Abstract
Background
The purpose was to determine whether the Gleason Score (GS) assigned at a comprehensive cancer center better predicts risk of biochemical failure (BF) following prostate radiotherapy (RT) compared to the GS of the referring institution (RI).
Methods
Between 1994 and 2007, 1,649 men received RT for prostate cancer at Fox Chase Cancer Center (FCCC). The Cox proportional hazard regression was used for inferences about the relationship of time to BF and GS. Harrell's C-index (HCI) was used to assess concordance in the Cox regression between predicted and observed events.
Results
The discordance rate was 26 % for any change in either major or minor Gleason pattern. In the RI GS 2-6 group, 79 (8 %) patients were upgraded to GS 7. Twenty percent of RI GS 7 patients were downgraded and 2% upgraded. In the RI GS 8-9 group, 58 % were downgraded to GS 6 (12 %) or GS 7 (88 %). The FCCC GS altered the NCCN risk group assignment in 144 men (9%): 92 (64%) men to lower risk and 52 (36%) to higher risk. FCCC GS was a stronger predictor of BF; the Hazard Ratios for GS 2-6 (ref), 3+4, 4+3 and 8-9 were 1.00 (ref), 1.82, 4.14 and 2.92, respectively. In contrast, the Hazard Ratios for the RI GS were 1.00 (ref), 1.53, 2.44 and 1.76, respectively. FCCC GS (HCI = 0.76) had improved performance compared to RI GS (HCI = 0.74).
Conclusion
Changes in GS were common and the GS assigned by a comprehensive cancer center provided better BF risk stratification and prognostication for patients. Changes in GS may impact treatment recommendations in 9 - 26 % of patients.
Keywords: (4-10) Prostate Cancer, Radiotherapy, Gleason Score, Risk Stratification
Introduction
The Gleason histopathologic grading system is one of three determinants of prostate cancer stage and is an important indicator of the biologic behavior and outcome after radical prostatectomy (RP), external beam radiation therapy, and brachytherapy. 1-4 Based on the study from the Veterans Administration Cooperative Urological Research Group, the Gleason Grading System, initially developed by Dr. Donald Gleason in 1966, is the dominant prostate cancer grading system used worldwide.5 Since its development, there have been several revisions in guidelines of pathological reporting, the most recent one being from 2005.6 The Gleason grading system is a measure of histological architectural differentiation of prostate cancer cells, from “1” – the most differentiated, to “5”- the undifferentiated. The most prevalent and second most prevalent patterns are added together to yield a Gleason Score (GS) with values between 2 and 10. Gleason score 2-6 is considered well to moderately well differentiated, GS 7 is moderately to poorly differentiated, and GS 8-10 is poorly differentiated to undifferentiated.7 Gleason score is an important prognostic factor, independent of initial PSA (iPSA) or T-stage, that is used in the American Joint Committee on Cancer (AJCC) staging 8 and the National Comprehensive Cancer Network (NCCN) risk grouping that are used to make treatment recommendations.9
Disagreement between individual readings of a single prostate biopsy slide has previously been reported.10-12 However, there are no official recommendations with regards to the need for a second, confirmatory pathology review (SPR) prior to treatment of prostate cancer. Furthermore, there are no reported series examining the impact of a SPR for men receiving external beam radiotherapy. Of the two series that examine SPR for patients undergoing brachytherapy, the GS change resulted in alterations of clinical risk level in 15-19% of their patient cohort.13, 14 The aim of our study was to retrospectively evaluate the impact of a SPR for men treated with external beam radiotherapy at an academic, comprehensive cancer center.
Materials and Methods
We examined 1,649 men diagnosed with prostate cancer at a referring institution (RI) by transrectal ultrasound-guided prostate biopsy. A RI was defined as any institution other than Fox Chase. Patients were definitively treated between 1994 and 2007 with either 3D conformal radiation therapy (3DCRT) or intensity modulated radiation therapy (IMRT) alone (no androgen deprivation therapy). The 3DCRT and IMRT techniques have been previously reported.15-17
Pathological slides of all patients diagnosed at RI were reviewed by the Pathology Department at Fox Chase by oncologic pathologist with special expertise in urologic pathology. Each slide was assigned a Gleason Score (GS), which is a measure of aggressiveness of prostate cancer.5 Pathologic specimens of prostate tissue are viewed under the microscope and graded based on the degree of differentiation of primary and secondary morphologic patterns of the tumor, each graded from 1 to 5. The GS is calculated by summing the major and minor Gleason grade patterns from each biopsied site, with the total score ranging from 2 to 10 (GS of 2 representing least aggressive and GS 10 most aggressive with highest potential to spread). Per NCCN guidelines, GSs were grouped according to the prognostic category as follows: GS 2-6 were low grade, GS 7 (3+4 and 4+3) was intermediate grade, and GS 8-9 (4+4; 4+5 and 5+4) were high grade.9 A panel of oncologic pathologists further reviewed cases with discrepancy in diagnosis of GS with the RI (a total of 429 cases (26%)) until a consensus diagnosis was reached.
Follow-up generally consisted of serial PSA determinations every 6 months and digital rectal exams annually. Biochemical failure (BF) was determined using the American Society for Radiation Oncology (ASTRO) consensus definition (i.e. Phoenix or PSA nadir + 2 ng/mL definition).
Statistical Methods
The agreement between pathologists at FCCC and RI was measured with the Kappa statistic, where a value of 0 indicates no agreement and 1 indicates perfect agreement. The Cox proportional hazard regression was used for inferences about the relationship of time to BF with GS for FCCC and RI separately, each model adjusting for T-stage (T1/2 versus T3), iPSA (continuous) and RT dose (continuous). In a separate model, the impact of FCCC GS review (upgrading or downgrading) on time to BCF while adjusting for the initial GS from the RI, T-stage, iPSA and dose was examined. Harrell's C-index (HCI) was used to assess concordance in the Cox regression between predicted and observed events. HCI of 1 indicates a perfect ability to rank the outcomes in the order they actually occurred, where 0.5 is a purely random ranking; this value is comparable to the area under the receiver operator characteristic curve. Statistical analyses were done using SAS software (version 9.2). Tests were twosided using a 0.05 level of significance. Kaplan Meier plots were generated using R, version 2.10.1.
Results
The median follow up was 64 months (range: 4-176) and the median follow-up PSA interval was 6.2 months (range: 0.5-95). The median patient age was 68 years (range: 36-88). Most patients (79.4 %) had T1/2 prostate cancer and PSA <10 (Table 1). The median RT dose was 76 Gy in 2 Gy fractions (range: 70-80). Table 2 illustrates the distribution of patients by year of treatment.
Table 1. Patient characteristics for the study population (n=1,649).
| Range | Median | Mean | SD | No. of Patients | % | ||
|---|---|---|---|---|---|---|---|
| Age | 36-88 | 68 | 67.1 | 7.4 | 1649 | ||
| FCCC-GS | |||||||
| 2-6 | 1143 | 69.3 | |||||
| 7 | 473 | 28.7 | |||||
| (3+4) | (290) | (17.6) | |||||
| (4+3) | (183) | (11.1) | |||||
| 8-10 | 33 | 2.0 | |||||
| RI-GS | |||||||
| 2-6 | 1139 | 69.1 | |||||
| 7 | 451 | 27.4 | |||||
| (3+4) | (268) | (16.3) | |||||
| (4+3) | (183) | (11.1) | |||||
| 8-10 | 59 | 3.6 | |||||
| iPSA (ng/mL) | 0.1-64.6 | 6.1 | 7.4 | 5.1 | |||
| ≤10 | 1334 | 80.9 | |||||
| >10-19.99 | 280 | 17.0 | |||||
| ≥20 | 35 | 2.1 | |||||
| T stage | |||||||
| T1/2 | 1615 | 97.9 | |||||
| T3 | 34 | 2.1 | |||||
| RT Dose (Gy) | 70-80 | ||||||
| ≤74 | 720 | 43.7 | |||||
| >74 | 929 | 56.3 | |||||
| NCCN Risk Groupings | |||||||
| Low | 847 | 51.4 | |||||
| Intermediate | 706 | 42.8 | |||||
| High | 96 | 5.8 | |||||
Abbreviations: FCCC = Fox Chase Cancer Center; RI = Referring Institution; GS = Gleason Score; iPSA = initial PSA; RT = radiotherapy; NCCN = National Comprehensive Cancer Network; SD = Standard Deviation; Low = T1c-T2a, PSA < 10 ng/mL, and GS 2-6; Intermediate = T2b-T2c or PSA 10-20 ng/mL or GS = 7; High = T3a-4, PSA > 20 ng/mL, or GS 8-10.
Table 2. Distribution of 1,649 patients by Year of RT (start of RT).
| Year | N | Percent |
|---|---|---|
| 1994 | 4 | 0.24 |
| 1995 | 26 | 1.58 |
| 1996 | 45 | 2.73 |
| 1997 | 70 | 4.24 |
| 1998 | 130 | 7.88 |
| 1999 | 190 | 11.52 |
| 2000 | 198 | 12.01 |
| 2001 | 166 | 10.07 |
| 2002 | 197 | 11.95 |
| 2003 | 165 | 10.01 |
| 2004 | 139 | 8.43 |
| 2005 | 122 | 7.4 |
| 2006 | 151 | 9.16 |
| 2007 | 46 | 2.79 |
Abbreviations: RT – Radiotherapy
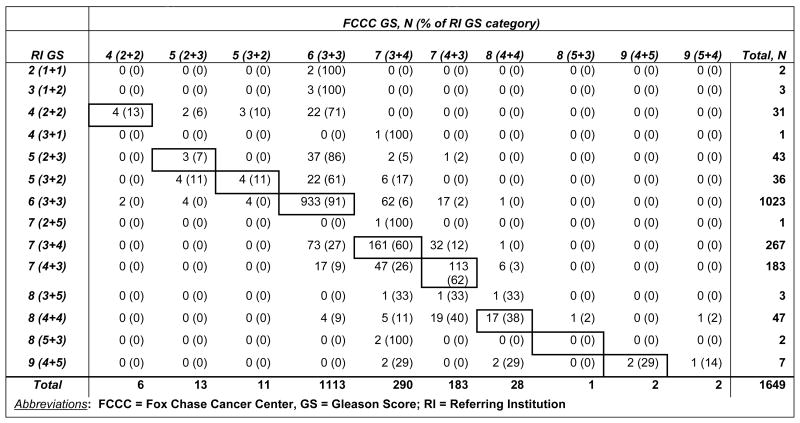
Table 3 summarizes changes of patient's GS upon FCCC pathology review. Agreement between FCCC-GS and RI-GS was moderate, kappa=0.53 (95% CI = 0.50 - 0.57). Overall, compared to RI-GS (i.e. 6 vs. 7 vs. 8-9), FCCC-GS was upgraded in 8% of patients and downgraded in 6% of patients. In the RI GS 6 (3+3) group, 79 (8%) patients were upgraded to the intermediate category: 62 patients (6%) upgraded to GS 3+4, and 17 (2%) upgraded to GS 4+3. A greater impact of the FCCC SPR was observed in the RI GS 7 group where 20% were downgraded to FCCC GS 6 and 2% upgraded to FCCC GS 8-9. Regarding changes within the GS 7 group, 12% of men with RI-GS 3+4 were reassigned FCCC-GS 4+3 and 14% of men with RI GS 4+3 were reassigned FCCC-GS 3+4. The greatest impact of the FCCC SPR was seen in the RI GS 8-9 group, where the majority of men (58%) were downgraded to FCCC GS 6 (12%) or 7 (88%). The FCCC GS altered the NCCN risk group assignment in 144 men (9%): 92 (64%) men to lower risk and 52 (36%) to higher risk.
Table 3. Detailed changes of GS between RI and FCCC pathology review.
|
Cox proportional hazards regression analysis was used for inferences about time to BF based on the RI-GS and FCCC-GS. Three different Cox multivariable analyses (MVA) were completed. The first two analyze RI-GS and FCCC-GS separately (Table 4), adjusting for T-stage (T1/2 v. T3), iPSA and RT dose. The MVA model with FCCC-GS shows the hazard risk (HR) for BF was greater than that for RI-GS that suggests the risk of BF based on FCCC-GS was greater compared to RI-GS. For FCCC GS 3+4 (vs GS 2-6) the HR was 1.82 (95% CI 1.25 - 2.65, p = 0.002), for GS 4+3 was 4.14 (95% CI 2.87 - 5.96, p < 0.0001), and for GS 8-9 was 2.92 (95% CI 1.45 - 5.87, p = 0.003). Comparatively, the HR for the RI GS 3+4 (vs. GS 2 - 6) was 1.53 (95% CI 1.05 - 2.24, p = 0.03), for GS 4+3 was 2.44 (95% CI 1.71 - 3.49, p < 0.0001), and for GS 8-9 was 1.76 (95% CI 0.96 - 3.24, p = 0.07). The FCCC GS was also highly predictive of BF for GS 3+4 versus 4+3 with a lower HR compared to the RI GS, suggesting that changes in primary Gleason pattern were also important (See Table 4).
Table 4. Cox Proportional Hazards Multivariable Analysis for Biochemical Failure.
| Model including FCCC Gleason | Model including RI Gleason | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||||||||
| GS | |||||||||||||
| 3+4 vs 2-6 | 1.82 | (1.254-2.653) | 0.002 | 1.53 | (1.05-2.241) | 0.03 | |||||||
| 4+3 vs 2-6 | 4.14 | (2.87-5.963) | <.0001 | 2.44 | (1.706-3.485) | <.0001 | |||||||
| 8-9 vs 2-6 | 2.92 | (1.454-5.867) | 0.003 | 1.76 | (0.957-3.238) | 0.07 | |||||||
| T-stage | |||||||||||||
| T2 vs T1 | 1.33 | (0.989-1.779) | 0.06 | 1.44 | (1.071-1.922) | 0.02 | |||||||
| T3 vs T1 | 4.04 | (2.192-7.452) | <.0001 | 3.90 | (2.125-7.168) | <.0001 | |||||||
| Log (iPSA) | |||||||||||||
| Continuous | 2.21 | (1.747-2.787) | <.0001 | 2.32 | (1.829-2.952) | <.0001 | |||||||
| RT Dose (Gy) | |||||||||||||
| Continuous | 0.97 | (0.92-1.03) | 0.4 | 1.01 | (0.96-1.06) | 0.7 | |||||||
| Additional comparison from model GS | |||||||||||||
| 4+3 vs 3+4 | 2.27 | (1.53-3.37) | <0.0001 | 1.59 | (1.03-2.46) | 0.04 | |||||||
Abbreviations: FCCC = Fox Chase Cancer Center; RI = Referring Institution; GS = Gleason Score; iPSA = initial PSA; RT = radiotherapy; CI = confidence interval
Regarding the concordance estimates of the two models, HCI was 0.76 for the model with FCCC-GS, slightly higher than HCI of 0.74 for the model with RI-GS. In summary, these data demonstrate that FCCC GS was better at assigning and discriminating risk of BF between GS groups compared to RI GS.
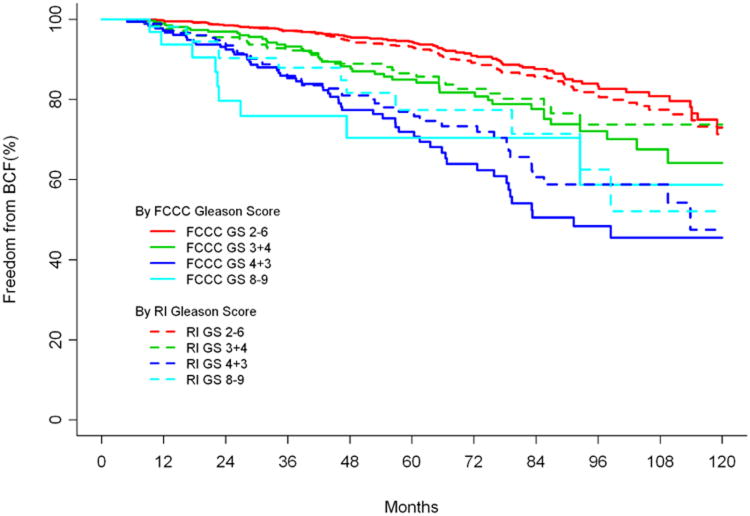
The Kaplan-Meier estimates of BF based on RI versus FCCC GS are shown in Figure 1. This figure further illustrates that estimates of BF according to the FCCC GS were generally higher for GS 7 and GS 8-9 compared to RI. The favorable, GS 2-6 group, had lower estimates of BF according to the FCCC GS versus RI GS.
Figure 1. Freedom from biochemical failure (BCF) for Fox Chase Cancer Center (FCCC) versus Referring Institution (RI) Gleason score (GS).
Table 5 shows the predictive value of a change (upgrading or downgrading) in GS based on FCCC SPR. Examining BF based on the change in GS, Table 5 shows that independent of the initial RIGS, GS upgrading was associated with poorer BF (p = 0.01) in comparison to the group whose GS stays the same or is lowered (p = 0.0004).
Table 5.
Cox Proportional Hazards Multivariable Analysis for Biochemical Failure Examining the Gleason Score Change.
| Parameter | HR | 95% HR | CI | p-value | |
|---|---|---|---|---|---|
| GS Change | FCCC lower vs same | 0.41 | 0.25 | 0.67 | 0.0004 |
| FCCC higher vs same | 1.58 | 1.11 | 2.24 | 0.01 | |
| RI-GS | 3+4 vs 2-6 | 2.02 | 1.36 | 2.98 | 0.0005 |
| 4+3 vs 2-6 | 3.59 | 2.44 | 5.28 | <.0001 | |
| 8-9 vs 2-6 | 3.03 | 1.6 | 5.75 | 0.0007 | |
| T-stage | T2 vs T1 | 1.39 | 1.04 | 1.86 | 0.03 |
| T3 vs T1 | 4.14 | 2.24 | 7.66 | <.0001 | |
| Log (iPSA) | Continuous | 2.25 | 1.78 | 2.84 | <.0001 |
| RT Dose (Gy) | Continuous | 1.00 | 0.99 | 1.00 | 0.8 |
Abbreviations: FCCC = Fox Chase Cancer Center; RI = Referring Institution; GS = Gleason Score; iPSA = initial PSA; RT = radiotherapy; CI = confidence interval
Discussion
In this study, the largest retrospective analysis of the impact of a SPR of GS for prostate cancer and only report of radiotherapy, we found that overall a SPR impacted approximately 13 % of patients when considering a change in the overall GS (i.e. 6 vs. 7 vs. 8-9). The FCCC GS altered the NCCN risk group assignment in 144 men (9%): 92 (64%) men to lower risk and 52 (36%) to higher risk. In general, the risk of BF was higher for FCCC GS 7 and GS 8-9 groups compared to the RI GS. However, there were a smaller proportion of men in the GS 7 and GS 8-9 groups according to the FCCC GS, suggesting that FCCC pathologists were very good at identifying men at higher risk of BF. Within the often-controversial intermediate risk group changes in GS 3+4 versus 4+3 were more prognostic according to the FCCC GS compared to the RI. When considering any change in either major or minor Gleason pattern, the discordance rate was 26%. With a median follow up of 64 months, our data shows an improvement in overall prediction of BF with FCCC GS compared to RI-GS. Overall, these data support a routine SPR, preferably at a dedicated, comprehensive cancer center with a pathologist specialized in the diagnosis of prostate cancer.
To our knowledge, there are no national guidelines that advise a SPR at a comprehensive cancer center. Gupta et al reported on the prevalence of inter-institutional anatomic pathology slide review for purpose of diagnosis confirmation prior to surgical interventions. Their survey requests with regards to requirements and/or performance of SPR from 300 academic and community-based hospitals revealed 126 usable surveys. The results showed that approximately 50 % of surveyed hospitals employed a mandatory second review of outside pathology specimens and about 38% encouraged it.18 Academic centers were more likely to have a mandatory policy in place. This study reported the prevalence of discrepancies up to 30%, with majority between 2% and 5%, for both oncologic and non-oncologic histology specimens.
Several studies have examined the impact of a SPR for cancer patients. A study from the University of Iowa College of Medicine looked at 5,629 second opinion surgical oncologic and nononcologic pathology specimens for most organ systems and found that there were major disagreements involving 132 (2.3%) of cases.19 Fourteen of the 132 cases involved GU histology. While specifics of Gleason scoring was not discussed, the authors did state that 6 (43%) of the 14 cases resulted in major alterations of treatment or prognosis. Kronz et al, reported their findings from a prospective review of mandatory pathology second opinion at the Johns Hopkins Hospital.20 Of the 6,171 reviewed cases, they found that the overall rate of discrepant cases, resulting in a major modification of therapy or prognosis, was 1.4% overall and 1.2% for GU cancers. The majority of discrepant cases involved a change between benign and malignant or a major change in tumor classification, while changes involving a modification of tumor grade or stage were not discussed.
More specific to prostate cancer and GS, Brimo et al evaluated 855 prostate cancer patients' biopsy specimens diagnosed elsewhere and referred to Johns Hopkins Hospital for radical prostatectomy. They found a GS discrepancy in 124 cases (15%), of which 57 (46%) were upgraded and 67 (54%) were downgraded to a different risk category (i.e. GS 6 vs 7 vs 8-10).21 Of the total 204 cases with GS 7, GS was changed from 3+4 to 4+3 in 13 (6%) cases and from 4+3 to 3+4 in 12 (6%) cases. A recent study by Kuroiwa et al. reported on their experience with regards to discrepancy between local and central pathological review of prostatectomy specimens.22 Their review of data from 50 institutions and 2015 radical prostatectomy specimens revealed undergrading and overgrading of GS in 25.9% and 19.2 % cases, respectively. The exact concordance rate for GS 8-10 was significantly lower than that of GS 5-6, 3+4 and 4+3. They found that high volume institutions showed significantly higher exact concordance rates between local and central review for radical prostatectomy GS (p<0.001). A separate report from the same institution investigated the significance of dedicated central pathologic review for GS correlation between the biopsy and radical prostatectomy specimens and the prediction of high-grade GS for 1629 patients.23 The authors found that central review significantly increased the exact concordance rate between the biopsy and RP specimens compared with local review. With regards to high-grade GS in the RP specimens, central review showed significantly greater sensitivity, positive predictive value, and negative predictive value than local review. A study from Canada evaluated the agreement of non-specialist and genitourinary pathologists in grading prostate cancer.24 The authors retrospectively evaluated 151 cases and found that 28% (42 of 151) had a change in risk category after expert review, thereby altering treatment options. The authors concluded that all referred patients should continue to have their pathology centrally reviewed prior to making final treatment decisions. Another report from the British Columbia Cancer Agency examined 1,323 men treated with prostate brachytherapy and found the GS increased in 21.6% with a SPR, decreased in 2.4% and in 1.2% of cases there was a change in diagnosis – from benign to prostate cancer.13 As a consequence, the authors reported a change in treatment, such as androgen deprivation therapy use, for 196 patients (14.8%) – those upgraded from GS 6 to 7. Patients with GS 8-10 were not brachytherapy candidates, and were therefore not included in this study, nor were patients whose GS changed from ≤7 to ≥8. The authors also did not elaborate on Gleason grading changes from 3+4 to 4+3 and vice versa. In the current study, a SPR resulted in a change in Gleason category (i.e. 6 versus 7 versus 8-9), which impacts treatment recommendations, in 14 %. When considering any change in the primary or secondary Gleason pattern, such as 3+4 versus 4+3, the discordance rate increases to 26%.
Several other studies have examined interobserver reproducibility in GS and reported discordance in 19-64% among interobservers that further supports the need to a SPR.10-12 Studies by Alsbrook et al, have demonstrated that the GS agreement among the urological pathologists fares much better than the agreement among general pathologists.10, 11 A recent report by Netto et al published their findings of TAX 3501 trial, a randomized, multinational trial.25 They looked at interobserver variability between central and local pathologists evaluating radical prostatectomy specimens, and found a 30 % GS discordance rate with an overall change of progression-free survival estimates for 13 % of patients. A study specifically designed to estimate the frequency of change in GS as a result of GU pathologists' review and subsequent cancer treatment recommendations was reported by Nguyen et al.26 They reported on the results of 602 patients' specimens initially diagnosed with prostate cancer at nonacademic institutions. GS was changed in 44% of cases: upgrades represented 81% of those cases. The patients' risk category was changed from low to intermediate or high risk in 8.2% and from intermediate or high risk to low risk in 1% of cases, thereby altering treatment decisions for approximately 10% of their patient cohort.
A limitation of our study is its retrospective nature and its associated caveats including the impact of patient selection, follow-up bias, and changes in GS grading overtime. Regarding the GS grading overtime, for example, we observed that the frequency of our pathologists concurring with a RI GS < 6 was higher earlier in our study period. This may in part be explained by Epstein et al's report on the inability to accurately measure the aggressiveness of prostate cancer when a GS 2-4 is assigned on prostate needle biopsy published in 2000.27 Another limitation is underlying assumption and broad generalization that the quality of GS categorization at RIs is equivalent. This is certainly not the case, and some RIs may employ a pathologist who specializes in genitourinary cancer; however a more detailed analysis comparing various RIs was beyond the scope of this analysis. Last, it is assumed that a risk stratification model based on GS seeks to achieve the greatest stratification of risk between groups. This analysis demonstrates that FCCC GS provided greater BF risk stratification compared to RI GS but, at the cost of the proportion of patients assigned to the higher risk groups. Specifically, the proportion of patients assigned to GS 7 and GS 8-9 were smaller with FCCC GS. From a population perspective, this is ideal but from a personal perspective, this may not be ideal, as higher risk patients may not be classified as such. The ideal distribution of patients among the various GS groups is not well established but, risk stratification models such as the American Joint Committee on Cancer staging grouping has radiated towards achieving the greatest risk stratification among risk groups.
Conclusion
A SPR at a dedicated, comprehensive cancer center by a pathologist specializing in genitourinary malignancies resulted in a change in GS grouping in 13 % and GS overall by 26 %. The FCCC GS altered the NCCN risk group assignment in 144 men (9%): 92 (64%) men to lower risk and 52 (36%) to higher risk. These changes all have the potential to alter management and prognosis. The GSs assigned upon the SPR provided greater prognostication of BF risk. Patients may benefit from national standards that encourage a SPR at a comprehensive cancer center.
Acknowledgments
This publication was supported by grant number P30 CA006927 from the National Cancer Institute, NIH. Its contents are solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The authors thank Dr. Gerald Hanks for his leadership in the establishment of the Fox Chase Cancer Center database for the treatment of prostate cancer reported herein and Ruth Peter and Teri Marino-White for their dedication to its maintenance.
Footnotes
Conflict of Interest: The Authors have no financial disclosures
References
- 1.Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27(26):4300–5. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Chen MH, McLeod D, Carroll PR, Moul JW, D'Amico AV. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. 2005;23(28):6992–8. doi: 10.1200/JCO.2005.01.2906. [DOI] [PubMed] [Google Scholar]
- 3.Potters L, Roach M, 3rd, Davis BJ, et al. Postoperative nomogram predicting the 9-year probability of prostate cancer recurrence after permanent prostate brachytherapy using radiation dose as a prognostic variable. Int J Radiat Oncol Biol Phys. 2010;76(4):1061–5. doi: 10.1016/j.ijrobp.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 4.Potters L, Purrazzella R, Brustein S, et al. The prognostic significance of Gleason Grade in patients treated with permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2003;56(3):749–54. doi: 10.1016/s0360-3016(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 5.Gleason DF. Histologic grading of prostate cancer: a perspective. Hum Pathol. 1992;23(3):273–9. doi: 10.1016/0046-8177(92)90108-f. [DOI] [PubMed] [Google Scholar]
- 6.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL, Committee IG. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29(9):1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 7.Epstein JI, Potter SR. The pathological interpretation and significance of prostate needle biopsy findings: implications and current controversies. J Urol. 2001;166(2):402–10. [PubMed] [Google Scholar]
- 8.Edge SB. AJCC cancer staging manual. New York: Springer; 2010. [Google Scholar]
- 9.Mohler JL. The 2010 NCCN Clinical Practice Guidelines in Oncology on Prostate Cancer. Journal of the National Comprehensive Cancer Network. 2010;8(2):145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 10.Allsbrook WC, Jr, Mangold KA, Johnson MH, et al. Interobserver reproducibility of Gleason grading of prostatic carcinoma: urologic pathologists. Hum Pathol. 2001;32(1):74–80. doi: 10.1053/hupa.2001.21134. [DOI] [PubMed] [Google Scholar]
- 11.Allsbrook WC, Jr, Mangold KA, Johnson MH, Lane RB, Lane CG, Epstein JI. Interobserver reproducibility of Gleason grading of prostatic carcinoma: general pathologist. Hum Pathol. 2001;32(1):81–8. doi: 10.1053/hupa.2001.21135. [DOI] [PubMed] [Google Scholar]
- 12.di Loreto C, Fitzpatrick B, Underhill S, et al. Correlation between visual clues, objective architectural features, and interobserver agreement in prostate cancer. Am J Clin Pathol. 1991;96(1):70–5. doi: 10.1093/ajcp/96.1.70. [DOI] [PubMed] [Google Scholar]
- 13.Thomas CW, Bainbridge TC, Thomson TA, McGahan CE, Morris WJ. Clinical impact of second pathology opinion: a longitudinal study of central genitourinary pathology review before prostate brachytherapy. Brachytherapy. 2007;6(2):135–41. doi: 10.1016/j.brachy.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Sooriakumaran P, Lovell DP, Henderson A, Denham P, Langley SE, Laing RW. Gleason scoring varies among pathologists and this affects clinical risk in patients with prostate cancer. Clin Oncol (R Coll Radiol) 2005;17(8):655–8. doi: 10.1016/j.clon.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Buyyounouski MK, Horwitz EM, Price RA, Hanlon AL, Uzzo RG, Pollack A. Intensity-modulated radiotherapy with MRI simulation to reduce doses received by erectile tissue during prostate cancer treatment. Int J Radiat Oncol Biol Phys. 2004;58(3):743–9. doi: 10.1016/S0360-3016(03)01617-1. [DOI] [PubMed] [Google Scholar]
- 16.Buyyounouski MK, Horwitz EM, Uzzo RG, et al. The radiation doses to erectile tissues defined with magnetic resonance imaging after intensity-modulated radiation therapy or iodine-125 brachytherapy. Int J Radiat Oncol Biol Phys. 2004;59(5):1383–91. doi: 10.1016/j.ijrobp.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 17.Hanks GE, Hanlon AL, Pinover WH, Horwitz EM, Schultheiss TE. Survival advantage for prostate cancer patients treated with high-dose three-dimensional conformal radiotherapy. Cancer J Sci Am. 1999;5(3):152–8. [PubMed] [Google Scholar]
- 18.Gupta D, Layfield LJ. Prevalence of inter-institutional anatomic pathology slide review: a survey of current practice. Am J Surg Pathol. 2000;24(2):280–4. doi: 10.1097/00000478-200002000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Manion E, Cohen MB, Weydert J. Mandatory second opinion in surgical pathology referral material: clinical consequences of major disagreements. Am J Surg Pathol. 2008;32(5):732–7. doi: 10.1097/PAS.0b013e31815a04f5. [DOI] [PubMed] [Google Scholar]
- 20.Kronz JD, Westra WH, Epstein JI. Mandatory second opinion surgical pathology at a large referral hospital. Cancer. 1999;86(11):2426–35. [PubMed] [Google Scholar]
- 21.Brimo F, Schultz L, Epstein JI. The value of mandatory second opinion pathology review of prostate needle biopsy interpretation before radical prostatectomy. J Urol. 2010;184(1):126–30. doi: 10.1016/j.juro.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Kuroiwa K, Shiraishi T, Ogawa O, et al. Discrepancy between local and central pathological review of radical prostatectomy specimens. J Urol. 2010;183(3):952–7. doi: 10.1016/j.juro.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Kuroiwa K, Shiraishi T, Naito S Clinicopathological Research Group for Localized Prostate Cancer I. Gleason score correlation between biopsy and prostatectomy specimens and prediction of high-grade Gleason patterns: significance of central pathologic review. Urology. 2011;77(2):407–11. doi: 10.1016/j.urology.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 24.D'Souza N, Loblaw DA, Mamedov A, Sugar L, Holden L. Prostate cancer pathology audits: is central pathology review still warranted? The Canadian journal of urology. 2012;19(3):6256–60. [PubMed] [Google Scholar]
- 25.Netto GJ, Eisenberger M, Epstein JI Investigators TAXT. Interobserver variability in histologic evaluation of radical prostatectomy between central and local pathologists: findings of TAX 3501 multinational clinical trial. Urology. 2011;77(5):1155–60. doi: 10.1016/j.urology.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen PL, Schultz D, Renshaw AA, et al. The impact of pathology review on treatment recommendations for patients with adenocarcinoma of the prostate. Urol Oncol. 2004;22(4):295–9. doi: 10.1016/S1078-1439(03)00236-9. [DOI] [PubMed] [Google Scholar]
- 27.Epstein JI. Gleason score 2-4 adenocarcinoma of the prostate on needle biopsy: a diagnosis that should not be made. Am J Surg Pathol. 2000;24(4):477–8. doi: 10.1097/00000478-200004000-00001. [DOI] [PubMed] [Google Scholar]