Abstract
One of the underlying mechanisms for metabolic individuality is genetic variation. Single nucleotide polymorphisms (SNPs) in genes of metabolic pathways can create metabolic inefficiencies that alter the dietary requirement for, and responses to, nutrients. These SNPs can be detected using genetic profiling and the metabolic inefficiencies they cause can be detected using metabolomic profiling. Studies on the human dietary requirement for choline illustrate how useful these new approaches can be, as this requirement is influenced by SNPs in genes of choline and folate metabolism. In adults, these SNPs determine whether people develop fatty liver, liver damage and muscle damage when eating diets low in choline. Because choline is very important for fetal development, these SNPs may identify women who need to eat more choline during pregnancy. Some of the actions of choline are mediated by epigenetic mechanisms that permit ‘retuning’ of metabolic pathways during early life.
Key Words: Choline, Development, Single nucleotide polymorphism, Epigenetics, Methylation
FOCUS
The study of choline provides insight into a varied array of nutrigenetic mechanisms that are clinically relevant for infant development
Key insights
This article highlights studies that illustrate the utility of genetic and metabolomic profiling, particularly looking at the human dietary requirement for, and the responses to, choline. It discusses the interaction of genes and maternal/infant diet, both of which can alter epigenetic marks that control gene expression throughout life.
Current knowledge
Genetic variation is one of the underlying mechanisms of metabolic individuality. Genetic polymorphisms, detected by genetic profiling, modify dietary requirements by creating metabolic inefficiencies, which are detected by using metabolomic profiling. In adults, these polymorphisms determine whether people develop fatty liver, liver damage, and/or muscle damage when eating low-choline diets. Choline is also important for fetal development of the brain. Some of the actions of choline are mediated by epigenetic mechanisms, which may permit the ‘retuning’ of metabolic pathways during early life.
Practical implications
A better understanding of important nutrient-gene interactions and nutrigenetic profiling can help clinicians to identify people with metabolic inefficiencies, and, in particular, identify women who need to eat more choline during pregnancy. Because the metabolic variation is a source of variance (noise), it should be considered in the design of future human nutrition research studies.
Recommended reading
Fischer LM, da Costa KA, Galanko J, Sha W, Stephenson B, Vick J, Zeisel SH: Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. Am J Clin Nutr 2010;92:336-346.
Key Messages
Metabolic variation is a source of variance (noise) that should be considered in the design of human nutrition research studies.
Genetic polymorphisms modify dietary requirements by creating metabolic inefficiencies.
Nutrigenetic profiling can help clinicians to identify people with metabolic inefficiencies.
Maternal and infant diet can alter epigenetic marks that control gene expression throughout life.
Introduction
The obstetrician and pediatrician care for mothers and infants during a period when interactions between diet and genes have very important effects on child development that modulate health status later in life [1]. Scientists are just beginning to understand which components of the diet have such effects, and what the underlying mechanisms for these effects are. At the same time, it is becoming apparent that gene expression modulates metabolic pathways and people can have large differences in metabolism, often reflecting inefficiencies in metabolic pathways caused by genetic variation. These new knowledge bases are evolving and, within a decade, the catalog of important nutrient-gene interactions should be large enough that it will be used in everyday clinical practice.
Genetic Variation and Gene Expression
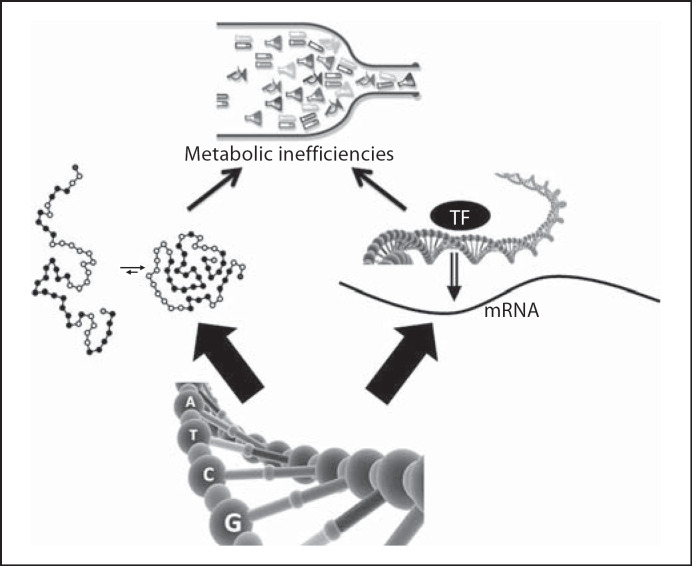
When the human genome was sequenced, it was thought that scientists would understand the genetic causes of diseases; unfortunately, the underlying biology was much more complicated than expected. Although all humans share much genetic code, there are many ways whereby people differ: some of these differences result in metabolic variation. Humans inherit, from their ancient ancestors, ‘misspellings’ of genes (these are called single nucleotide polymorphisms or SNPs); more than 1 million common SNPs are known and each person has approximately 50,000 of them [2]. Many of these SNPs have no known function, but many others alter the expression of critical genes involved in metabolism. In the exonic region of a gene which encodes for a protein, a SNP can lead to a substitution of an amino acid in the protein; this causes a change in structure of the protein and can result in loss or gain of function. In the regulatory region of a gene that contains the switches (transcription factor binding sites) that turn the gene on or off, a SNP can change gene expression and thereby alter the amount of protein available to perform its function in metabolism. Such SNPs often decrease the amount of functional protein because they inhibit gene expression, but some SNPs can increase gene expression if they result in a defective region of the gene that normally acts to inhibit gene expression (fig. 1).
Fig. 1.
Gene misspellings (SNPs) can result in metabolic inefficiencies. SNPs can result in changes in the amino acid composition of an enzyme that leads to misfolding and poor function, or SNPs can lead to defective transcription factor (TF) binding sites and inability to activate gene expression.
When SNPs occur in genes critical for metabolism, they create metabolic inefficiencies that can influence requirements for, and responses to, a nutrient. This concept is a familiar one in personalized medicine where clinicians are now aware of the effects of SNPs in genes of drug metabolism. There are fast and slow metabolizers of the drug warfarin that can be defined by SNPs, and doses of this drug need to be modified accordingly for optimal outcome [3]. Pertinent to nutrition, there are SNPs that determine whether we have fast or slow metabolism of caffeine [4], whether we have higher dietary requirements for folate [5], choline [6,7], vitamin C [8], or vitamin D [8], and whether we have increased serum cholesterol concentrations after we eat a high-fat meal [9].
This concept is a familiar one in personalized medicine where clinicians are now aware of the effects of SNPs in genes of drug metabolism.
Epigenetic Modulation of Gene Expression
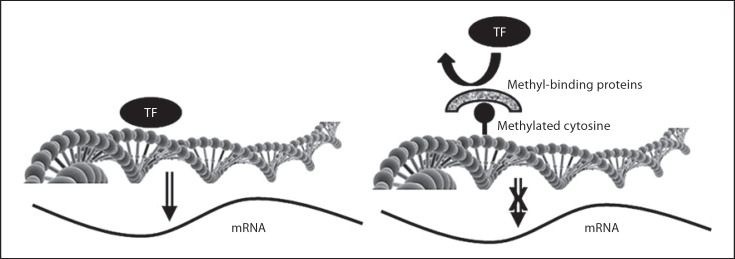
Genetic complexity goes beyond the effects of SNPs. There are marks (small chemical groups) that get added to genes or chromosomal proteins that can modulate gene expression [10]. These epigenetic marks are added to genes during the period starting when a stem cell begins to divide and ending when the daughter cells differentiate; thereafter, they are faithfully copied for the rest of the life of the cell. Because most stem cells enter this critical period during fetal life and perhaps during the first few years after birth, these are the periods of infant development when epigenetic marks are most susceptible to being influenced by the environment, especially by nutrition [11]. This is fortunate because it permits the developmental system to escape the rigidity of genetic coding by switching certain genes on and off, thereby ‘retuning’ gene expression to achieve some metabolic flexibility. If the world outside the womb does not match expectations, epigenetic mechanisms permit some degree of adaptation. As noted earlier, these epigenetic marks become fixed after early life, and the retuning of metabolism that was helpful in early life, may become a problem when environment or diet changes later in life. This is thought to be one of the underlying mechanisms for how exposures in early life influence disease risks in the adult [11]. Epigenetic marking is such an important process that nature has evolved multiple reinforcing pathways for these processes. Methyl-groups added to cytosine nucleotides in DNA act to attract a set of proteins to their vicinity, and these capping proteins block access for transcription factors that must bind to DNA to turn on gene expression; thus, DNA methylation usually inhibits gene expression (fig. 2). DNA is normally tightly coiled around a series of proteins called histones, and methyl-groups added to lysines in these proteins influence how tightly DNA can be packed in these coils. More methylation leads to more tightly coiled DNA. When DNA is tightly coiled, access is blocked for transcription factors that must bind to DNA to turn on gene expression; thus, gene expression is usually inhibited by histone methylation. DNA methylation and histone methylation are regulated by pathways that talk to each other, and usually are coordinated to result in reinforcing inhibitory signals. The complexity does not end here. Scientists are discovering that there is a third mechanism for epigenetic control of gene expression; there are microRNAs that bind to complementary sequences on genetic DNA and block access for transcription factors needed to turn on the genes [12].
Fig. 2.
Epigenetic regulation of gene expression. Normally, genes are expressed when transcriptions factors (TF) bind to DNA and activate the gene (left panel). One mechanism for controlling gene expression uses methylation of cytosines in DNA. When this methylation is present, proteins are attracted to the methylated site, and this blocks off access for the transcription factor and the gene cannot be turned on.
Diet is a potent modulator of epigenetic marks, especially during prenatal and early postnatal life. Diets high in choline, methionine, folate, and vitamins B6 and B12 increase DNA and histone methylation, alter gene expression, and can result in permanent changes in development. Feeding pregnant mice a diet high in these nutrients can alter insulin-like growth factor signaling [13], the color of hair [14,15], body weight [14,16], and even kinking of the tail [17] in the infants born of these mothers. Later, we discuss how dietary choline during pregnancy in mice influences neurogenesis and angiogenesis in fetal brain [18,19].
Concrete examples can help to solidify the implications of research for clinical practice. An excellent example of how nutrigenetic mechanisms influence development is provided by the recent research on the nutrient choline.
Genes, Metabolic Variation and the Requirement for and Responses to Choline
The study of choline provides insight into a varied array of nutrigenetic mechanisms that are clinically relevant for infant development. Choline, a nutrient found in many foods (especially in eggs, milk and meats; see the US Department of Agriculture's list of choline-containing foods at www.nal.usda.gov/fnic/foodcomp/Data/Choline/Choln02.pdf), is important for making the neurotransmitter acetylcholine, for production of the membrane phospholipids phosphatidylcholine and sphingomyelin, and it is an important source of methyl-groups [reviewed in ref. [20].
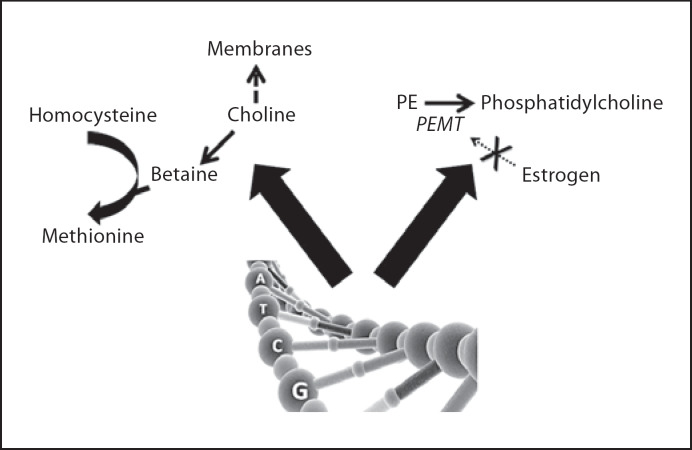
For many years, nutrition scientists thought that humans could meet their needs for choline through a metabolic pathway, present mainly in liver, which formsphosphatidylcholine (a source of choline) from phosphatidylethanolamine, using S-adenosylmethionine as a methyl-group donor. This pathway is encoded for by the gene PEMT[21]. However, when this idea was tested experimentally, it was observed that men, postmenopausal women and some premenopausal women developed liver and/or muscle damage when fed a low-choline diet [22,23,24]. This tissue damage resolved quickly when choline was reintroduced into their diets. Most premenopausal women were different: when they were fed low-choline diets for periods as long as 7 weeks, they did not develop liver or muscle damage [22]. Why are these women resistant to choline deficiency? The explanation lies in the pathway for endogenous synthesis of phosphatidylcholine (fig. 3); expression of the gene PEMT is increased by estrogen [25]. It is interesting that this activation of the gene begins at estrogen concentrations present in premenopausal women and is maximal at the higher estrogen concentrations achieved during pregnancy [25]. This is important for the later discussion of how choline is important for fetal development. Why then do some premenopausal women (about 44* [22]) still need to eat choline despite having this estrogen-activated pathway for forming choline? It is because they have one or more SNPs that increased the dietary requirement for this nutrient [6,7]. For example, an SNP in a gene important for folate metabolism (MTHFD1) decreased the availability of methyltetrahydrofolate and created an extra demand for betaine (a choline metabolite) an alternative methyl-group donor needed for the formation of methionine from homocysteine [7]. This shunting of choline into betaine formation decreased the availability of choline for making membranes and acetylcholine (fig. 3). People with two variant alleles for MTHFD1 were 85× more likely to develop fatty liver when fed a low-choline diet [7]. Another SNP of interest was in the PEMT gene; this SNP prevents estrogen activation of the gene, thereby preventing the increased expression of this gene that is normally seen in premenopausal women [26]. Women with this SNP were 24× more likely to develop fatty liver when fed a low choline diet [7]. This PEMT SNP is very common, with approximately 20* of the North Carolina population having two variant alleles [6].
Fig. 3.
SNPs can increase the dietary requirement for choline. In humans, SNPs in the gene MTHFD1 increase the demand for betaine as a methyl-donor, thereby increasing the dietary requirement for choline. Another SNP in the gene PEMT prevents the activation of this gene by estrogen, thereby decreasing endogenous production of phosphatidylcholine (a source of choline) in the liver and increasing the dietary requirement for choline. Both of these SNPs are common. PE = phosphatidylethanolamine.
The cumulative effect of SNPs in genes of choline and 1-carbon metabolic pathways was to create inefficiencies in the metabolism of choline. This could be detected as changes in the small molecules produced by metabolism. Plasma was analyzed using metabolomic profiling methods in people fed a standardized diet containing choline. Differences in plasma metabolites on this baseline diet accurately predicted who would develop fatty liver when these people were switched to a diet low in choline [27]; thus, people with SNPs were already metabolically different before being challenged. The altered metabolites were not restricted to the obvious expected changes in metabolites directly involved in choline and 1-carbon metabolism, but also included changes in carnitine, lipid, keto and amino acid metabolism [27].
Choline and Fetal Development
Evidence shows that specific systems in women are designed to deliver choline to the fetus and young infant, suggesting that this nutrient is important for normal human development. First, as noted earlier, young women have a special capacity to form phosphatidylcholine that serves to buffer them from vagaries of diet intake [25,26]. Second, transport systems in the placenta and mammary gland deliver large amounts of choline from mother to the fetus and infant, respectively [28,29]. This results in plasma and tissue choline concentrations that are much higher in the fetus and young infant than they are in adults [30,31]. Unfortunately for the mother, this extra demand for choline provided to the baby increases the choline requirements of the mother and likely exceeds the capacity for endogenous production of choline in the liver [32].
In rodents, the availability of choline to the fetus is very important for brain (specifically hippocampal) development. This area of the brain is important for memory function. If pregnant rodents are fed a diet supplemented with choline, their offspring perform as much as 30* better on tests of visuospatial and auditory memory [33,34,35] and this improvement lasts for their entire lifetime. Conversely, when pregnant rodents are fed diets low in choline, their offspring have decremented visuospatial and auditory memory [36]. Fetuses from mothers fed a choline-supplemented diet have almost twice the rate of neurogenesis in their hippocampi when compared to fetuses from mothers fed a low choline diet [18,37,38]. In addition, fetuses from mothers fed a choline-supplemented diet have half the rate of neuronal apoptosis (programmed cell death) in their hippocampi when compared to fetuses from mothers fed a low choline diet [18,37,38]. Thus, maternal ingestion of a high-choline diet during pregnancy increases neuronal proliferation and decreases neuronal death in fetal brain. This may explain the observed effects on memory function. It appears that low choline causes neurons to differentiate and mature earlier, thereby shortening the period during which they can divide [18,39]. In addition to influencing brain neurogenesis, choline availability has similar effects on brain angiogenesis. Fetuses from mothers fed a choline-supplemented diet have more blood vessels in their hippocampi when compared to fetuses from mothers fed a low choline diet [19].
Thus, maternal ingestion of a high-choline diet during pregnancy increases neuronal proliferation and decreases neuronal death in fetal brain.
How could mothers' dietary choline influence fetal brain? As noted earlier, choline is an important source of the methyl-groups needed for epigenetic marking of DNA and histones. Some of the genes that control cell cycling are regulated epigenetically. For example, a gene encoding for an inhibitor of cell cycling (CDKN3) is expressed when the gene is undermethylated, and is suppressed when the gene is methylated [40,41]. Fetuses from mothers fed a choline-supplemented diet have more highly methylated DNA in CDKN3 in their hippocampi when compared to fetuses from mothers fed a low-choline diet [40]. When CDKN3 is highly methylated the gene is not expressed and an important brake on cell cycling is removed, hence the increased neurogenesis seen in choline-supplemented fetal brain. Thus, maternal diet changes epigenetic marks in fetal brain and this, in turn, changes brain structure and function. This is not the only example of such epigenetic effects on the fetus caused by altering maternal diet. There is a mouse strain that has a gene encoding for kinky tails; this gene is epigenetically regulated and is suppressed when methylated. When pregnant mice are fed a high-choline, high-methionine diet, their offspring had more straight tails, and when pregnant dams are fed a low choline diet their offspring had more permanently kinked tails [17]. For both brain development and tail kinkiness, the epigenetic retuning that occurred when stem cells were dividing became fixed once the cells differentiated and were faithfully copied in all future cell divisions; thus, the effect on the offspring persisted into later life.
The effects of choline during pregnancy may not be limited to the development of the hippocampus. It is interesting that some of the developmental abnormalities characteristic of the fetal alcohol syndrome in rats were diminished if pregnant dams consumed supplemental choline [42]. Choline is important for neural tube closure [43,44], and a population-based case-control study in 440 cases and 400 controls in California found that risk for neural tube defects in the baby was significantly reduced in pregnant women consuming diets in the highest quartile for choline and betaine content compared to those consuming the lowest quartile for these nutrients [45]. Similar observations were made for the risk of orofacial clefts [46]. These studies are the first evidence that choline is important for normal brain development in humans, and since neural tube closure involves neurogenesis and migration, these data strongly suggest that the effects of choline on neurogenesis and apoptosis that occur in rodents may well be robust enough to have importance in humans. Unfortunately, prospective randomized control studies are hard to perform in humans, and there are no adequately powered studies that demonstrate an effect of choline during pregnancy on human hippocampal function in babies. One observational study [47] reported that there was no association between cord blood choline concentrations and scores on gross intelligence tests in 5-year-olds, but these tests were not designed to detect subtle differences in hippocampal function, and cord blood choline likely does not reflect maternal diet accurately. Future studies need to consider the complex interactions between diet, genetic variation, and epigenetics that lead to choline-mediated effects on brain development. It is possible that only the mothers (or babies?) with SNP-induced metabolic inefficiencies are sensitive to dietary intake of choline. Only a properly designed and powered randomized control trial can determine if choline supplementation has beneficial effects on memory development in humans.
Unfortunately, prospective randomized control studies are hard to perform in humans, and there are no adequately powered studies that demonstrate an effect of choline during pregnancy on human hippocampal function in babies.
Conclusion
The study of the human requirement for choline and of the actions of choline on brain development provides examples of how studies on metabolic variation can be designed and interpreted. The elucidation of the factors that result in metabolic individuality promises to refine our understanding of how nutrition influences health.
Disclosure Statement
The authors declares that no financial or other conflict of interest exists in relation to the content of this article. The writing of this article was supported by Nestlé Nutrition Institute. Dr. Zeisel received grant support from the Balchem and the Egg Nutrition Research Center for studies other than those described in this paper. Dr. Zeisel is on the Scientific Advisory Board for Solae, American Pistachio Growers, Dupont, Metabolon and GenoVive.
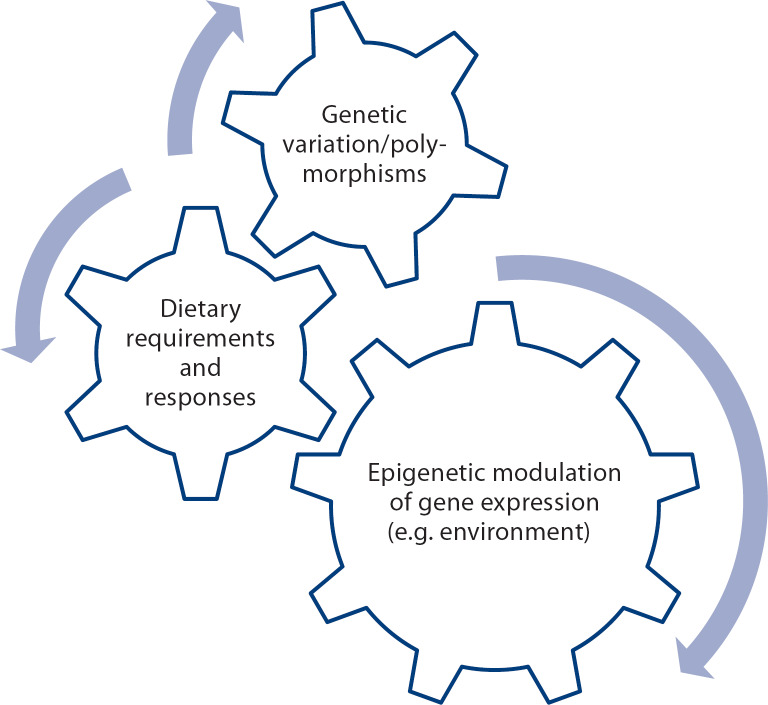
The components that modulate metabolic variation, which could lead to clinically relevant individual metabolic inefficiencies in early life and in adults (see text for details).
Acknowledgments
Support for this work was provided by grants from the National Institutes of Health (DK55865 and DK56350).
References
- 1.Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807–813. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 2.Crawford DC, Nickerson DA. Definition and clinical importance of haplotypes. Annu Rev Med. 2005;56:303–320. doi: 10.1146/annurev.med.56.082103.104540. [DOI] [PubMed] [Google Scholar]
- 3.Carlquist JF, Anderson JL. Using pharmacogenetics in real time to guide warfarin initiation: a clinician update. Circulation. 2011;124:2554–2559. doi: 10.1161/CIRCULATIONAHA.111.019737. [DOI] [PubMed] [Google Scholar]
- 4.Cornelis MC, El-Sohemy A. Coffee, caffeine, and coronary heart disease. Curr Opin Lipidol. 2007;18:13–19. doi: 10.1097/MOL.0b013e3280127b04. [DOI] [PubMed] [Google Scholar]
- 5.Bailey LB, Gregory JF., 3rd Polymorphisms of methylenetetrahydrofolate reductase and other enzymes: metabolic significance, risks and impact on folate requirement. J Nutr. 1999;129:919–922. doi: 10.1093/jn/129.5.919. [DOI] [PubMed] [Google Scholar]
- 6.da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. 2006;20:1336–1344. doi: 10.1096/fj.06-5734com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohlmeier M, da Costa KA, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci USA. 2005;102:16025–16030. doi: 10.1073/pnas.0504285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahill LE, Fontaine-Bisson B, El-Sohemy A. Functional genetic variants of glutathione S-transferase protect against serum ascorbic acid deficiency. Am J Clin Nutr. 2009;90:1411–1417. doi: 10.3945/ajcn.2009.28327. [DOI] [PubMed] [Google Scholar]
- 9.Mattei J, Demissie S, Tucker KL, Ordovas JM. Apolipoprotein A5 polymorphisms interact with total dietary fat intake in association with markers of metabolic syndrome in Puerto Rican older adults. J Nutr. 2009;139:2301–2308. doi: 10.3945/jn.109.109900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisel SH. Epigenetic mechanisms for nutrition determinants of later health outcomes. Am J Clin Nutr. 2009;89:1488S–1493S. doi: 10.3945/ajcn.2009.27113B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Zhao X, Hsieh J, Wichterle H, Impey S, Banerjee S, Neveu P, Kosik KS. MicroRNA regulation of neural stem cells and neurogenesis. J Neurosci. 2010;30:14931–14936. doi: 10.1523/JNEUROSCI.4280-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waterland RA, Lin JR, Smith CA, Jirtle RL. Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (Igf2) locus. Hum Mol Genet. 2006;15:705–716. doi: 10.1093/hmg/ddi484. [DOI] [PubMed] [Google Scholar]
- 14.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–957. [PubMed] [Google Scholar]
- 16.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes (Lond) 2008;32:1373–1379. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waterland RA, Dolinoy DC, Lin JR, Smith CA, Shi X, Tahiliani KG. Maternal methyl supplements increase offspring DNA methylation at Axin fused. Genesis. 2006;44:401–406. doi: 10.1002/dvg.20230. [DOI] [PubMed] [Google Scholar]
- 18.Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr. 2003;133:3614–3618. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehedint MG, Craciunescu CN, Zeisel SH. Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc Natl Acad Sci USA. 2010;107:12834–12839. doi: 10.1073/pnas.0914328107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vance DE, Walkey CJ, Cui Z. Phosphatidylethanolamine N-methyltransferase from liver. Biochim Biophys Acta. 1997;1348:142–150. doi: 10.1016/s0005-2760(97)00108-2. [DOI] [PubMed] [Google Scholar]
- 22.Fischer LM, daCosta K, Kwock L, Stewart P, Lu TS, Stabler S, Allen R, Zeisel S. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. 2007;85:1275–1285. doi: 10.1093/ajcn/85.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Costa KA, Gaffney CE, Fischer LM, Zeisel SH. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am J Clin Nutr. 2005;81:440–444. doi: 10.1093/ajcn.81.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeisel SH, da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–2098. [PubMed] [Google Scholar]
- 25.Resseguie M, Song J, Niculescu MD, da Costa KA, Randall TA, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007;21:2622–2632. doi: 10.1096/fj.07-8227com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Resseguie ME, da Costa KA, Galanko JA, Patel M, Davis IJ, Zeisel SH. Aberrant estrogen regulation of PEMT results in choline deficiency-associated liver dysfunction. J Biol Chem. 2011;286:1649–1658. doi: 10.1074/jbc.M110.106922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sha W, da Costa KA, Fischer LM, Milburn MV, Lawton KA, Berger A, Jia W, Zeisel SH. Metabolomic profiling can predict which humans will develop liver dysfunction when deprived of dietary choline. FASEB J. 2010;24:2962–2975. doi: 10.1096/fj.09-154054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweiry JH, Yudilevich DL. Characterization of choline transport at maternal and fetal interfaces of the perfused guinea-pig placenta. J Physiol. 1985;366:251–266. doi: 10.1113/jphysiol.1985.sp015795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer LM, da Costa KA, Galanko J, Sha W, Stephenson B, Vick J, Zeisel SH. Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. Am J Clin Nutr. 2010;92:336–346. doi: 10.3945/ajcn.2010.29459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeisel SH, Epstein MF, Wurtman RJ. Elevated choline concentration in neonatal plasma. Life Sci. 1980;26:1827–1831. doi: 10.1016/0024-3205(80)90585-8. [DOI] [PubMed] [Google Scholar]
- 31.Ilcol YO, Ozbek R, Hamurtekin E, Ulus IH. Choline status in newborns, infants, children, breast-feeding women, breast-fed infants and human breast milk. J Nutr Biochem. 2005;16:489–499. doi: 10.1016/j.jnutbio.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Zeisel SH, Mar MH, Zhou Z, da Costa KA. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr. 1995;125:3049–3054. doi: 10.1093/jn/125.12.3049. [DOI] [PubMed] [Google Scholar]
- 33.Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front Integr Neurosci. 2007;1:7. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 35.Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988;21:339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- 36.Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain Res. 1999;118:51–59. doi: 10.1016/s0165-3806(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 37.Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res. 1999;113:13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- 38.Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res. 1999;115:123–129. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 39.Albright CD, Mar MH, Craciunescu CN, Song J, Zeisel SH. Maternal dietary choline availability alters the balance of netrin-1 and DCC neuronal migration proteins in fetal mouse brain hippocampus. Brain Res Dev Brain Res. 2005;159:149–154. doi: 10.1016/j.devbrainres.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J Neurochem. 2004;89:1252–1259. doi: 10.1111/j.1471-4159.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. FASEB J. 2002;16:619–621. doi: 10.1096/fj.01-0564fje. [DOI] [PubMed] [Google Scholar]
- 44.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Inhibitors of choline uptake and metabolism cause developmental abnormalities in neurulating mouse embryos. Teratology. 2001;64:114–122. doi: 10.1002/tera.1053. [DOI] [PubMed] [Google Scholar]
- 45.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–109. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 46.Shaw GM, Carmichael SL, Laurent C, Rasmussen SA. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology. 2006;17:285–291. doi: 10.1097/01.ede.0000208348.30012.35. [DOI] [PubMed] [Google Scholar]
- 47.Signore C, Ueland PM, Troendle J, Mills JL. Choline concentrations in human maternal and cord blood and intelligence at 5 y of age. Am J Clin Nutr. 2008;87:896–902. doi: 10.1093/ajcn/87.4.896. [DOI] [PMC free article] [PubMed] [Google Scholar]