | 0.04 |
| Day 6 | 1.00 (0.00–1.00)a | 0.55 (0.25–1.00)a,b | 0.00 (0.00–1.00)b | 9.50 | 0.009 | 0.43 |
| Male not in same cage-half as any pup (proportion) | ||||||
| Day 2 | 0.05 (0.00–0.50) | 0.20 (0.00–1.00) | 0.20 (0.00–0.90) | 2.58 | 0.275 | 0.12 |
| Day 3 | 0.00 (0.00–0.00) | 0.00 (0.00–0.15) | 0.00 (0.00–0.10) | 1.15 | 0.562 | 0.05 |
| Day 4 | 0.05 (0.00–0.95) | 0.35 (0.00–0.75) | 0.05 (0.00–0.30) | 2.34 | 0.310 | 0.11 |
| Day 6 | 0.00 (0.00–0.10)a | 0.10 (0.00–0.65)a,b | 0.80 (0.00–1.00)b | 12.19 | <0.001 | 0.55 |
| Female w/in 10 cm of pups (proportion) | ||||||
| Day 2 | 0.48 (0.00–1.00) | 0.25 (0.00–1.00) | 0.70 (0.00–1.00) | 0.22 | 0.897 | 0.01 |
| Day 3 | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | <0.01 | >0.999 | <0.01 |
| Day 4 | 1.00 (0.00–v1.00) | 1.00 (0.10–1.00) | 0.93 (0.05–1.00) | 0.60 | 0.743 | 0.03 |
| Day 6 | 1.00 (1.00–1.00) | 1.00 (0.85–1.00) | 1.00 (0.85–1.00) | 1.13 | 0.568 | 0.05 |
| Female not in same cage-half as any pup (proportion) | ||||||
| Day 2 | 0.00 (0.00–1.00) | 0.30 (0.00–0.65) | 0.20 (0.00–0.70) | 0.12 | 0.943 | <0.01 |
| Day 3 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | <0.01 | >0.999 | <0.01 |
| Day 4 | 0.00 (0.00–1.00) | 0.00 (0.00–0.55) | 0.00 (0.00–0.30) | 0.87 | 0.649 | 0.04 |
| Day 6 | 0.00 (0.00–0.00) | 0.00 (0.00–.015) | 0.00 (0.00–0.15) | 1.13 | 0.568 | 0.05 |
Effect size estimate, calculated as Χ2/(N-1) (Green and Salkind, 2008)
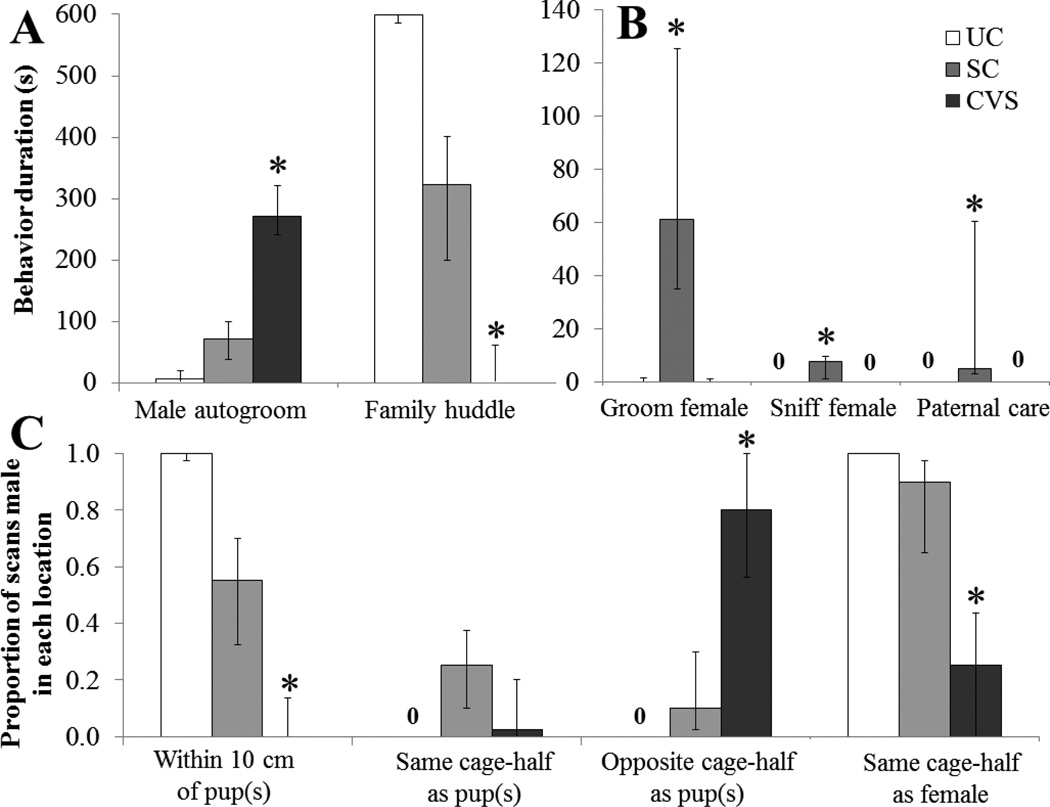
Post-hoc tests indicated that immediately following separation from the family and exposure to a restraint stressor (day 6), CVS fathers tended to spend more time engaging in self-directed behavior and less time interacting with their mate and pups compared to undisturbed control fathers. Specifically, CVS fathers spent significantly more time autogrooming (Fig. 2A) and significantly less time in a family huddle (Fig. 2A) than did UC fathers (P<0.05 for both comparisons) but not SC fathers; UC and SC fathers did not differ in either of these measures. In contrast, separation from the family without exposure to an additional stressor appeared to increase social and parental behavior in separation control fathers. Specifically, SC fathers spent more time engaging in paternal care and more time grooming their mate than did both CVS and UC fathers (P<0.05 in all cases; Fig. 2B); UC and CVS father did not differ in either behavior. Additionally, SC fathers spent more time sniffing their mate than did UC fathers (P<0.05; Fig. 2B), but neither group differed from CVS fathers in this behavior. In terms of location, CVS fathers spent significantly less time in the same cage-half as the female, in the same cage-half as the pups, and within 10 cm of pup(s) than did UC fathers (P<0.05 for all comparisons; Fig. 2C) but not SC fathers; UC and SC fathers did not differ in any of these measures. Both SC and CVS fathers performed more rears and spent less time sitting still/resting than did UC fathers (P<0.05 in all cases), but SC and CVS fathers did not differ in these behaviors.
Figure 2.
Behavioral data from 10-min observation of first-time California mouse fathers on day 6 of a 7-day experimental protocol. Fathers in the chronic variable stress condition (CVS, n=8) were observed immediately following a 10-min stressor, separation control fathers (SC, n=7) were observed following 10 min of separation from the mate and pups, and unmanipulated control fathers (UC, n=8) were observed without prior manipulation. All bars are medians with first and third quartiles. A) CVS males spent significantly more time autogrooming and less time huddling with the family when compared to UC males (*CVS vs. UC, P<0.05). B) SC fathers spent significantly more time grooming the female pairmate and engaging in paternal care (combination of lick pup, huddle pup, and kyphosis) than did CVS and UC males (*SC vs. CVS and UC, P<0.05), while SC fathers spent more time sniffing the mate than did UC fathers (*SC vs. UC, P<0.05), but did not differ from CVS fathers. C) CVS fathers spent more time in the opposite cage-half as the pups and the female, and spent less time within 10 cm of the pups, than did UC fathers (*CVS vs. UC, P<0.05).
Effect of fathers’ treatment group on pups and subsequent pregnancy
Pup body mass
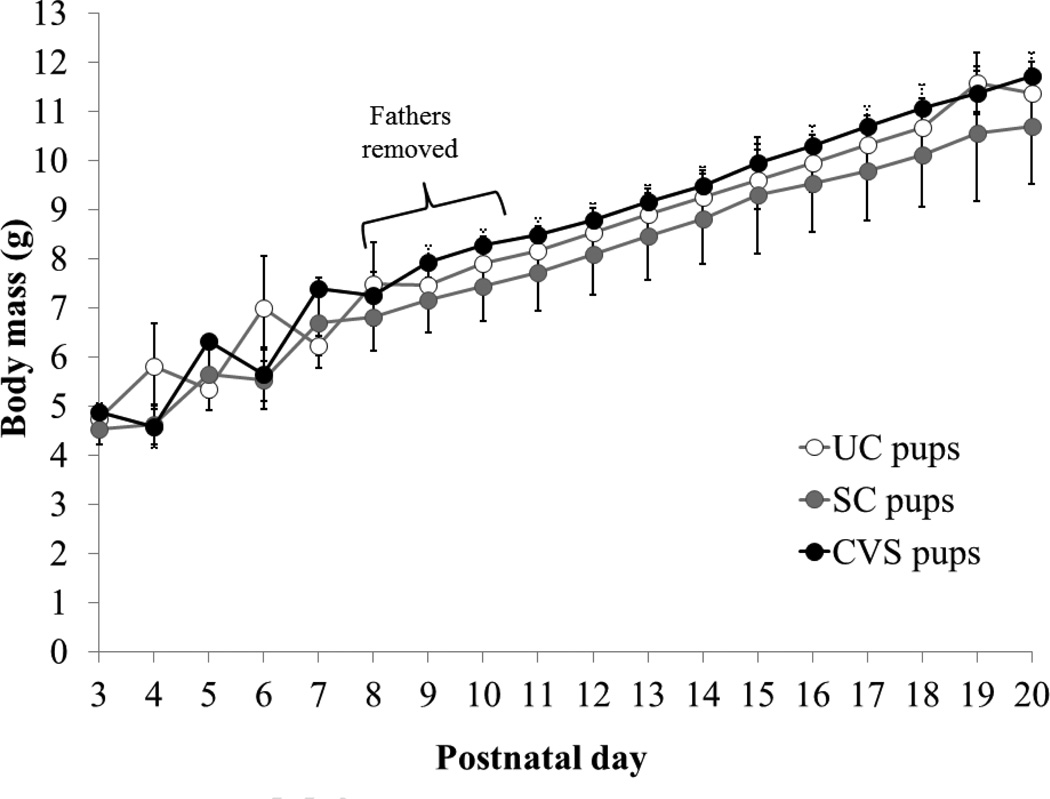
Pup mass increased significantly over time (F17,496=279.80, P<0.0001; Fig. 3), but was not affected by father’s treatment group (F2,35=0.44, P=0.650; time*group interaction F34,496=0.65, P=0.936). Fisher’s LSD post-hoc tests revealed that pup body mass differed significantly between all time points (P<0.05 for all comparisons) except for PND4 vs. PND5 (P=0.114), PND6 vs. PND7 (P=0.059), PND10 vs. PND11 (P=0.092), and PND19 vs. PND20 (P=0.218). Pup body masses were consistent with other reports in this species (Harris et al., 2011; Wright and Brown, 2002).
Figure 3.
Body mass of pups raised for the first 8–10 days post-partum by chronically stressed (CVS, n=14 pups from 8 litters) separation control (SC, n=11 pups from 7 litters), or unmanipulated control (UC, n=13 pups from 7 litters) fathers. Data are presented as mean ± SEM. Pups’ body mass increased over time (main effect of time: P<0.001; see results section for time point comparisons) but was not influenced by fathers’ treatment condition. Fathers were euthanized 8 days after the start of the experiment (morning of postnatal day 8, 9, or 10 for pups); pups were euthanized on postnatal day 20.
Day of eye opening
Day of eye opening did not differ among pups of CVS, SC, and UC fathers (UC: 14.62 ± 0.31, SC: 15.55 ± 0.34, CVS: 15.43 ± 0.30 days; F2,35=2.62, P=0.087) and was consistent with published literature on California mouse pups (Vieira and Brown, 2003).
Basal and post-stress corticosterone concentrations
After pups were exposed to predator urine for 5 min on PND 20, plasma corticosterone levels increased above baseline values (back-transformed mean and 95% confidence interval: basal 43.55 ng/ml [1.52, 1.76] vs. post-stress 384.59 ng/ml [2.53, 2.64]; F1,35=272.06, P<0.001); however, pups’ corticosterone levels were not affected by their father’s treatment group (F2,35=1.83, P=0.176; time*group interaction, F2,35=1.18, P=0.318).
Proportion of pregnant females
The proportion of females with detectable pregnancies following dissection did not differ significantly among females that had been paired with an unmanipulated control male (1 of 3), with a separation control male (4 of 6), and with a chronically stressed male (3 of 7; P=0.100, Fisher’s Exact test).
Discussion
This experiment is the first, to our knowledge, to experimentally determine the effects of persistent stress on paternal care in a monogamous, biparental mammal. Since stress is suggested to mediate a trade-off between current and future reproduction (Ketterson and Nolan, 1999; Moore and Hopkins, 2009; Ricklefs and Wikelski, 2002; Zera and Harshman, 2001), we predicted that stress in California mouse fathers would result in decreased paternal behavior. Consequently, we predicted that pups of stressed fathers would exhibit reduced survival rates, decreased growth, delayed development, and altered corticosterone release.
Even though 7 days is relatively short for a chronic stress protocol (but see Bhatnagar and Dallman, 1998; Molina et al., 1994; Murua et al., 1991; Ostrander et al., 2006), stressed fathers lost body mass over the course of the experiment, showed altered organ masses (decreased thymus mass and increased adrenal mass [CVS vs. UC]), and had increased baseline corticosterone levels on day 4 of the 7-day chronic variable stress paradigm, as compared to the control groups, which confirms that the paradigm was stressful (see de Jong et al., 2013). The stress protocol produced behavioral differences as well: stressed fathers were less likely to be located in the cage-half containing pups during instantaneous scans occurring in the dark phase of days 2–4, as compared to both UC and SC fathers, and stressed fathers differed from controls in several behaviors during the 10-min observation sessions. Behavioral differences observed following stress (or separation) on days 2 and 6 demonstrate that separation control fathers were more likely to investigate or interact with their mate than fathers from the other groups: following reunion after a brief (10- or 15-min) separation from the mate and pups, separation control fathers spent more time grooming and sniffing the female and more time engaging in paternal care than did UC or CVS males, whereas chronically stressed fathers did not differ from controls in these measures. CVS fathers spent significantly more time autogrooming and less time huddling with the family when compared to control fathers, but did not differ from SC fathers in these behaviors. Significant behavioral differences occurred during sessions immediately following stress exposure (or separation) regardless of time of day (dark vs. light phase), suggesting that acute stressors (or separation) had transient effects on behavior. Additionally, duration of the CVS paradigm and/or time of day may be important in generating behavioral changes, as several behaviors differed among groups on day 6 (0800h, lights-on, post-stress/separation), but only one behavioral difference was evident among groups during 10-min observations on days 2, 3 or 4 (on day 2 [2000h, lights-off, post-stress/separation], separation control fathers spent more time sniffing the mate than did unmanipulated control fathers). Contrary to the strong effects of stress and glucocorticoids on reproductive behavior and offspring survival predicted in the literature (see discussions in Bonier et al., 2009a,b; Breuner et al., 2008; Sapolsky et al., 2000; Wingfield and Sapolsky, 2003), no father was seen displaying direct aggression towards the mate or pups at any time, and no pups were harmed.
Notably, in many of the behavioral analyses, separation control fathers, rather than CVS fathers, were the group that most clearly differed from the others. Both CVS and SC fathers were separated from their pairmate and pups immediately before behavioral observations on days 2 and 6, but only SC fathers engaged in paternal care and increased the duration of time interacting with the female pairmate upon return, as compared to undisturbed controls. Despite being awake and active at the time of reunion, CVS fathers engaged in relatively few interactions with the family; instead, they spent most of their time autogrooming. CVS fathers did not differ from UC fathers in paternal behavior or in duration of time interacting with the female, but this is likely due to the fact that all but one of the UC fathers were sleeping at this time. Day 6 data were collected during lights-on, a time when these mice are usually asleep, evidenced by the UC fathers’ high rates of group huddle (six of the seven UC fathers spent >90% of the observation time in a group huddle), low rates of locomotion, and the high proportion of scans during which mice were noted to be sleeping or still. Additionally, UC males were almost always in the same cage as the female and pups; CVS males were not.
These finding indicate that SC fathers were quick to return to their pairmate and pups and to engage in social and parental behavior following reunion, whereas CVS males were not. Thus, CVS blocks the separation-induced increase in social behavior that normally occurs following a brief separation of the father from the family. Brief separation has previously been shown to increase parental care in California mouse fathers (Bredy et al., 2004) and in rat mothers (Boccia and Pendersen, 2001), and stress negatively impacting parental behavior is consistent with studies on rats, where both acute and chronic stress (with or without separation) have been shown to decrease maternal care (without separation: Ivy et al., 2008; Nephew and Bridges, 2011; Roth and Sullivan, 2005; Sukikara et al., 2010; with separation: Yamada et al., 2002).
In the wild, it is possible that chronically stressed fathers would be more likely to abandon the family or to be predated if they were spending more time away from the nest. Field studies of birds have found that both stress and chronic elevation of glucocorticoids via implants can decrease parental care and/or increase rates of nest abandonment by both mothers and fathers (Almasi et al., 2008; Angelier et al., 2009; Love et al., 2004; Silverin 1986,1998; Spee et al., 2011; Wingfield and Silverin, 1986; Wingfield and Kitaysky, 2002). In California mice, fathers’ disappearance from the nest would likely have severe fitness consequences, as mother-only California mouse families have been shown to wean fewer pups than two-parent families (Cantoni and Brown, 1997a,b; Gubernick and Teferi, 2000). Less dramatically, stress could also reduce fathers’ social and parental behaviors following return of the father from a foraging bout away from the nest. This decrease in paternal interaction with pups might have negative consequences for pup growth and development, as fathers’ presence has been shown to enhance these measures when mothers were removed from the cage for a period of time each day (Dudley, 1974).
While we have shown that chronic stress can subtly alter paternal and social behavior in the laboratory setting, the mechanism by which this alteration occurs is not known. Previous work in rats (Brummelte et al., 2006), common marmosets (Callithrix jacchus; Saltzman and Abbott, 2009), and pied flycatchers (Ficedula hypoleuca; Silverin 1986, 1988) suggests that chronically elevated glucocorticoid levels, outside of stress, can result in offspring abandonment and decreased parental care; however, evidence suggests that strictly glucocorticoid-mediated mechanisms are unlikely to account for altered paternal behavior observed on days 2 and 6 in our study. Glucocorticoids most notably elicit their effects by binding to intracellular receptors and altering DNA transcription and protein synthesis, a process taking roughly 1–2 h (Beato, 1989; Strahle et al., 1988; Zakon, 1998). In the present study, behavioral differences were mainly apparent immediately after a 10- or 15-min stressor, thus not allowing time for genomic effects from acute corticosterone elevation to occur. Moreover, circulating plasma corticosterone levels were elevated in stressed fathers on day 4, but no behavioral differences were apparent during the observation session on that day, suggesting that glucocorticoid elevation alone did not alter behavior in stressed fathers. Previous work from our lab supports this idea: a single corticosterone injection did not alter paternal behavior in California mouse fathers 90–120 min later, suggesting that acute elevation of corticosterone (and presumably glucocorticoid-induced changes in gene expression) is not sufficient to inhibit paternal behavior under otherwise undisturbed conditions (Harris et al., 2011).
In addition to genomic effects, however, corticosterone may act via membrane-bound receptors to cause rapid (within minutes), second-messenger-mediated changes in behavior (Borski, 2000; Groeneweg et al., 2011; Joels and Baram, 2009; Losel and Wehling, 2003). It may be that corticosterone altered behavior in the present study by binding to membrane-bound receptors. However, we previously found that a 5-min stressor did not alter paternal behavior but did increase circulating corticosterone levels (Chauke et al., 2011), suggesting that a single, acute stressor causing elevation of corticosterone was not sufficient to rapidly (non-genomically) alter paternal behavior.
Another possibility is that chronic elevation of baseline corticosterone levels induced or was associated with neuroendocrine modification of stress-responsive brain regions in CVS fathers, explaining why more behavioral differences were observed on day 6 as compared to day 2. Circulating and central concentrations of numerous hormones and neuropeptides, many of which are implicated in behavioral regulation, can be altered by stress and by changes in glucocorticoid levels (e.g., corticotropin-releasing hormone [CRH], arginine vasopressin [AVP], prolactin, oxytocin, opioids, testosterone, serotonin; Herman and Cullinan, 1997; Insel and Young, 2000; Sapolsky et al., 2000) and therefore may be important in stress-induced modification of behavior. In California mice, the stress-reactive peptide AVP may be particularly relevant. In our larger CVS study (CVS and UC fathers from the present experiment, in addition to males pair-housed with either another male or a tubally ligated female; see de Jong et al., 2013), chronically stressed males across all three housing conditions had elevated levels of AVP mRNA, but not CRH mRNA, in the paraventricular nucleus of the hypothalamus (PVN) when compared to unmanipulated control males (de Jong et al., 2013). (Brains of the SC males were not characterized.)
In other rodent species, increased AVP expression in the PVN is associated with increased anxiety (Landgraf and Wigger, 2003; Pan et al., 2009; Wigger et al., 2004), suggesting that increased AVP in our chronically stressed fathers may be indicative of increased anxiety in these animals as compared to their non-stressed counterparts. In line with this possibility, a previous study on virgin male California mice found that males with higher AVP mRNA expression in the PVN took significantly longer to contact a foster pup, and that males that approached pups more quickly appeared to be less anxious (as determined by a urine-marking test; de Jong et al., 2012). Additionally, chronically stressed fathers in the present study spent significantly more time autogrooming than control fathers, and this behavior has been associated with high levels of anxiety in other rodents (Ferre et al., 1995; Kalueff and Touhimaa, 2005). Taken together, these results suggest that anxiety, rather than stress per se, might underlie alterations in social behavior and pup responsiveness in this species (but see results in Chauke et al., 2012).
In the present study, chronic stress in fathers and the resulting alterations in social and paternal behavior did not result in any detectable changes in pups, including survival, development (measured as day of eye opening), growth, basal or stress-induced plasma corticosterone concentrations during the first 20 postnatal days, suggesting that slight decreases in current reproductive investment (paternal care) do not necessarily have a measurable consequence on parental fitness. One caveat is that all fathers were sacrificed when pups were around 10 days old, but pups were studied until 20 days of age. It may be that the early disappearance of the father masked any changes caused by having a chronically stressed father. Additionally, it is possible that mothers altered their behavior to compensate for the reduction in paternal care (although we did not find evidence of this in the behavioral data), and that this prevented any differences in pup outcomes. Alternatively, being raised by a stressed father might have altered pup variables not measured in this study. For example, the amount of licking and grooming received by California mouse pups has been shown to influence spatial learning and memory in adulthood, and pups raised by both parents receive more licking/grooming than do pups raised by the mother only (Bredy et al., 2004). More recently, evidence for non-genomic transmission of paternal care from father to son has been found in this species. Sons raised with an experimentally manipulated ‘good’ father spent more time licking and grooming their own pups later in life compared to those males raised with a ‘less attentive’ father (Gleason and Marler, 2013). Considering that the stressed males in our study spent less time behaving paternally than separation control males (despite both groups being away from the family for the same duration), future studies investigating longer-term developmental variables, including memory, learning and parental behavior of adult offspring, would be informative.
In conclusion, we found that chronic stress in a monogamous, biparental male mammal can reduce mate- and pup-directed behavior that normally follows separation and reunion, but effects were subtle and did not translate to detectable changes in pup outcomes or parental fitness. Due to logistical constraints, we were not able to collect extensive behavioral data, and this may have limited our ability to detect group differences in paternal behavior. Moreover, our study was conducted under controlled laboratory conditions, which might have reduced or eliminated any effects of paternal stress on pup survival and development. Future studies should characterize long-term measures of pup development and behavior, and possibly superimpose chronic stress on more challenging living conditions (e.g., requiring animals to work for food, housing animals at cold ambient temperature; Cantoni and Brown, 1997a,b; Gubernick et al., 1993; Wright and Brown, 2002). Subsequent studies should also characterize behavior of the female pairmate to test for behavioral compensation by the mother and look for more nuanced behavioral changes in fathers. Additionally, work investigating the epigenetic effects of chronic stress should be conducted, as recent evidence shows that chronically stressed male house mice produce sperm with altered microRNA, resulting in long-term germ cell reprogramming and changes in offspring neuroendocrine function (Rodgers et al., 2013).
Highlights.
Fathers were subj ected to 7-days of chronic variable stress (CVS), separation or no manipulation
CVS decreased thymus and body mass, elevated plasma GCs, and increased adrenal mass
CVS blocked separation-induced increase in paternal and social behavior; increased autogrooming
Most behavioral differences were noted 6 of the 7-day protocol and the role of GCs is not clear
Pups did not differ in body mass gain, day of eye opening, or basal or post-stress corticosterone levels.
Acknowledgments
We would like to thank the UCR vivarium staff for their assistance with animal care. We would also like to thank the many people who helped perform this study: Omar Aldaas, Trey Amador, Radhika Chandramouli, Julia Cho, Gavrielle Concepcion, Saif Hossain, Juan Pablo Perea-Rodriguez, Aaron Stamp, and Samantha Zamora, and the three anonymous reviewers who provided constructive comments on a previous draft of the manuscript. This work was supported by NIH grant R21MH087806 awarded to W. Saltzman and by a UCR Undergraduate Research Grant to V. Yang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almasi B, Roulin A, Jenni-Eiermann S, Jenni L. Parental investment and its sensitivity to corticosterone is linked to melanin-based coloration in barn owls. Horm. Behav. 2008;54:217–223. doi: 10.1016/j.yhbeh.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Angelier F, Clément-Chastel C, Welcker J, Gabrielsen GW, Chastel O. How does corticosterone affect parental behaviour and reproductive success? A study of prolactin in black-legged kittiwakes. Funct. Ecol. 2009;23:784–793. [Google Scholar]
- Armario A. The hypothalamic-pituitary-adrenal axis: what can it tell us about stressors? CNS Neurol. Disord. Drug Targets. 2006;5:485–501. doi: 10.2174/187152706778559336. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kramer KM, Lewis-Reese AD, Carter CS. Effects of stress on parental care are sexually dimorphic in prairie voles. Physiol. Behav. 2006;87:424–429. doi: 10.1016/j.physbeh.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Balshine-Earn S. The benefits of uniparental versus biparental mouth brooding in Galilee St. Peter's fish. J. Fish Biol. 1997;50:371–381. [Google Scholar]
- Barnett SA, Dickson RG. A paternal influence on survival of wild mice in the nest. Nature. 1985;317:617–618. [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Berg SJ, Wynne-Edwards KE. Changes in testosterone, cortisol, and estradiol levels in men becoming fathers. Mayo Clin. Proc. 2001;76:582–592. doi: 10.4065/76.6.582. [DOI] [PubMed] [Google Scholar]
- Berger DE, Selhorst SC. A simulation comparison of univariate and multivariate analysis of a multi-factor repeated measures design. Claremont, CA: The Claremont Graduate School; 1983. ERIC Service No. ED 229 389. [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamicpituitary-adrenal responses to a novel stressor after chronic stress. Neurosci. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Blumstein D, Daniel JC. Quantifying Behavior the JWatcher Way. Sunderland, MA: Sinauer Associates; 2007. [Google Scholar]
- Boccia ML, Pedersen CA. Brief vs. long maternal separations in infancy: contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26:657–672. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Bonier F, Martin PR, Moore IT, Wingfield JC. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 2009a;24:634–642. doi: 10.1016/j.tree.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Bonier F, Moore IT, Martin PR, Robertson RJ. The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen. Comp. Endocrinol. 2009b;163:208–213. doi: 10.1016/j.ygcen.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Borski RJ. Nongenomic membrane actions of glucocorticoids in vertebrates. Trends Endocrinol. Metab. 2000;11:427–436. doi: 10.1016/s1043-2760(00)00325-8. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Lee AW, Meaney MJ, Brown RE. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus) Horm. Behav. 2004;46:30–38. doi: 10.1016/j.yhbeh.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Patterson SH, Hahn TP. In search of relationships between the acute adrenocortical response and fitness. Gen. Comp. Endocrinol. 2008;157:288–295. doi: 10.1016/j.ygcen.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Pawluski JL, Galea LA. High post-partum levels of corticosterone given to dams influence postnatal hippocampal cell proliferation and behavior of offspring: a model of post-partum stress and possible depression. Horm. Behav. 2006;50:370–382. doi: 10.1016/j.yhbeh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Cantoni D, Brown RE. Male influence on interbirth interval in the monogamous California mouse when required to forage for food. Ann. N.Y. Acad. Sci. 1997a;807:486–489. doi: 10.1111/j.1749-6632.1997.tb51946.x. [DOI] [PubMed] [Google Scholar]
- Cantoni D, Brown RE. Paternal investment and reproductive success in the California mouse, Peromyscus californicus . Anim. Behav. 1997b;54:377–386. doi: 10.1006/anbe.1996.0583. [DOI] [PubMed] [Google Scholar]
- Carlson AA, Manser MB, Young AJ, Russell AF, Jordan NR, McNeilly AS, Clutton-Brock T. Cortisol levels are positively associated with pup-feeding rates in male meerkats. Proc. R. Soc. B. 2006;273:571–577. doi: 10.1098/rspb.2005.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos GP. Endocrinology of the stress response. Annu. Rev. Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Chauke M, de Jong TR, Garland T, Jr, Saltzman W. Paternal responsiveness is associated with, but not mediated by reduced neophobia in male California mice (Peromyscus californicus) Physiol. Behav. 2012;107:65–75. doi: 10.1016/j.physbeh.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauke M, Malisch JL, Robinson C, de Jong TR, Saltzman W. Effects of reproductive status on behavioral and endocrine responses to acute stress in a biparental rodent, the California mouse (Peromyscus californicus) Horm. Behav. 2011;60:128–138. doi: 10.1016/j.yhbeh.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong TR, Chauke M, Harris BN, Saltzman W. From here to paternity: Neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus) Horm. Behav. 2009;56:220–231. doi: 10.1016/j.yhbeh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Measor KR, Chauke M, Harris BN, Saltzman W. Brief pup exposure induces Fos expresssion in the lateral habenula and serotonergic caudal dorsal raphe nucleus of paternally experienced male California mice (Peromyscus californicus) J. Neurosci. 2010;169:1094–1104. doi: 10.1016/j.neuroscience.2010.06.012. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Korosi A, Harris BN, Perea-Rodriguez JP, Saltzman W. Individual variation in paternal responses of virgin California mice (Peromyscus californicus): behavioral and physiological correlates. Physiol. Biochem. Zool. 2012;85:740–751. doi: 10.1086/665831. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Harris BN, Perea-Rodriguez JP, Saltzman W. Physiological and neuroendocrine responses to chronic variable stress in male California mice (Peromyscus californicus): Influence of social environment and paternal state. Psychoneuroendocrinol. 2013;38:2023–2033. doi: 10.1016/j.psyneuen.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley D. Contributions of paternal care to the growth and development of the young in Peromyscus californicus . Behav. Biol. 1974;11:155–166. doi: 10.1016/s0091-6773(74)90305-8. [DOI] [PubMed] [Google Scholar]
- Espejo EF. Structure of the mouse behaviour on the elevated plus-maze test of anxiety. Behav. Brain Res. 1997;86:15–112. doi: 10.1016/s0166-4328(96)02245-0. [DOI] [PubMed] [Google Scholar]
- Ferré P, Fernández-Teruel A, Escorihuela RM, Driscoll P, Corda MG, Giorgi O, Tobena A. Behavior of the Roman/Verh high-and low-avoidance rat lines in anxiety tests: relationship with defecation and self-grooming. Physiol. Behav. 1995;58:1209–1213. doi: 10.1016/0031-9384(95)02068-3. [DOI] [PubMed] [Google Scholar]
- Gleason ED, Marler CA. Non-genomic transmission of paternal behavior between fathers and sons in the monogamous and biparental California mouse. Proc. R. Soc. B. 2013 doi: 10.1098/rspb.2013.0824. http://dx.doi.org/10.1098/rspb.2013.0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good TC, Harris KK, Ihunnah CA. Corticosteroids as potential mechanism regulating variability in reproductive success in monogamous oldfield mice (Peromyscus polionotus) Physiol. Behav. 2005;86:96–102. doi: 10.1016/j.physbeh.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joëls M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 2011;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- Green SB, Salkind NJ. Using SPSS for Window and Macintosh: Analyzing and understanding data. Upper Saddle River, NJ: Pearson Prentice Hall; 2008. [Google Scholar]
- Gross MR, Shine R. Parental care and mode of fertilization in ectothermic vertebrates. Evolution. 1981;35:775–793. doi: 10.1111/j.1558-5646.1981.tb04937.x. [DOI] [PubMed] [Google Scholar]
- Gubernick DJ, Wright SL, Brown RE. The significance of father’s presence for offspring survival in the monogamous California mouse, Peromyscus californicus . Anim. Behav. 1993;46:539–546. [Google Scholar]
- Gubernick DJ, Teferi T. Adaptive significance of male paternal care in a monogamous mammal. Proc. Biol. Sci. 2000;267:147–150. doi: 10.1098/rspb.2000.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BN, Perea-Rodriguez JP, Saltzman W. Acute effects of corticosterone injection on paternal behavior in California mouse (Peromyscus californicus) fathers. Horm. Behav. 2011;60:666–675. doi: 10.1016/j.yhbeh.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Harris BN, Saltzman W, de Jong TR, Milnes MR. Hypothalamic-pituitary-adrenal (HPA) axis function in the California mouse (Peromyscus californicus): Changes in baseline activity, reactivity, and fecal excretion of glucocorticoids across the diurnal cycle. Gen. Comp. Endocrinol. 2012;179:436–450. doi: 10.1016/j.ygcen.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BN, Saltzman W. Effect of reproductive status on hypothalamic-pituitaryadrenal (HPA) activity and reactivity in male California mice (Peromyscus californicus) Physiol. Behav. 2013;112–113:70–76. doi: 10.1016/j.physbeh.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. Neuropeptides and the evolution of social behavior. Curr. Opin. Neurobiol. 2000;10:784–789. doi: 10.1016/s0959-4388(00)00146-x. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. J. Neurosci. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamic-pituitary-adrenocortical function during acute and chronic stress. Ann. N.Y. Acad. Sci. 2009;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nat. Rev. Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J. Neurosci. Method. 2005;143:169–178. doi: 10.1016/j.jneumeth.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V., Jr Adaptation, exaptation, and constraint: a hormonal perspective. Am. Nat. 1999;154:S4–S25. doi: 10.1086/303280. [DOI] [PubMed] [Google Scholar]
- Kleiman DG, Malcolm JR. The evolution of male parental investment in mammals. In: Gubernick DJ, Klopfer PH, editors. Parental Care in Mammals. New York: Plenum Press; 1981. pp. 347–388. [Google Scholar]
- Lack D. Ecological adaptations for breeding in birds. London, UK: Chapman & Hall; 1968. [Google Scholar]
- Lamb ME. How do fathers affect children’s development?: Let me count the ways. In: Lamb ME, editor. The role of the father in child development. Fifth edition. Hoboken NJ: Wiley; 2010. pp. 1–26. [Google Scholar]
- Landgraf R, Wigger A. Born to be anxious: neuroendocrine and genetic correlates of trait anxiety in HAB rats. Stress. 2003;6:111–119. doi: 10.1080/1025389031000104193. [DOI] [PubMed] [Google Scholar]
- Léonhardt M, Matthews SG, Meaney MJ, Walker CD. Psychological stressors as a model of maternal adversity: Diurnal modulation of corticosterone responses and changes in maternal behavior. Horm. Behav. 2007;51:77–88. doi: 10.1016/j.yhbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Lösel R, Wehling M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 2003;4:46–55. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol. Behav. 2007;90:29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- McCabe TT, Blanchard BD. Three species of Peromyscus. Santa Barbara, CA: Rood Associates; 1950. [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Dev. Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- McEwn BS. Stress, adaptation, and disease: Allostasis and allostatic load. N. Eng. J. Med. 1998;338:171–179. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stressed or stressed out: what is the difference? J. Psychiatry Neurosci. 2005;30:315–318. [PMC free article] [PubMed] [Google Scholar]
- Molina VA, Heyser CJ, Spear LP. Chronic variable stress or chronic morphine facilitates immobility in a forced swim test: reversal by naloxone. Psychopharmacol. 1994;114:433–440. doi: 10.1007/BF02249333. [DOI] [PubMed] [Google Scholar]
- Moore IT, Hopkins WA. Interactions and trade-offs among physiological determinants of performance and reproductive success. Integr. Comp. Biol. 2009;49:441–451. doi: 10.1093/icb/icp081. [DOI] [PubMed] [Google Scholar]
- Murua VS, Gomez RA, Andrea ME, Molina VA. Shuttle-box deficits induced by chronic variable stress: Reversal by imipramine administration. Pharm. Biochem. Behav. 1991;38:125–130. doi: 10.1016/0091-3057(91)90599-w. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress. 2011;14:677–684. doi: 10.3109/10253890.2011.605487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, French JA. Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets (Callithrix kuhlii) Horm. Behav. 2001;39:70–82. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinol. 2006;147:2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovtscharoff W, Jr, Helmeke C, Braun K. Lack of paternal care affects synaptic development in the anterior cingulate cortex. Brain Res. 2006;1116:58–63. doi: 10.1016/j.brainres.2006.07.106. [DOI] [PubMed] [Google Scholar]
- Pan Y, Liu Y, Young KA, Zhang Z, Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci. Lett. 2009;454:67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Reburn CJ, Wynne-Edwards KE. Hormonal changes in males of a naturally biparental and uniparental mammal. Horm. Behav. 1999;36:163–176. doi: 10.1006/hbeh.1998.1509. [DOI] [PubMed] [Google Scholar]
- Ribble DO. The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav. Ecol. Sociobiol. 1991;29:161–166. [Google Scholar]
- Ribble DO. Lifetime reproductive success and its correlates in the monogamous rodent, Peromyscus californicus . J. Anim. Ecol. 1992a;61:457–468. [Google Scholar]
- Ribble DO. Dispersal in a monogamous rodent, Peromyscus californicus . Ecology. 1992b;73:859–866. [Google Scholar]
- Ribble DO, Salvioni M. Social-organization and nest co-occupancy in Peromyscus californicus, a monogamous rodent. Behav. Ecol. Sociobiol. 1990;26:9–15. [Google Scholar]
- Ricklefs RE, Wikelski M. The physiology/life-history nexus. Trends Ecol. Evol. 2002;17:462–468. [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposures alters sperm microRNA content and reprograms offspring stress axis regulation. J. Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol. Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Abbott DH. Effects of elevated circulating cortisol concentrations on maternal behavior in common marmoset monkeys (Callithrix jacchus) Psychoneuroendocrinology. 2009;34:1222–1234. doi: 10.1016/j.psyneuen.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Endocrinology of the Stress-Response. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. Cambridge, Massachusetts: Bradford Book: Massachusetts Institute of Technology; 2002. pp. 409–450. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schradin C, Pillay N. The influence of the father on offspring development in the striped mouse. Behav. Ecol. 2004;16:450–455. [Google Scholar]
- Shellman-Reeve JS. Advantages of biparental care in the wood-dwelling termite Zootermopsis nevadensis . Anim. Behav. 1997;54:163–170. doi: 10.1006/anbe.1996.0412. [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ., Jr . Nonparametric Statistics for the Behavioral Sciences. New York: McGraw-Hill Book Company; 1988. [Google Scholar]
- Silverin B. Corticosterone-binding proteins and behavioral effects of high levels of corticosterone during the breeding period in the pied flycatcher. Gen. Comp. Endocrinol. 1986;64:67–74. doi: 10.1016/0016-6480(86)90029-8. [DOI] [PubMed] [Google Scholar]
- Silverin B. Behavioural and hormonal responses of the pied flycatcher to environmental stressors. Anim. Behav. 1998;55:1411–1420. doi: 10.1006/anbe.1997.0717. [DOI] [PubMed] [Google Scholar]
- Simpkiss JL, Devine DP. Response of the HPA axis after chronic variable stress: effects of novel and familiar stressors. Neuroendocrinol. Lett. 2003;24:97–103. [PubMed] [Google Scholar]
- Spée M, Marchal L, Lazin D, Maho YL, Chastel O, Beaulieu M, Raclot T. Exogenous corticosterone and nest abandonment: A study in a long-lived bird, the Adélie Penguin. Horm. Behav. 2011;60:362–370. doi: 10.1016/j.yhbeh.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Strähle U, Schmid W, Schütz G. Synergistic action of the glucocorticoid receptor with transcription factors. EMBO J. 1988;7:3389. doi: 10.1002/j.1460-2075.1988.tb03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukikara MH, Mota-Ortiz SR, Baldo MV, Felicio LF, Canteras NS. The periaqueductal gray and its potential role in maternal behavior inhibition in response to predatory threats. Behav. Brain. Res. 2010;209:226–233. doi: 10.1016/j.bbr.2010.01.048. [DOI] [PubMed] [Google Scholar]
- Torres S, Nowson C. Relationship between stress, eating behavior and obesity. Nutri. 2007;23:887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Vieira ML, Brown RE. Effects of the presence of the father on pup development in California mice (Peromyscus californicus) Dev. Psychobiol. 2003;42:246–251. doi: 10.1002/dev.10097. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Barash DP. Reproductive suppression among female mammals: Implications for biomedicine and sexual selection theory. Q. Rev. Biol. 1983;58:513–538. doi: 10.1086/413545. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog. Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Wigger A, Sánchez MM, Mathys KC, Ebner K, Frank E, Liu D, Kresse A, Neumann ID, Holsboer F, Plotsky PM, Landgraf R. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology. 2004;29:1–14. doi: 10.1038/sj.npp.1300290. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Silverin B. Effects of corticosterone on territorial behavior of free-living male song sparrows Melospiza melodia . Horm. Behav. 1986;20:405–417. doi: 10.1016/0018-506x(86)90003-6. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. Ecological bases of hormone-behavior interactions: The “Emergency Life History Stage.”. Amer. Zool. 1998;38:191–206. [Google Scholar]
- Wingfield JC, Kitaysky AS. Endocrine responses to unpredictable environmental events: stress or anti-stress hormones? Integ. Comp. Biol. 2002;42:600–609. doi: 10.1093/icb/42.3.600. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: when and how. J Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- Wright SL, Brown RE. The importance of paternal care on pup survival and pup growth in Peromyscus californicus when required to work for food. Behav. Proc. 2002;60:41–52. doi: 10.1016/s0376-6357(02)00101-8. [DOI] [PubMed] [Google Scholar]
- Yamada K, Santo-Yamada Y, Wada K. Restraint stress impaired maternal behavior in female mice lacking neuromedin B receptor (NMB-R) gene. Neurosci. Lett. 2002;330:163–166. doi: 10.1016/s0304-3940(02)00771-1. [DOI] [PubMed] [Google Scholar]
- Zakon HH. The effects of steroid hormones on electrical activity of excitable cells. Trends Neurosci. 1998;21:202–207. doi: 10.1016/s0166-2236(97)01209-5. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Bautista CJ, Deas M, Martínez-Samayoa PM, González-Zamorano M, Ledesma H, Morales J, Larrea F, Nathanielsz PW. A low maternal protein diet during pregnancy and lactation has sex-and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J. Physiol. 2006;571:221–230. doi: 10.1113/jphysiol.2005.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zera AJ, Harshman LG. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 2001;32:95–126. [Google Scholar]