Abstract
Carbamyl phosphate synthetase (from Escherichia coli) consists of a 7.3S protomeric unit that contains one heavy polypeptide chain (molecular weight about 130,000) and one light chain (molecular weight about 42,000). The heavy and light chains were separated by gel filtration in the presence of 1 M potassium thiocyanate. In contrast to the native enzyme and the reconstituted enzyme (prepared by mixing the separated heavy and light chains), the heavy chain does not catalyze glutamine-dependent carbamyl phosphate synthesis, although it does catalyze the synthesis of carbamyl phosphate from ammonia. The heavy chain also catalyzes two of the partial reactions catalyzed by the intact enzyme; i.e., the bicarbonate-dependent cleavage of ATP and the synthesis of ATP from ADP and carbamyl phosphate. Both positive (ammonia, ornithine, IMP) and negative (UMP) allosteric regulatory sites are located on the heavy chain. The only catalytic activity exhibited by the light chain is the hydrolysis of glutamine. A model is presented according to which glutamine binds to the light chain, which is followed by release of nitrogen from the amide group for use by the heavy chain. The findings suggest that glutamine-dependent carbamyl phosphate synthetase (and perhaps other glutamine amidotransferases) arose in the course of evolution by a combination of a primitive ammonia-dependent synthetic enzyme and a glutaminase; this combination may have been associated with a change from ammonia to glutamine as the principal source of nitrogen.
Keywords: nonidentical subunits, salt dissociation, E. coli
Full text
PDF

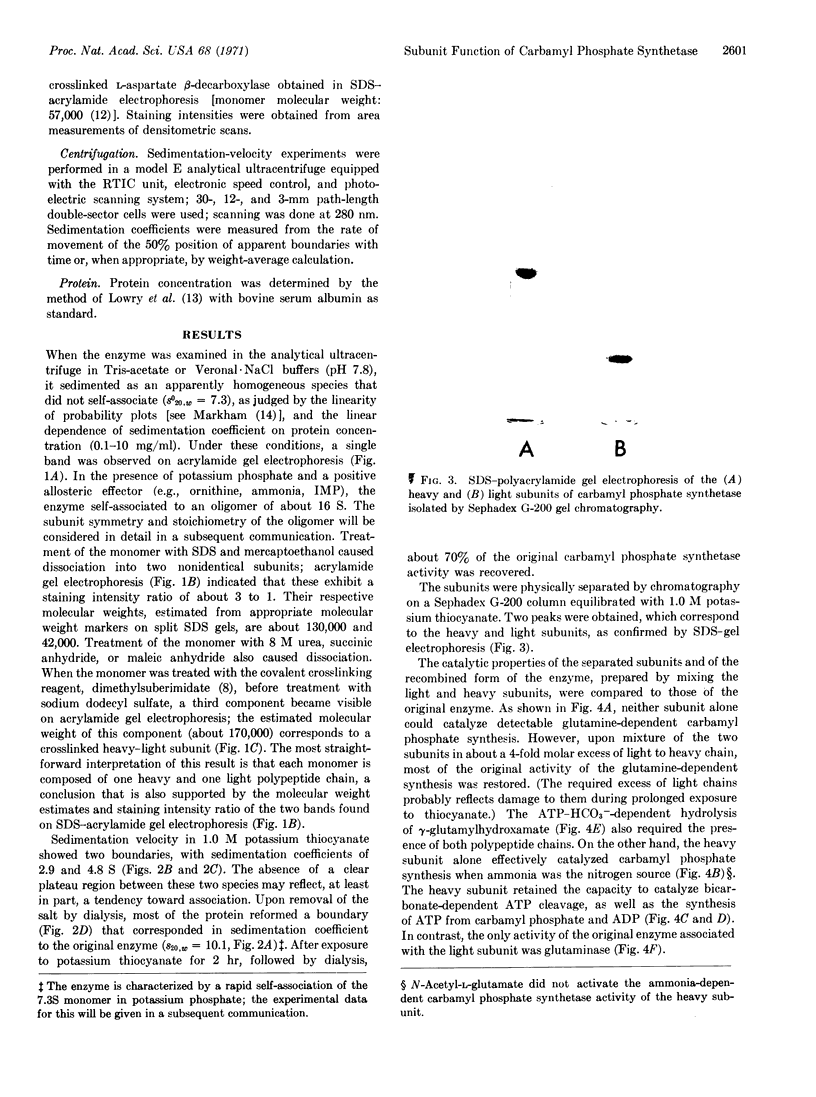
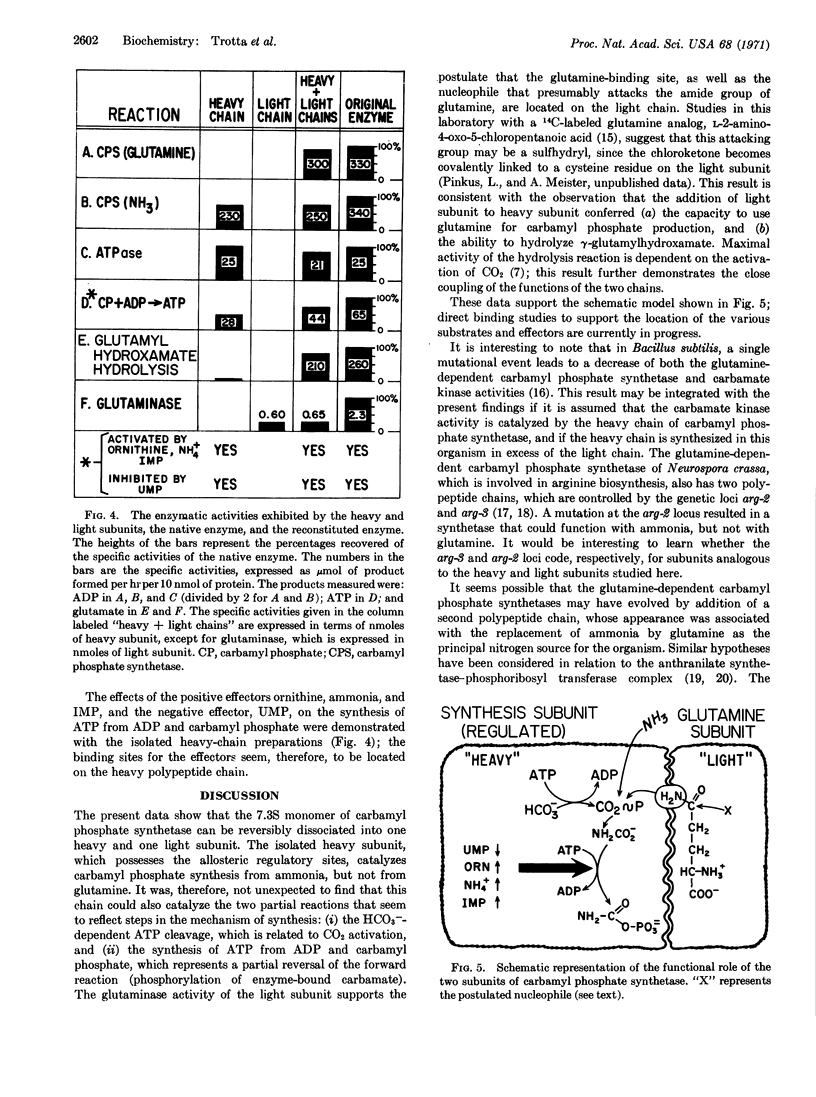

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Marvin S. V. Effect of ornithine, IMP, and UMP on carbamyl phosphate synthetase from Escherichia coli. Biochem Biophys Res Commun. 1968 Sep 30;32(6):928–934. doi: 10.1016/0006-291x(68)90116-2. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Bicarbonate-dependent cleavage of adenosine triphosphate and other reactions catalyzed by Escherichia coli carbamyl phosphate synthetase. Biochemistry. 1966 Oct;5(10):3157–3163. doi: 10.1021/bi00874a012. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Control of Escherichia coli carbamyl phosphate synthetase by purine and pyrimidine nucleotides. Biochemistry. 1966 Oct;5(10):3164–3169. doi: 10.1021/bi00874a013. [DOI] [PubMed] [Google Scholar]
- Bowers W. F., Czubaroff V. B., Haschemeyer R. H. Subunit structure of L-aspartate beta-decarboxylase from Alcaligenes faecalis. Biochemistry. 1970 Jun 23;9(13):2620–2625. doi: 10.1021/bi00815a009. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Carbamyl phosphate synthesis in Neurospora crassa. II. Genetics, metabolic position, and regulation of arginine-specific carbamyl phosphokinase. Biochim Biophys Acta. 1965 Aug 24;107(1):54–68. doi: 10.1016/0304-4165(65)90388-0. [DOI] [PubMed] [Google Scholar]
- Huang M., Gibson F. Biosynthesis of 4-aminobenzoate in Escherichia coli. J Bacteriol. 1970 Jun;102(3):767–773. doi: 10.1128/jb.102.3.767-773.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang L. H., Zalkin H. Multiple forms of anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase from Salmonella typhimurium. J Biol Chem. 1971 Apr 25;246(8):2338–2345. [PubMed] [Google Scholar]
- Issaly I. M., Issaly A. S., Reissig J. L. Carbamyl phosphate biosynthesis in Bacillus subtilis. Biochim Biophys Acta. 1970 Mar 18;198(3):482–494. doi: 10.1016/0005-2744(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Khedouri E., Anderson P. M., Meister A. Selective inactivation of the glutamine binding site of Escherichia coli carbamyl phosphate synthetase by 2-amino-4-oxo-5-chloropentanoic acid. Biochemistry. 1966 Nov;5(11):3552–3557. doi: 10.1021/bi00875a024. [DOI] [PubMed] [Google Scholar]
- LEVENBERG B. Role of L-glutamine as donor of carbamyl nitrogen for the enzymatic synthesis of citruline in Agaricus bisporus. J Biol Chem. 1962 Aug;237:2590–2598. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nagano H., Zalkin H., Henderson E. J. The anthranilate synthetase-anthranilate-5-phosphorribosylpyrophosphate phosphoribosyltransferase aggregate. On the reaction mechanism of anthranilate synthetase from Salmonella typhimurium. J Biol Chem. 1970 Aug 10;245(15):3810–3820. [PubMed] [Google Scholar]
- PAMILJANS V., KRISHNASWAMY P. R., DUMVILLE G., MEISTER A. Studies on the mechanism of glutamine synthesis; isolation and properties of the enzyme from sheep brain. Biochemistry. 1962 Jan;1:153–158. doi: 10.1021/bi00907a023. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]




