Abstract
Co-infection of hepatitis B virus (HBV) with hepatitis C virus (HCV) is quite common, leading to an increase in morbidity and mortality. As such, HBV vaccination is recommended in HCV-infected individuals. HBV vaccine responses in HCV-infected individuals, however, are often blunted when compared to uninfected populations. The mechanism for this failure of vaccine response in HCV-infected subjects remains unclear. In this study, we investigated the expression and function of an inhibitory receptor, killer cell lectin-like receptor subfamily G member 1 (KLRG1), in regulation of CD4+ T cells and HBV vaccine responses during HCV infection. We demonstrated that KLRG1 was over-expressed on CD4+ T cells from HCV-infected, HBV vaccine non-responders (HBV-NR) compared to those responders (HBV-R). The capacity of CD4+ T cell to proliferate and secrete IL-2 cytokine was inversely associated with the level of KLRG1 expression. Importantly, blocking KLRG1 signaling resulted in a significant improvement of CD4+ T cell proliferation and IL-2 production in HCV-infected, HBV-NR in response to T cell receptor (TCR) stimulation. Moreover, blockade of KLRG1 increased the phosphorylation of Akt (Ser473) and decreased the expression of cell cycle inhibitors p16ink4a and p27kip1, which subsequently enhanced CDK 2 and cyclin E expressions. These results suggest that the KLRG1 pathway impairs CD4+ T cell responses to neo-antigen and induces a state of immune senescence in individuals with HCV infection, raising the possibility that blocking this negative signaling pathway might improve HBV vaccine responses in the setting of chronic viral infection.
Keywords: CD4+ T cells, hepatitis B vaccine, hepatitis C infection, KLRG1, p16ink4a, p27kip1
Introduction
Hepatitis C virus (HCV) infection is a global public health problem, with approximately 200 million peoples chronically infected worldwide. HCV-mediated impairment of the host innate to adaptive immune system is imperative for the development of persistent viral infection and poor vaccine responses, although the underlying mechanisms for this failure require further study [1]. It is well known that antigen presenting cells (APCs) and CD4+ T cells play a pivotal role in the host immune responses to pathogenic infection and vaccination [2–3]. We have previously reported that a differential secretion of IL-12/IL-23 by APCs drives TH17 cell development that may be involved in the vaccine failure observed during HCV infection [2]. In this study, we further explored the mechanisms of CD4+ T cell dysfunction using a model of hepatitis B vaccine failure in the setting of chronic HCV infection in humans.
Given the shared risk factors for transmission, co-infection of hepatitis B virus (HBV) with HCV is common and may lead to a higher rate and more rapid progression to liver cirrhosis and liver cancer [4–5]. Thus, HBV vaccine is recommended to prevent HBV super-infection and its associated increases in morbidity and mortality in HCV-infected individuals. However, vaccine response in this setting is often blunted, with poor response rates to a standard course of HBV vaccinations in chronically HCV-infected patients, especially in those with advanced liver fibrosis and cirrhosis, when compared to healthy populations (40~60% versus 90~95%) [6–7]. Our recent data suggest that even in the setting of relatively preserved hepatic function, seroconversion in HCV-infected individuals is much lower than age-matched healthy subjects [6]. The reasons for vaccine non-response in 5~10% of healthy subjects and 40~60% of HCV-infected individuals remain poorly understood, although several factors are known to play a role, including age (HBV hypo-responsiveness is strongly correlated with aging, with seroconversion rates showing declines as early as age 35 and markedly waning over the ensuing decades), gender, smoking, obesity, and certain human leukocyte antigen (HLA) alleles [8–10]. Given the fact that these HBV vaccine non-responders also have poor recall responses to tetanus toxoid or Candida, it has been suggested that HBV vaccine failure may be due to a defect in CD4+ helper T cells [11–14], regulatory T cells [15], or in antigen presenting cells (APCs) [16–17]; this has, however, remained controversial [18–19]. A number of clinical studies have attempted to correct vaccine non-response by adding adjuvants, altering doses, and administering vaccine through different routes or strategies [20– 24]. These approaches have led to varying degrees of improvement in healthy subjects, but have had limited success in virally-infected individuals, in part due to our incomplete understanding of the mechanisms that inhibit vaccine responses in this setting.
Recently, it has been reported that an inhibitory receptor and marker for cell aging - killer cell lectin-like receptor subfamily G member 1 (KLRG1) - increases substantially on T cells and NK cells during pathogenic infections [25–35]. KLRG1 is a transmembrane protein with an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic domain and a C-type lectin-like domain in extracellular region. The expression and function of KLRG1 during chronic viral infection remain elusive, and thus further defining its role in immune responses in a clinically relevant disease model is significant and timely. In this report, we focus on exploring the role of KLRG1 in regulating CD4+T cell responses to HBV vaccine in HCV-infected individuals. We found that the expression of KLRG1 was significantly up-regulated on CD4+ T cells, leading to an over-expression of cell cycle inhibitors (p16ink4a/p27kip1) and impaired cellular functions, which were more prominent in HBV-vaccine non-responders (HBV-NR) compared with HBV-vaccine responders (HBV-R) during chronic HCV infection.
Materials and Methods
Subjects
The study protocol was approved by a joint institutional review board at East Tennessee State University and James H. Quillen VA Medical Center (ETSU/VA IRB, Johnson City, TN). A total of 48 HCV-infected subjects and 16 uninfected controls without serologic evidence of prior exposure to HBV or HAV were recruited into this study to receive either Engerix® HBV (if HAV antibody negative), or Twinrix® HAV/HBV combination vaccines as appropriate. The HCV-infected subjects comprised 24 HBV vaccine responders (HBV-R, defined as hepatitis B surface antibody titer >10 IU/ml at 1–6 months following a standard course of HBV vaccination), and 24 HBV vaccine non-responders (HBV-NR, defined as hepatitis B surface antibody titer <10 IU/ml at 1–6 months following a standard course of HBV vaccination). All infected subjects were virologically and serologically positive for HCV, prior to the antiviral treatment, with HCV genotype 1 (70%) and type 2 or 3 (30%), and viral load ranging from 12,300 ~ 500,000 IU/ml. The HCV-uninfected subjects comprised 1 spontaneously resolved individual (SRI), 4 sustained virological responder (SVR) following antiviral treatment, and 11 healthy subjects (HS). Written informed consent was obtained from all participants. The mean age of HCV-infected HBV-NR was comparable to HBV-R and control subjects (P>0.05).
Cell isolation and culture
Human peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood of study subjects by Ficoll-density centrifugation with lympho-H (Atlanta biological, Lawrenceville, GA), and then viably cryopreserved in freezing medium in liquid nitrogen. If indicated, CD4+ T cells were further purified from isolated PBMCs by negative selection with magnetic beads using a CD4+ T cell Isolation Kit (Cell purity > 95%, Miltenyi Biotec, Auburn, CA, USA). Cells were cultured with RPMI 1640 containing 10% fetal bovine serum (FBS, LifeTechnologies, Gaithersburg, MD), 100 mg/ml penicillin-streptomycin (Thermo Scientific, Logan, Utah), and 2 mM L-glutamine (Thermo Scientific, Logan, Utah) at 37 °C with 5% CO2 atmosphere for the subsequent experiments.
Flow cytometry
Procedures for intracellular and cytokine staining were performed essentially as described previously [2]. Briefly, purified CD4+ T cells or PBMCs were incubated with or without anti-CD3/CD28 antibodies (1 µg/ml, eBioscience, San Diego, CA) or hepatitis B surface antigen (HBsAg, 2.5 µg/ml, Biospasific, Emeryville, CA) as indicated in the results. For IL-2 intracellular staining, 1 µg/ml Brefeldin A (BioLegend, San Diego, CA) was added 4 h prior to cell harvesting to halt cytokine secretion. The cells were stained for surface marker expression and then fixed and permeabilized using an Inside Stain kit (Miltenyi Biotec, Aubum, CA) according to the manufacturer’s instructions. Four-color flow cytometric analysis was performed using the following antibodies: Alexa Fluor 488-conjugated KLRG1 (13F12) (gift from Dr. Hanspeter Pircher), APC-CD4 / PE-IL-2 (eBioscience, San Diego, CA), and Alexa Fluor 488-Phospho-Akt (ser473) (193H12; Cell Signaling, Danvers, MA). For Akt phosphocytometry staining, purified CD4+ T cells were incubated with KLRG1 blocking or control IgG antibodies in the presence of 1 µg/ml anti-CD3/CD28 for 72 h. The cells were re-stimulated with 3 µg/ml anti-CD3/CD28 and incubated on ice for 15 min, then fixed in 4% paraformaldehyde for 10 min and permeabilized with 90% methanol on ice for 30 min. The cells were sequentially incubated with pAkt (ser473) (D9E, Cell Signaling, Boston, MA) or rabbit isotype control (DA1E, Cell Signaling, Boston, MA) for 1 h at room temperature. Fluorescence minus one (FMO) strategy was used to determine background levels of staining and adjust multicolor compensation for cell gating. The cells analysis was performed on a FACSCalibur or Accuri™ C6 flow cytometer (BD, Franklin Lakes, NJ) using CELLQuest or FlowJo software (Tree Star, Inc., Ashland, OR).
KLRG1 blockade
Purified CD4+ T cells or PBMCs from HCV patients were incubated with anti-human KLRG1 Ab (4 µg/ml, gift from Dr. Hanspeter Pircher) or isotype control IgG in the presence of various concentrations of anti-CD3/CD28 for different times as indicated in the results, then subjected to flow cytometric analysis of intracellular IL-2 and pAkt expressions, CFSE assay, and Western blot.
Proliferation assay
PBMCs were labeled with CFSE (2.5 µM, Invitrogen) for 10 min at 37°C per manufacture’s instruction, washed with complete medium, and cultured (5 × 104) in a 96-well plate in the presence anti-CD3 (1 µg/ml), anti-CD28 (µg/ml) and rhIL-2 (100 U/ml, R&D System). After culture for 5 d, the cells were immune-stained with PE-CD4, ALEXA FLUOR® 488-KLRG1, and analyzed with a FACSCalibur flow cytometer and FlowJo software.
Western blot
Purified CD4+ T cells from HBV-NR of HCV patients were incubated with anti-human KLRG1 or control IgG Ab (4 µg/ml) in the presence of anti-CD3/CD28 (1 µg/ml) for 3 d. The expression of P16ink4a, P27kip1, CDK2, Cyclin E in CD4+ T cell were measured by Western blot. Briefly, the cells were lysed in 1x RIPA lysis buffer (Boston BioProducts Inc, Ashland, MA) supplied with protease inhibitors/phosphorylase inhibitors (Thermo Scientific, Rockford, IL) and EDTA on ice. Cell lysates were centrifuged for 15 min at 4°C and the protein concentrations were measured. Protein samples were thereafter combined with 4x Laemmli sample buffer (Boston BioProducts, Ashland, MA), denatured, and separated by SDS-PAGE. Following transfer to an Amersham Hybond-P membrane (GE Heathcare, Piscataway, NJ), the membrane was blocked by 3% BSA-TBST and probed with P16ink4a (Bethyl Laboratories, Montgomery, TX), P27kip1 (BioLegend, San Diego, CA), Cyclin E (BioLegend), CDK2 (BioLegend) or β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. Finally, the membrane was incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (Millipore, Temecula, CA) and developed by Amersham™ ECL™ Prime Western Blotting Detection Reagents (GE Healthcare Biosciences, Pittsburgh, PA) on Kodak X-OMAT-LS X-ray film (Sigma-Aldrich, St. Louis, MO). Specific bands were quantified by an AlphaEaseFC software (Alpha Innotech Corporation).
RT-PCR
Purified CD4+ T cells from HS, HCV-infected HBV-NR and HBV-R were subject to RT-PCR assay for mRNA level of p16ink4a. Total RNAs were isolated using QIAGEN Rnasy Mini Kit (QIAGEN, Valencia, CA). 1 µg/ml of RNAs was reverse-transcribed (Ambion, Austin, TX) and the cDNAs were amplified by PCR using the following conditions: 95°C 10 min followed by 95°C 45 s, 60°C 45 s, 72°C 45 s for 30 cycles, and then 72°C 5 min. The primers for p16ink4a gene amplifications are sense: 5’-CCA TCA TCA TGA CCT GGA TCG-3’; antisense 5’-AGC ATG GAG CCT TCG GCT GA-3’ (Integrated DNA Technologies, Coralville, IA). β-actin gene serves as control for normalization. The amplified products were analyzed by electrophoresis on 2% agarose gels. Optimal densitometry (OD) value of the DNA products is determined by Gal-Pro Analyzer (Version 4.0, Media Cybernetics, LP).
Statistical analysis
Study results were summarized for each group and results were expressed as the mean ± standard deviation (SD). Comparison between two groups was performed by multiple comparisons testing/least significant difference or Tukey’s procedure depending on the ANOVA F test Prism software (version 4; GraphPad Software) by a nonparametric Mann–Whitney U test. A pairwise t test was used to compare the significance of changes in KLRG1 blockage experiments. Correlations between KLRG1 expression on CD4+ T cells and IL-2 expressions were analyzed using a Pearson Correlation program. Values of p <0.05 (*), p<0.01(**), and p<0.001(***) were considered significant or very significant, respectively.
Results
KLRG1 is over-expressed on CD4+ T cells in HCV-infected, HBV-NR versus HBV-R
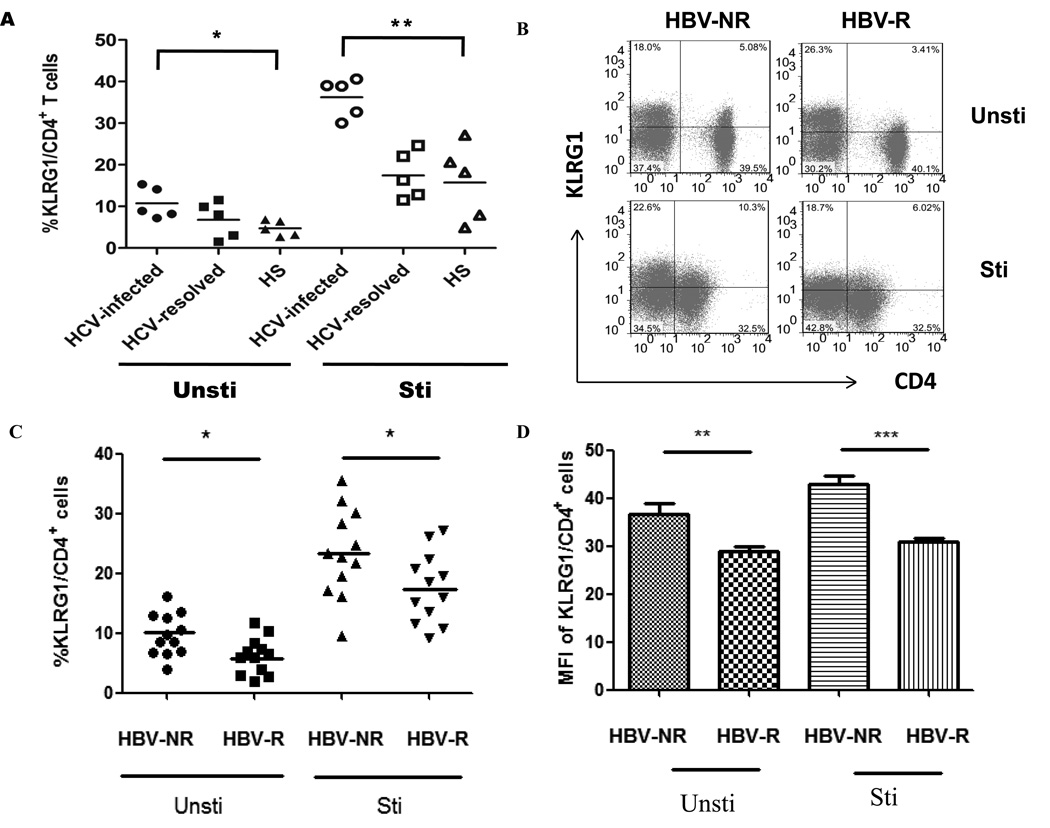
We have previously demonstrated that an impaired APC and T cell functions might play a role in blunting HBV vaccine response in individuals with HCV infection, though the underlying mechanisms for these cell defects is unclear [2, 6]. KLRG1 is an inhibitory receptor expressed on T cells and NK cells and is known as a marker for cell aging or immune senescence [28–30]. As an initial approach to study the role of KLRG1 on CD4+ T cell function and HBV vaccine responses during HCV infection, we first examined KLRG1 expression on CD4+ T cells from HCV-infected versus HCV-uninfected subjects, with or without anti-CD3/CD28 stimulation ex vivo. As shown in Fig. 1A, KLRG1 expression on CD4+ T cells derived from HCV-infected patients was much higher than those HCV-uninfected individuals, but no difference of KLRG1 expression was observed between HCV-resolved and healthy subjects; and as we and others have previous reported for the relationship between viral load and clinical disease progression or immunological changes, no apparent correlation was found between the KLRG1 expression and HCV RNA level. We then examined KLRG1 expression on CD4+T cells from HCV-infected, HBV-NR and HBV-R. As shown in Fig. 1B representative dot plots, after co-stimulation of T cell receptor (TCR) with anti-CD3 and anti-CD28, KLRG1 expression on CD4+ T cells increased in both HBV-NR and HBV-R; however, KLRG1 expression on CD4+ T cells in HBV-NR was significantly higher than that observed in HBV-R with HCV infection, either with or without TCR stimulation. This hold true in terms of the percentage of KLRG1+ CD4+ T cell populations (Fig 1C, P<0.05) as well as the mean fluorescence intensity (MFI) of KLRG1 expression levels on CD4+ T cells from HBV-NR compared to HBV-R, regardless of TCR stimulation (Fig. 1D, P<0.01). These results suggest that HCV infection may induce T cell aging or senescence by up-regulation of KLRG1 expression on CD4+ T cells, which may contribute to HBV vaccine non-responsiveness.
Fig. 1. Higher levels of KLRG1 expression on CD4+ T cells in HBV-NR versus HBV-R in chronically HCV-infected individuals.
PBMCs from HCV-infected, HBV-NR and HBV-R, and HCV-uninfected subjects were stimulated with or without anti-CD3/CD28 for 18 h, immune stained with CD4 and KLRG1, followed by flow cytometric analysis. A) Summary data of percentages of KLRG1+ cell frequency in gated CD4+ T cells from HCV-infected and uninfected individuals, including HCV-resolved and healthy subjects, with or without TCR stimulation ex vivo. B) Representative dot plots of KLRG1 expression on CD4+ T cells from HCV-infected, HBV-NR versus HBV-R, without or with TCR ex vivo stimulation. C) Summary data of percentages of KLRG1+ cell frequency in gated CD4+ T cells from each group. D) MFI of KLRG1 expression level in CD4+ T cells from each group. *P<0.05; **P<0.01; ***P<0.001.
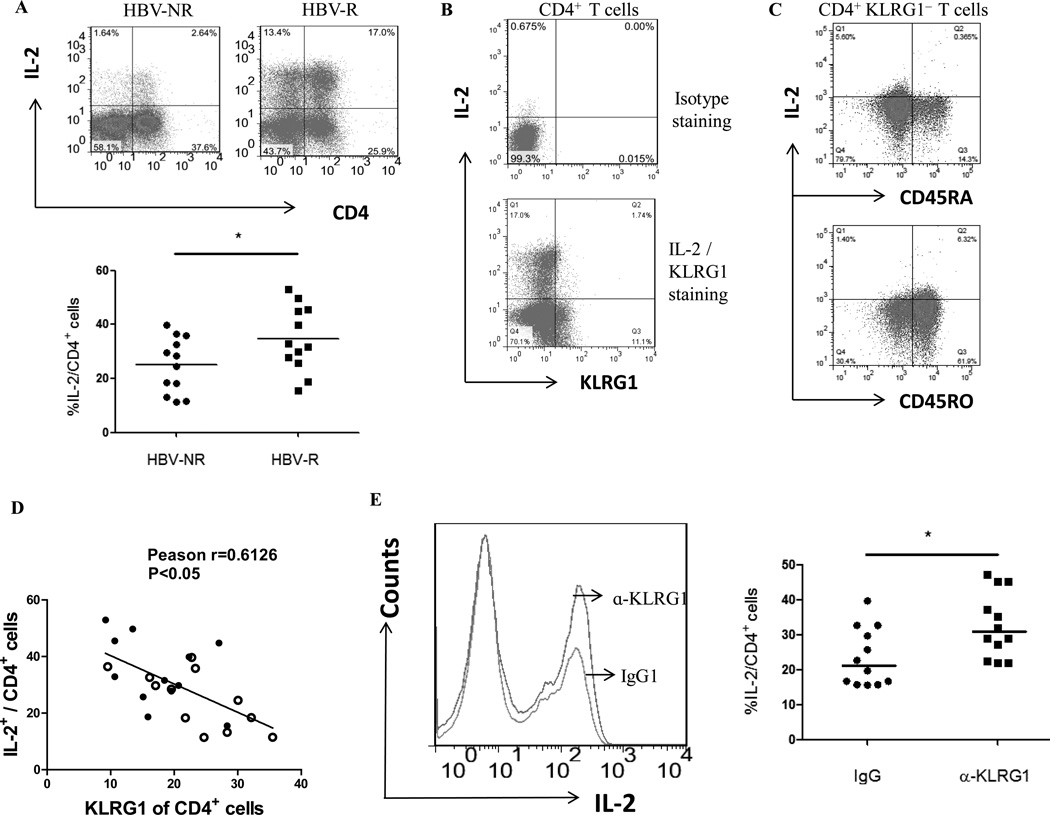
KLRG1 expression is inversely associated with IL-2 expression by CD4+ T cells in HCV-infected, HBV-R versus HBV-NR
Previous work has implicated that a higher level of KLRG1 expression on CD4+ or CD8+ T cells lead to an anergic or senescent status characterized by a decreased level of IL-2 production or cell proliferation [25, 35]. To better understand the effect of KLRG1 expression on human CD4+ T cell function and its role in vaccine responses in the setting of persistent viral infection, we examined IL-2 expression by CD4+ T cells from HBV-NR and HBV-R with chronic HCV infection. As shown in Fig. 2A the representative dot plots and summary data of IL-2 expression by CD4+ T cells, HCV-infected HBV-NR exhibited significantly less IL-2 production when compared with those from HBV-R. We then analyzed the relationship between KLRG1 expression and IL-2 production by purified CD4+ T cells in response to TCR stimulation. As shown in Fig. 2B isotype and IL-2 versus KLRG1 staining, almost all IL-2-producing cells were KLRG1− T cells, whereas majority of KLRG1+ helper T cells failed to produce IL-2. To determine whether IL-2 was produced by antigen-specific CD4+ T cells, we stimulated PBMCs from HCV-infected HBV-R ex vivo with HBsAg for 20 hrs, followed by FACS staining and gated on CD4+ KLRG1− cells, and then analyzed IL-2 expression by CD45RA (naive) versus CD45RO (memory) T cells. As shown in Fig. 2C, IL-2 was primarily expressed by memory rather than naive CD4+ KLRG1− T cells from HBV-R stimulated with HBsAg ex vivo, and the data were reproducible in repeated experiments. Notably, KLRG1 expression was inversely associated with IL-2 production by CD4+ T cells during HCV infection, with HBV-NR CD4+ T cells expressing more KLRG1 and producing less IL-2 compared with that HBV-R (Fig 2D). Importantly, blockade of KLRG1 signaling with specific anti-KLRG1 significantly increased IL-2 production by CD4+ T cells from HBV-NR of HCV-infected individuals when compared with those treated with IgG control (Fig 2E). These results indicate that i) HBV-NR exhibit poor IL-2 production by their CD4+ T cells when compared with HBV-R in HCV-infected individuals; ii) KLRG1 expression is negatively associated with IL-2 production in HCV infected patients and; iii) KLRG1 blockade can recover the impaired IL-2 production by CD4+ T cells from HCV-infected, HBV-NR.
Fig. 2. KLRG1 expression is inversely associated with IL-2 expression by CD4+ T cells in HCV-infected, HBV-NR and HBV-R.
A) PBMCs from chronically HCV-infected, HBV-NR (n=12) and HBV-R (n=12) were stimulated with or without CD3/CD28 for 18 h, immune stained with conjugated antibodies to human IL-2 and CD4, followed by flow cytometric analysis. The representative dot plots of IL-2 expression in CD4+ T cells from HCV-infected, HBV-NR and HBV-R are shown on the left. The percentage of IL-2+ cell frequency in gated CD4+ T cells from HCV infected HBV-NR and HBV-R is shown on the right. Each symbol represents an individual subject, and the horizontal bars represent median values. *P<0.05. B) Representative dot plots of isotype staining and IL-2 versus KLRG1 staining in purified CD4+ T cells from a patient with HCV infection. C) Representative dot plots of IL-2 expression by naive versus memory CD4+ KLRG1− T cells from HCV-infected HBV-R stimulated with HBsAg ex vivo. PBMCs from HCV-infected HBV-R were ex vivo stimulated with HBsAg for 20 h, followed by FACS staining, gated on CD4+ KLRG1− cells, and then analyzed for IL-2 expression by CD45RA (naive) versus CD45RO (memory) T cells. D) The relationship between KLRG1 expression and IL-2 production by CD4+ T cells from HBV-NR (open circles) and HBV-R (filled circles) of HCV-infected individuals, analysis by Pearson Correlation with 2-tailed significance. *P<0.05. E) Purified CD4+ T cells from chronically HCV-infected HBV-NR (n=12) were incubated with anti-KLRG1 or control IgG in the presence of TCR stimulated for 72 h, immune stained with conjugated antibodies to human IL-2, followed by flow cytometric analysis. The representative histogram of IL-2 expression by CD4+ T cells treated with anti-KLRG1 versus isotype IgG control is shown on the left. The percentages of IL-2-expressing CD4+ T cells treated with IgG and anti-KLRG1 are shown on the right. Each symbol represents an individual subject, and the horizontal bars represent median values. *P<0.05.
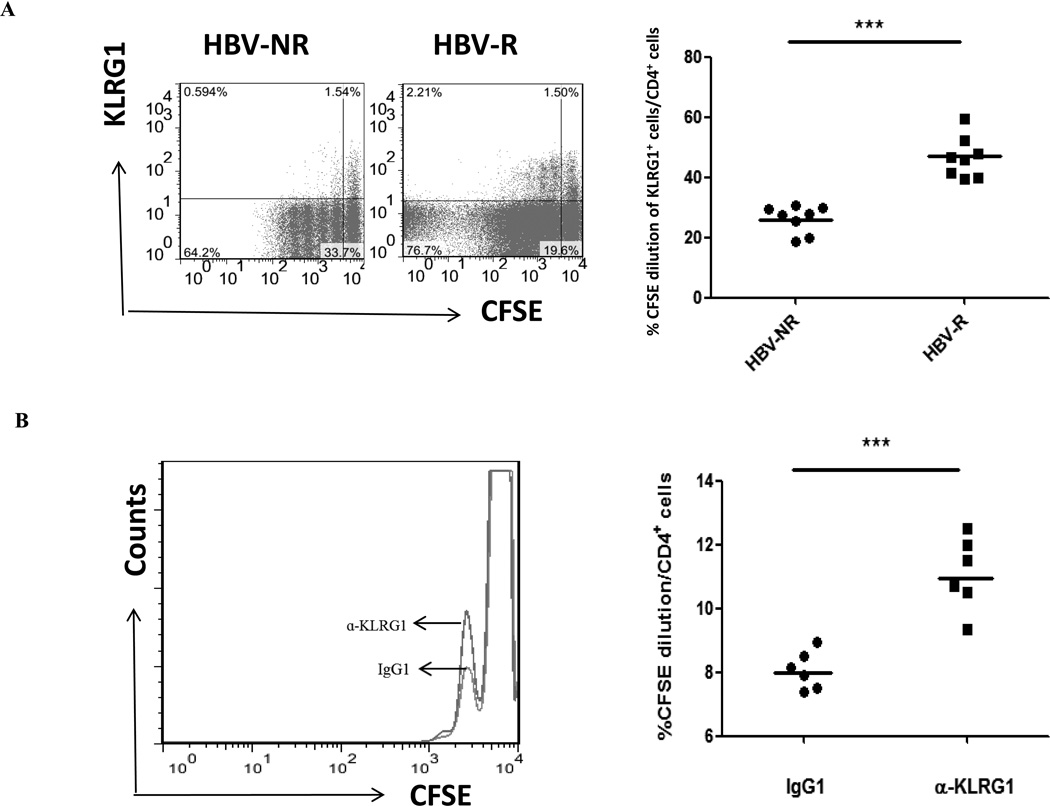
KLRG1 negatively regulates the proliferative capacity of CD4+ T cells that are more significantly suppressed in HCV-infected, HBV-NR than HBV-R
The ability of KLRG1 to inhibit human T cell proliferative capacity is crucial for T cell aging and immune senescence. Although KLRG1 expression on CD8+ T cells has been shown to inversely correlate with their proliferative capacity [25, 35], a role for KLRG1 in regulation of CD4+ T cell proliferation has not been demonstrated in the setting of HCV infection. Here, we compared the relationship of KLRG1 expression and proliferation of CD4+ T cells from HCV-infected, HBV-NR and HBV-R in response to TCR stimulation using CFSE dilution to track cell division. To this end, we labeled PBMCs with CFSE, stimulated with anti-CD3/CD28 in the presence of IL-2 for 5 days, and then double stained with anti-human CD4 and KLRG1 conjugates. CFSE dilution in KLRG1+/− populations in gated CD4+ T cells was shown in Fig. 3A. Consistent with their ability to produce IL-2, the proliferative capacity of CD4+ T cells from HBV-NR was much lower than what seen in HBV-R with HCV infection (P<0.001). Interestingly, most of the proliferative CD4+ T cells were KLRG1− populations, whereas KLRG1+ T cells proliferate with less cycle following 5 days of TCR stimulation compared with KLRG1− T cells; this was particularly true for HBV-NR when compared to HBV-R.
Fig. 3. KLRG1 negatively regulates the proliferative capacity of CD4+ T cells that are repressed more in HBV-NR than HBV-R in HCV-infected individuals.
A) PBMCs isolated from HCV-infected, HBV-NR and HBV-R were labeled with CFSE, stimulated with anti-CD3/CD28 in the presence of IL-2 ex vivo for 5 days, and then double stained with conjugated antibodies to CD4 and KLRG1, followed by flow cytometry analysis. Representative dot plots of cell division as CFSE-dilution in KLRG1+/− populations in the gated CD4+ T cells from HBV-NR and HBV-R are shown on the left panel, and summary data of the KLRG1+ CD4+ T cell CFSE dilution are shown on the right. Each symbol represents an individual subject, and the horizontal bars represent median values. ***p<0.001. B) Overlaid histograms showing the proliferation of purified CD4+ T cells from HBV-NR treated with isotype control IgG1 and anti-KLRG1 mAb. The right panel shows the percentage of CFSE dilution in CD4+ T cells treated with control IgG1 and anti-KLRG1 from 6 HBV-NR. Each symbol represents an individual subject, and the horizontal bars represent median values. ***p<0.001.
To further investigate the role of KLRG1 in control of T cell proliferation, we carried out the T cell proliferation assay with CFSE dilution concomitant with KLRG1 blockade in CD4+ T cells from HBV-NR with HCV infection. In this case, purified CD4+ T cells were used for blocking experiments to avoid the secondary effects from accessory cells in bulk PBMCs. Notably, T cells treated with anti-KLRG1 alone (without TCR stimulation) failed to proliferate, suggesting that the blocking antibody inhibits negative signaling by KLRG1, rather than by directly activating T cells, and its effect requires TCR stimulation to drive cell proliferation. As shown in Fig. 3B, inhibition of KLRG1 signaling in conjunction with TCR stimulation significantly enhanced the proliferative capacity of purified CD4+ T cells, although the cell cycle progression using purified CD4+ T cells was much less than those observed in bulk PBMCs (Fig. 3A), likely due to the lack of other cytokine stimulations from accessory cells. Nevertheless, the cell division events were markedly increased in purified CD4+ T cells from 6 HBV-NR with anti-KLRG1 blockade compared to those treated with isotype control IgG (P<0.001). These results indicate that HBV-NR CD4+ T cells proliferate inadequately, and KLRG1 negatively controls CD4+ T cell proliferation; as such, blocking this inhibitory pathway can rescue T cell proliferation in HCV-infected, HBV-NR.
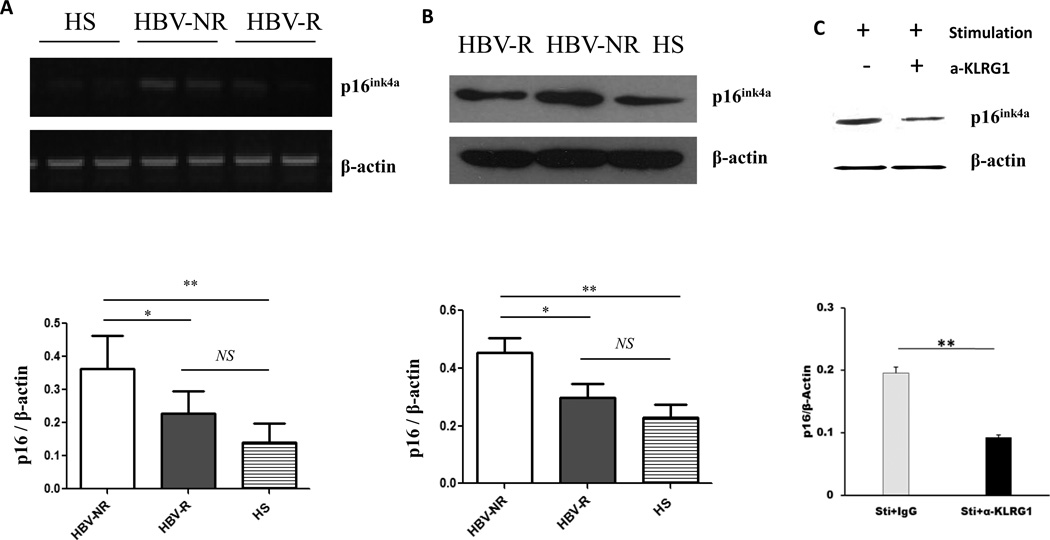
KLRG1 impairs T cell responses via the p16ink4a pathway in HBV-R and HBV-NR with HCV infection
To determine how KLRG1 inhibits CD4+T cell proliferation, we further examined the downstream signaling molecules that control T cell cycle progression. p16ink4a is a well-known cell cycle inhibitor and marker of cell aging [36–38]. The INK4a pathway regulates cell-cycle progression by blocking the cyclin-dependent kinase (CDK) 4/6-Cyclin D complex. This complex increases the phosphorylation of RB, causing it to release the transcription factor E2F. E2F mediates the transcription of several cellular genes that are involved in G1/S progression [38]. Given its key role in T cell proliferation, we thus examined p16ink4a mRNA by RT-PCR and protein expression by Western blot using purified CD4+ T cells from HCV-infected, HBV-R and HBV-NR, and compared with HS. As shown in Fig. 4A, the level of p16ink4a mRNA expression in CD4+ T cells from HBV-NR was significantly higher than that of HBV-R (P<0.05) and HS (P<0.01). Although a relatively higher level of p16ink4a mRNA was detected in CD4+ T cells from HCV-infected, HBV-R versus HS, it was not significantly so. This difference of mRNA expression from three groups of subjects was reproducible in repeated experiments by RT-PCR, and was confirmed by its protein expression levels detected by Western blot (Fig. 4B). Since both KLRG1 and p16ink4a are regarded as markers for cell aging and immune senescence [28–30, 36–38], we next determined whether these two molecules are functionally linked by blocking KLRG1 signaling in CD4+ T cells and subsequently detecting p16ink4a level by Western blot. As shown in Fig. 4C, p16ink4a expression was inhibited in T cells following TCR stimulation in the presence of anti-KLRG1 compared with those treated with IgG control antibody. These results indicate that intracellular p16ink4a is involved in the KLRG1-mediated T cell dysfunction and HBV vaccine non-responsiveness during chronic HCV infection.
Fig. 4. KLRG1 impairs T cell responses via p16ink4a pathway in HBV-R and HBV-NR of HCV-infected individuals.
Purified CD4+ T cells from HCV-infected HBV-NR and HBV-R or HS were incubated with anti-CD3/CD28 for 3 days, total protein and mRNA were extracted from the cell lysates, followed by RT-PCR analysis of p16ink4a mRNA expression (A) and Western blot for p16ink4a protein expression (B). Purified CD4+ T cells from HCV-infected HBV-NR incubated with anti-CD3/CD28 in the presence of anti-KLRG1 or control IgG for 3 days were subjected to Western blot analysis of p16ink4a protein expression (C). β-actin serves as loading control. Representative imaging are shown in the upper panel, and summary densitometry data of p16ink4a expression, corrected by β-actin level, from three independent experiments are shown in the lower panel. *P<0.05; **P<0.01; NS=no significance.
KLRG1 inhibits TCR-mediated Akt (Ser473) phosphorylation and downstream signaling pathways in CD4+ T cells during HCV infection
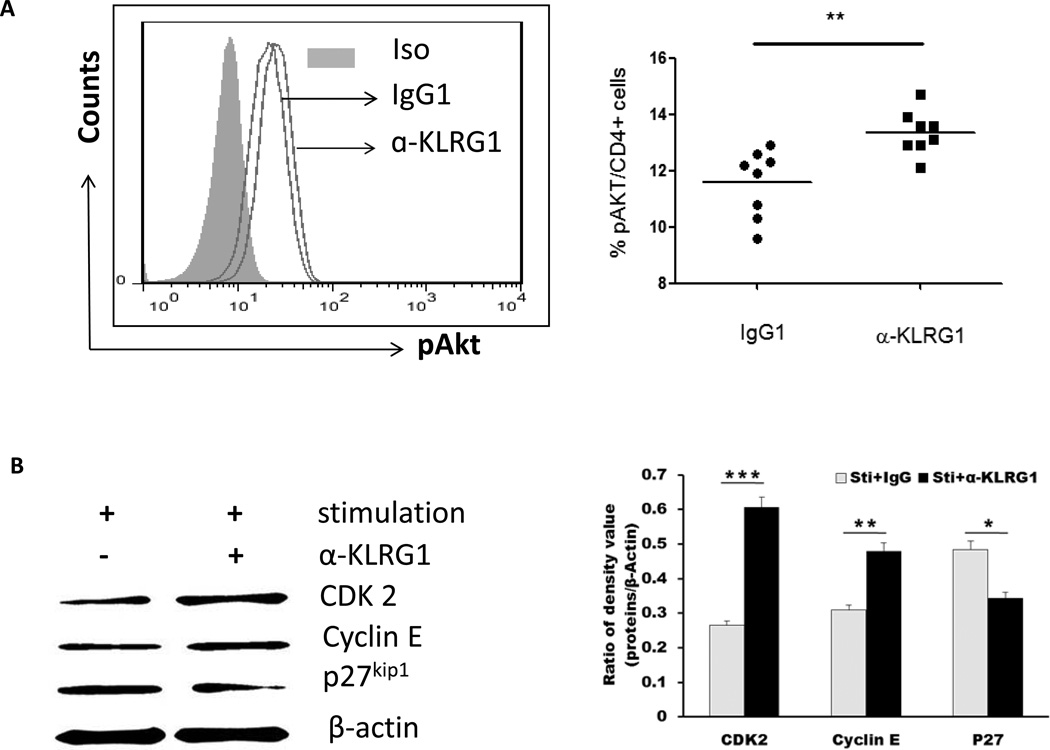
Akt (Thr308 or Ser 473) phosphorylation is the initial event of T cell activation upon TCR stimulation [35–36]. Recent evidence suggests an enhancement of Akt (Ser473) phosphorylation in CD8+CD28−CD27− senescent T cells when PBMCs were stimulated with anti-CD3 in the presence of E-cadherin blocking antibody [35]. To further define the underlying mechanisms involved in the improvement of senescent CD4+ T cell activation and proliferation following blockade of KLRG1 signaling, we detected the phosphorylation of Akt in CD4+ T cells from HCV-infected, HBV-NR by flow cytometry following TCR stimulation in conjunction with KLRG1 blockade. As shown in Fig. 5A, compared with cells treated with control IgG, blockade of KLRG1 signaling significantly enhanced the phosphorylation of pAkt (Ser473) in purified CD4+ T cells from HBV-NR with chronic HCV infection. The data were reproducible using purified CD4+ T cells from 8 HCV-infected, HBV-NR and ex vivo stimulated with anti-CD3/CD28 in the presence of anti-KLRG1 versus control IgG (P<0.01).
Fig. 5. KLRG1 inhibits Akt (Ser473) phosphorylation and downstream signaling pathways in CD4+ T cells during HCV infection.
A) KLRG1 blockade increases pAkt (ser473) phosphorylation in purified CD4+ T cells from HCV-infected HBV-NR. Left panel: representative histograms of pAkt (ser473) expression in the CD4+ T cells treated with control IgG1 or anti-KLRG1. Grey-filled area is isotype control staining. Right panel: summary of the percentages of pAkt (ser473) phosphorylation in CD4+ T cells from HBV-NR following treatment with anti-KLRG1 or control IgG. The horizontal bars indicate the median values for 8 HCV infected HBV-NR. **P<0.01. B) T cells of PBMCs from HCV-infected HBV-NR were stimulated by anti-CD3/CD28 in the presence of anti-KLRG1 or control IgG for 3 days, and total protein were extracted from the cell lysates, followed by Western blot analysis of CDK2, Cyclin E and p27kip1 expressions. β-actin serves as loading control. Representative imaging are shown in the left panel, and summary densitometry data of CDK2, Cyclin E and p27kip1 expressions, corrected by β-actin level, from three independent experiments are shown in the right panel. *P<0.05; **P<0.01; ***P<0.001.
We have previously shown that HCV core protein inhibits T cell cycle progression through Akt/p27kip1 pathway [39– 40]. Thus, the immune senescence mediated by inhibitory receptors, such as KLRG1, may prevent TCR-mediated PI3K/Akt phosphorylation [25, 35]. This in turn lifts the block on forkhead box O (FOXO) transcription factors and activates p27kip1, causing G1-S phase growth arrest by blocking the activations of cyclins and CDKs. Therefore, we expect that improved Akt phosphorylation by blocking KLRG1 signaling will subsequently decrease p27kip1 expression and enhance cyclin and CDK activation. To test this hypothesis, T cells from HCV-infected, HBV-NR were stimulated with anti-CD3/CD28 in the presence of anti-KLRG1 or control IgG. p27kip1 as well as cyclin E and CDK 2 were detected by Western blot. As shown in Fig. 5B, the expression level of p27kip1 was decreased, whereas cyclin E and CDK 2 increased, in cells treated with anti-KLRG1 versus IgG. The results were reproducible in 3 independent experiments using purified cells from different HBV-NR with HCV infection. These results indicate that KLRG1 negatively regulates CD4+ T cell functions by affecting multiple intrinsic regulators, including Akt/p27kip1-related cell cycle proteins. Thus, manipulating these signaling molecules may provide an alternative approach to improving HBV vaccine responsiveness in HCV-infected individuals.
Discussion
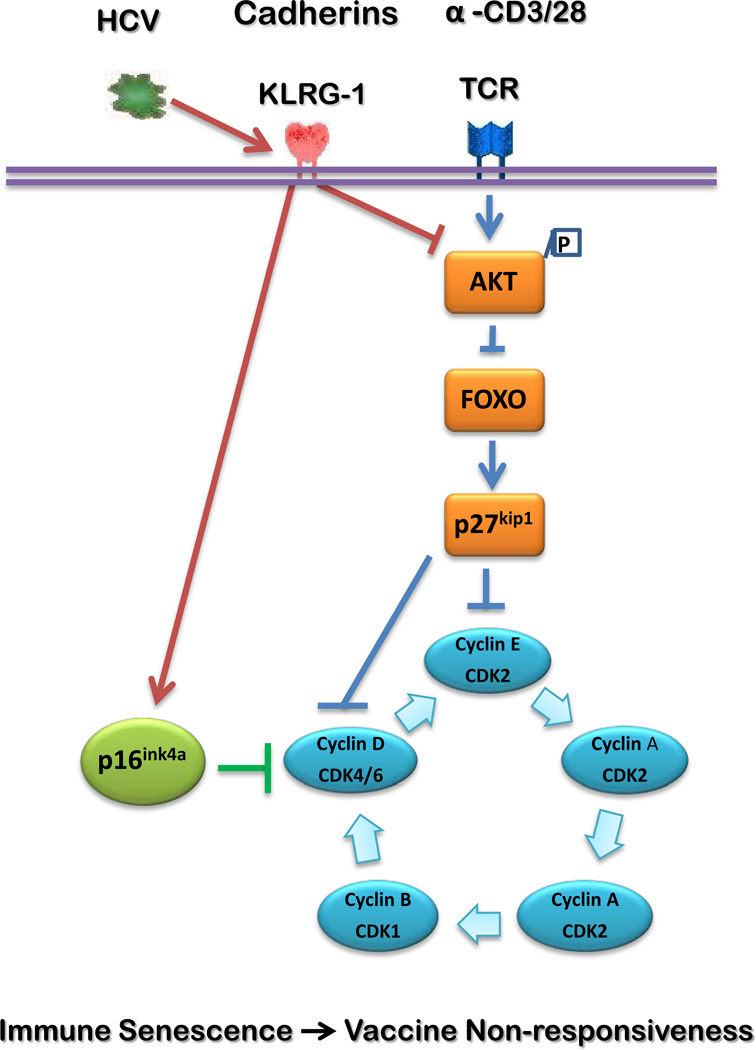
HCV infection is a world-wide infectious disease that can lead to chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. After decades of studies on this immunomodulatory virus, it has become evident that HCV-mediated host immune dysfunction plays a major role in viral persistence as well as disease progression. Notably, like HIV infection, individuals with HCV infection often do not respond well to HBV vaccinations, and efforts to boost vaccine response have proven to be futile - in part due to our poor understanding of the mechanisms that inhibit vaccine response in this setting. Here we use the model of HBV vaccine failure in HCV-infected individuals to explore the role of KLRG1 in regulating CD4+ T cell functions and to examine whether blocking KLRG1 pathway affects immune responses in HCV patients who have failed HBV vaccinations. Our data show that KLRG1 is over-expressed on CD4+ T cells from HBV-NR compared with HBV-R in HCV-infected individuals. Moreover, HCV-infected HBV-NR exhibit a more profound dysfunction of CD4+ T cell proliferation and secretion of IL-2 cytokine when compared with HBV-R, which is inversely associated with the level of KLRG1 expression. Importantly, blocking KLRG1 signaling leads to a significant improvement of CD4+ T cell proliferation and IL-2 production in HCV-infected, HBV-NR in response to TCR stimulation. Additionally, blockade of KLRG1 increases the phosphorylation of Akt (Ser473) and decreases the expression of cell cycle inhibitors p16ink4a and p27kip1, which subsequently enhances CDK 2 and cyclin E expressions. These results suggest that KLRG1 impairs CD4+ T cell responses via p16ink4a and p27kip1 pathways, and the blunted HBV vaccine response during HCV infection might be a result, at least in part, of virus-mediated premature cell aging through KLRG1 signaling pathway. Based on this study, we propose a model, as depicted in Fig. 6, to illustrate the role of KLRG1 in impairing CD4+ T cell function and HBV vaccine responses in the setting of chronic HCV infection.
Fig. 6. A putative model for HCV-induced, KLRG1-mediated CD4+ T cell dysfunction via p16ink4a and p27kip1 pathways.
HCV-driven up-regulation of KLRG1 expression on CD4+ T cells inhibits TCR-induced Akt phosphorylation. This in turn lifts the block on forkhead box O (FOXO) transcription factors and activates p27kip1, causing G1 growth arrest by blocking the activations of cyclins and cyclin-dependent kinases (CDKs) [39, 40]. HCV infection also up-regulates p16ink4a expression in CD4+ T cells, which is linked to KLRG1 signaling. This in turn blocks the activations of Cyclins and CDKs, causing G1 growth arrest [1]. This KLRG1-mediated p16ink4a/p27kip1 alteration inhibits CD4+ T cell responses that are likely involved in the vaccine responses during chronic HCV infection. Therefore, blocking KLRG1 and its downstream signaling molecules may provide a novel approach to boosting vaccine responses in virally infected individuals.
While the role of KLRG1 in immune senescence has been emerging, its link to chronic infection and vaccine response is rather novel. Recently, Lindenstrom et al. [41] employed a BCG vaccine model to demonstrate that CD4+KLRG1− IL-2 secreting subsets were central to increased protective efficacy. Their data suggested that the waning of memory immunity that occurred as tuberculous infections became chronic was associated with a loss of IL-2-producing CD4 cells and an increase in KLGR1+ anergic T cells. These findings could be reversed by vaccine boosting leading to selection induction, expansion and maintenance of CD4+ KLRG1− memory T cells. Our study supports this concept, in that chronic HCV infection leads to blunted vaccine responses characterized by high KLRG1 expression on CD4+T cells with impaired ability to proliferate and to produce IL-2.
It is well-recognized that elderly individuals are more susceptible to infections and have decreased responses to vaccinations [42]. In general, the immune responses in the elderly are significantly less robust than responses by younger individuals in magnitude, duration, and quality of response, leading to generally poor efficacy in terms of vaccine responses [43–45]. Remarkably, vaccine responses, such as HBV, influenza, and pneumococcal vaccine, are also impaired in individuals with chronic viral infection, noted in the setting of HIV, HCV, and CMV infections [46–47]. It would be imperative to characterize the mechanisms underlying vaccine non-responsiveness in chronically viral infected individuals, as they are the individuals most susceptible to super-infection-mediated increases in morbidity and mortality. Most vaccines aim to develop good neutralizing antibody, which involves the activation of APCs, the interaction of the activated CD4+ T cells with their cognate B cells to form germinal centers, and maturation of the B cells to produce specific antibodies. Each of these steps can be affected by viral infection and/or cell aging. The mechanisms for virus-mediated impairment of CD4+ T cells response, in particular its ability of proliferation and IL-2 production, is a fascinating yet unclear research theme because it bridges the innate and adaptive immune (vaccine) responses [45].
To elucidate the mechanisms by which persistent viral infection mediates host immune dysfunction, and ultimately leads to blunted vaccine responses, we have previously explored the role of HCV-mediated immune exhaustion in HBV vaccine responses during HCV infection. We have shown that PD-1 and Tim-3, markers for cell exhaustion, are over-expressed on APC and T cells of HBV-NR versus HBV-R in HCV-infected individuals [2, 6]. In addition to inducing immune exhaustion that impairs essential functional activity; persistent viral infections can also lead to immune senescence, with accelerated premature cell aging due to telomere erosion or unrepaired DNA damage [26, 48]. Here we further demonstrate that KLRG1 and p16ink4a, markers for cell aging, are up-regulated in CD4+ T cells in HBV-NR compared with HBV-R with HCV infection. We thus believe that HCV may employ two critical cell regulatory mechanisms, cell exhaustion and cell aging, through up-regulation of two different inhibitory pathways, PD-1/Tim-3 and KLRG1/p16ink4a, to dampen the functions of immune cells to respond appropriately to vaccines during chronic infection.
It is possible that modulating these inhibitory receptors like Tim-3 and KLRG1 that are preferentially expressed in highly differentiated T cells during viral infection may boost immune responses. Although there has been substantial progress in identifying the mechanisms that regulate both processes separately, it is unclear how these processes interrelate, and whether blocking pathways that maintain either the exhausted or the senescent state, or both, can boost vaccine responses, especially in virally-infected individuals. A recent study demonstrated that young HIV-infected patients, with less than 4 years duration of infection, have early immune exhaustion leading to premature aging and senescence comparable to the elderly, suggesting virus induces premature immune senescence associated with high rates of immune exhaustion following short-term infection [48]. We are also exploring the mechanisms underlying how HCV infection induces immune exhaustion and immune senescence that are essential for developing specific strategies to improve vaccine responses in the setting of chronic viral infection. These studies have led to an intersecting field of virus-mediated immune exhaustion and immune senescence with regard to vaccine responses.
In summary, this study delineates the mechanisms of persistent infection-induced immune senescence or cell aging by measuring T cell response to neo-antigen stimulation. Our findings suggest that KLRG1 expression is up-regulated and associated with CD4+ T cell dysfunctions that are more prominent in HBV-NR compared to HBV-R in HCV-infected individuals. HCV-induced KLRG1 expression impairs CD4+ T cell responses via p16ink4a and p27kip1 pathways (Fig 6); thus inhibition of the KLRG1 pathway and downstream signaling molecules in CD4+T cell can be therapeutically exploited for improving human immune (vaccine) responses. Our data presented here suggest that KLRG1 may be used as a potential predictor for vaccine responses and progression of immune status in chronic HCV infection. Further characterization of KLRG1 expression and functional changes in CD4+ T cells will not only help us to better understand the pathogenesis of chronic HCV infection, but may reveal a potential therapeutic target for managing this disease.
Acknowledgements
We greatly appreciate the generous support from Dr. Hanspeter Pircher, Institute of Medical Microbiology and Hygiene, Department of Immunology, University of Freiburg, Freiburg, Germany, for providing anti-KLRG1 antibodies. This publication is the result of work supported with resources and the use of facilities at the James H. Quillen Veterans Affairs Medical Center. The contents in this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
This work was supported by an NIH NIDDK grant to ZQY/JPM (R01DK093526). Dr. Shi L is a visiting scholar, partially supported by the Guanghua Foundation of Xian Jiaotong University, China. Dr. Cheng YQ is a visiting scholar, partially supported by Beijing 302 Hospital, Beijing, China; Dr. Ying RS, a visiting scholar, holds a grant for viral hepatitis research from Guangzhou Municipal Health Bureau, China.
References
- 1.Yao ZQ, Moorman JP. Immune exhaustion and immune senescence – two distinct pathways for HBV vaccine failure during HCV and/or HIV infection (Review) AITE. 2013;61:193–201. doi: 10.1007/s00005-013-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang JM, Ma CJ, Li GY, Wu XY, Thayer P, Greer P, Smith AM, High KP, Moorman JP, Yao ZQ. Tim-3 alters the balance of IL-12/IL-23 and drives TH17 cells: Role in hepatitis B vaccine failure during hepatitis C infection. Vaccine. 2013;31:2238–2245. doi: 10.1016/j.vaccine.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moorman JP, Wang JM, Zhang Y, Ji XJ, Ma CJ, Wu XY, Jia ZS, Wang KS, Yao ZQ. Tim-3 pathway controls regulatory and effector T cell balance during hepatitis C virus infection. J Immunol. 2012;189:755–766. doi: 10.4049/jimmunol.1200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarski JP, Bohn B, Bastie A, Pawlotsky JM, Baud M, Bost-Bezeaux F, Tran VNJ, Seigneurin JM, Buffet C, Dhumeaux D. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27–33. doi: 10.1016/s0168-8278(98)80198-0. [DOI] [PubMed] [Google Scholar]
- 5.Duberg AS, Törner A, Davidsdóttir L, Aleman S, Blaxhult A, Svensson A, Hultcrantz R, Bäck E, Ekdahl K. Cause of death in individuals with chronic HBV and/or HCV infection, a nationwide community-based register study. J Viral Hepat. 2008;15:538–550. doi: 10.1111/j.1365-2893.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 6.Moorman JP, Zhang CL, Ni L, Ma CJ, Zhang Y, Wu XY, Thayer P, Islam TM, Borthwick T, Yao ZQ. Impaired hepatitis B vaccine responses during chronic hepatitis C infection: involvement of the PD-1 pathway in regulating CD4+ T cell responses. Vaccine. 2011;29:3169–3176. doi: 10.1016/j.vaccine.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer ES, Hofmann C, Smith PG, Shiffman ML, Sterling RK. Response to hepatitis A and B vaccine alone or in combination in patients with chronic hepatitis C virus and advanced fibrosis. Dig Dis Sci. 2009;54:2016–2025. doi: 10.1007/s10620-009-0867-4. [DOI] [PubMed] [Google Scholar]
- 8.Fisman DN, Agrawal D, Leder K. The effect of age on immunologic response to recombinant hepatitis B vaccine: a meta-analysis. Clin Infect Dis. 2002;35:1368–1375. doi: 10.1086/344271. [DOI] [PubMed] [Google Scholar]
- 9.Godkin A, Davenport M, Hill AV. Molecular analysis of HLA class II associations with hepatitis B virus clearance and vaccine non-responsiveness. Hepatology. 2005;41:1383–1390. doi: 10.1002/hep.20716. [DOI] [PubMed] [Google Scholar]
- 10.De Silvestri A, Pasi A, Martinetti M, Belloni C, Tinelli C, Rondini G, Salvaneschi L, Cuccia M. Family study of non-responsiveness to hepatitis B vaccine confirms the importance of HLA class III C4A locus. Genes Immun. 2001;2:367–372. doi: 10.1038/sj.gene.6363792. [DOI] [PubMed] [Google Scholar]
- 11.Salazar M, Deulofeut H, Granja C, Deulofeut R, Yunis DE, Marcus-Bagley D, Awdeh Z, Alper CA, Yunis EJ. Normal HBsAg presentation and T-cell defect in the immune response of non-responders. Immunogenetics. 1995;41:366–374. doi: 10.1007/BF00163994. [DOI] [PubMed] [Google Scholar]
- 12.Albarran B, Goncalves L, Salmen S, Borges L, Fields H, Soyano A, Montes H, Berrueta L. Profiles of NK, NKT cell activation and cytokine production following vaccination against hepatitis B. APMIS. 2005;113:526–535. doi: 10.1111/j.1600-0463.2005.apm_191.x. [DOI] [PubMed] [Google Scholar]
- 13.Goncalves L, Albarran B, Salmen S, Borges L, Fields H, Montes H, Soyano A, Diaz Y, Berrueta L. The non-response to hepatitis B vaccination is associated with impaired lymphocyte activation. Virology. 2004;326:20–28. doi: 10.1016/j.virol.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 14.Bauer T, Jilg W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine. 2006;24:572–577. doi: 10.1016/j.vaccine.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 15.Bauer T, Günther M, Bienzle U, Neuhaus R, Jilg W. Vaccination against hepatitis B in liver transplant recipients: pilot analysis of cellular immune response shows evidence of HBsAg-specific regulatory T cells. Liver Transpl. 2007;13:434–442. doi: 10.1002/lt.21061. [DOI] [PubMed] [Google Scholar]
- 16.Höhler T, Stradmann-Bellinghausen B, Starke R, Sänger R, Victor A, Rittner C, Schneider PM. C4A deficiency and nonresponse to hepatitis B vaccination. J Hepatol. 2002;37:387–392. doi: 10.1016/s0168-8278(02)00205-2. [DOI] [PubMed] [Google Scholar]
- 17.Verkade MA, van Druningen CJ, Op de Hoek CT, Weimar W, Betjes MG. Decreased antigen-specific T-cell proliferation by moDC among hepatitis B vaccine non-responders on haemodialysis. Clin Exp Med. 2007;7:65–71. doi: 10.1007/s10238-007-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desombere I, Cao T, Gijbels Y, Leroux-Roels G. Non-responsiveness to hepatitis B surface antigen vaccines is not caused by defective antigen presentation or a lack of B7 co-stimulation. Clin Exp Immunol. 2005;140:126–137. doi: 10.1111/j.1365-2249.2004.02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desombere I, Hauser P, Rossau R, Paradijs J, Leroux-Roels G. Nonresponders to hepatitis B vaccine can present envelope particles to T lymphocytes. J Immunol. 1995;154:520–529. [PubMed] [Google Scholar]
- 20.Jacques P, Moens G, Desombere I, Dewijngaert J, Leroux-Roels G, Wettendorff M, Thoelen S. The immunogenicity and reacogenicity profile of a candidate hepatitis B vaccine in an adult vaccine non-responder population. Vaccine. 2002;20:3644–3649. doi: 10.1016/s0264-410x(02)00397-3. [DOI] [PubMed] [Google Scholar]
- 21.Kim MJ, Nafziger AN, Harro CD, Keyserling HL, Ramsey KM, Drusano GL, Bertino JS., Jr Revaccination of healthy nonresponders with hepatitis B vaccine and prediction of seroprotection response. Vaccine. 2003;21:1174–1179. doi: 10.1016/s0264-410x(02)00626-6. [DOI] [PubMed] [Google Scholar]
- 22.Ramon JM, Bou R, Oromi J. Low-dose intramuscular revaccination against hepatitis B. Vaccine. 1996;14:1647–1650. doi: 10.1016/s0264-410x(96)00134-x. [DOI] [PubMed] [Google Scholar]
- 23.Rahman F, Dahmen A, Herzog-Hauff S, Böcher WO, Galle PR, Löhr HF. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology. 2000;31:521–527. doi: 10.1002/hep.510310237. [DOI] [PubMed] [Google Scholar]
- 24.Nyström J, Cardell K, Björnsdottir TB, Fryden A, Hultgren C, Sällberg M. Improved cell mediated immune responses after successful re-vaccination of non-responders to the hepatitis B virus surface antigen (HBsAg) vaccine using the combined hepatitis A and B vaccine. Vaccine. 2008;26:5967–5972. doi: 10.1016/j.vaccine.2008.08.054. [DOI] [PubMed] [Google Scholar]
- 25.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100:3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 26.Ibegbu CC, Xu YX, Harris W, Maggio D, Miller JD, Kourtis AP. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J Immunol. 2005;174:6088–6094. doi: 10.4049/jimmunol.174.10.6088. [DOI] [PubMed] [Google Scholar]
- 27.Robbins SH, Terrizzi SC, Sydora BC, Mikayama T, Brossay L. Differential regulation of killer cell lectin-like receptor G1 expression on T cells. J Immunol. 2003;170:5876–5885. doi: 10.4049/jimmunol.170.12.5876. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang Q, Wagner WM, Voehringer D, Wikby A, Klatt T, Walter S, Müller CA, Pircher H, Pawelec G. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1) Exp Gerontol. 2003;38:911–920. doi: 10.1016/s0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 29.Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 30.McMahon CW, Zajac AJ, Jamieson AM. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8(+) T cells. J Immunol. 2002;169:1444–1452. doi: 10.4049/jimmunol.169.3.1444. [DOI] [PubMed] [Google Scholar]
- 31.Wilson DC, Matthews S, Yap GS. IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1 effector subpopulations during Toxoplasma gondii infection. J Immunol. 2008;180:5935–5945. doi: 10.4049/jimmunol.180.9.5935. [DOI] [PubMed] [Google Scholar]
- 32.Thimme R, Appay V, Oschella MK, Panther E, Roth E, Hislop AD, Rickinson AB, Rowland-Jones SL, Blum HE, Pircher H. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J Virol. 2005;79:12112–12116. doi: 10.1128/JVI.79.18.12112-12116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bengsch B, Spangenberg HC, Kersting N, Neumann-Haefelin C, Panther E, von Weizsacker F, Blum HE, Pircher H, Thimme R. Analysis of CD127 and KLRG1 expression on Hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver. J Virol. 2007;81:945–952. doi: 10.1128/JVI.01354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thumme R. Co-expression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathogens. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henson SM, Franzese O, Macaulay R, Libri V, Azevedo RI, Kiani-Alikhan S, Plunkett FJ, Masters JE, Jackson S, Griffiths SJ, Pircher HP, Soares MV, Akbar AN. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;113:6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 36.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathway regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin N, Raguz S, Dharmalingam G, Gil J. Co-regulation of senescence-associated genes by oncogenic homeobox proteins and polycomb repressive complexes. Cell Cycle. 2013;12(14):2194–2199. doi: 10.4161/cc.25331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiocca EA. Oncolytic viruses. Nat Rev Cancer. 2002;2:938–950. doi: 10.1038/nrc948. [DOI] [PubMed] [Google Scholar]
- 39.Yao ZQ, Eisen-Vandervelde A, Waggoner S, Cale EM, Hahn YS. Direct binding of HCV core to gC1qR on CD4+ and CD8+ T cells leads to impaired activation of Lck and Akt. J Virol. 2004;78:6409–6419. doi: 10.1128/JVI.78.12.6409-6419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao ZQ, Eisen-Vandervelde A, Ray S, Hahn YS. HCV core/gC1qR interaction arrests T cell cycle progression through stabilization of the cell cycle inhibitor p27kip1. Virology. 2003;314:271–282. doi: 10.1016/s0042-6822(03)00419-7. [DOI] [PubMed] [Google Scholar]
- 41.Lindenstrom T, Hell Knudsen NP, Agger EM, Andersen P. Control of Chronic Mycobacterium tuberculosis Infection by CD4 KLRG1- IL-2 Secreting Central Memory Cells. J Immunol. 2013;190:6311–6319. doi: 10.4049/jimmunol.1300248. [DOI] [PubMed] [Google Scholar]
- 42.Wick G, Jansen-Durr P, Berger P, Blasko I, Grubeck-Loebenstein B. Diseases of aging. Vaccine. 2000;18:1567–1583. doi: 10.1016/s0264-410x(99)00489-2. [DOI] [PubMed] [Google Scholar]
- 43.Hayward AR, Buda K, Levin MJ. Immune response to secondary immunization with live or inactivated VZV vaccine in elderly adults. Viral Immunol. 1994;7:31–36. doi: 10.1089/vim.1994.7.31. [DOI] [PubMed] [Google Scholar]
- 44.Stepanova L, Naykhin A, Kolmskog C, Jonson G, Barantceva I, Bichurina M, Kubar O, Linde A. The humoral response to live and inactivated influenza vaccines administered alone and in combination to young adults and elderly. J Clin Virol. 2002;24:193–201. doi: 10.1016/s1386-6532(01)00246-3. [DOI] [PubMed] [Google Scholar]
- 45.Lefebvre JS, Haynes L. Vaccine strategies to enhance immune responses in the aged. Cur Opin Immu. 2013;25:1–6. doi: 10.1016/j.coi.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, Kottilil S, Gezmu M, Follmann D, Vodeiko GM, Levandowski RA, Mican JM, Fauci AS. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Barradas MC, Alexandraki I, Nazir T, Foltzer M, Musher DM, Brown S, Thornby J. Response of human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy to vaccination with 23-valent pneumococcal polysaccharide vaccine. Clin Infect Dis. 2003;37:438–447. doi: 10.1086/375841. [DOI] [PubMed] [Google Scholar]
- 48.Ferrando-Martínez S, Ruiz-Mateos E, Romero-Sánchez MC, Muñoz-Fernández MA, Viciana PP, Genebat M, Leal M. HIV Infection-Related Premature Immunosenescence: High Rates of Immune exhaustion after short time infection. Curr HIV Res. 2011;9:289–294. doi: 10.2174/157016211797636008. [DOI] [PubMed] [Google Scholar]