Summary
Autism spectrum disorder (ASD), a neurodevelopmental disorder affecting nearly 1 in 88 children, is thought to result from aberrant brain connectivity. Remarkably, there have been no systematic attempts to characterize whole-brain connectivity in children with ASD. Here, we use neuroimaging to show there are more instances of greater functional connectivity in the brains of children with ASD compared with typically developing children. Hyper-connectivity in ASD was observed at the whole-brain and subsystems level, across long- and short-range connections, and was associated with higher levels of fluctuations in regional brain signals. Brain hyper-connectivity predicted symptom severity in ASD such that children with greater functional connectivity exhibited more severe social deficits. We replicated these findings in two additional independent cohorts, demonstrating again that at earlier ages, the brain in ASD is largely functionally hyper-connected in ways that contribute to social dysfunction. Our findings provide novel insights into brain mechanisms underlying childhood autism.
Introduction
Autism spectrum disorder (ASD), a neurodevelopmental disorder that affects nearly 1 in 88 children (Baio, 2012), is thought to impact multiple inter-connected brain regions (Minshew and Williams, 2007). Knowledge of brain connectivity in ASD and its relation to core symptoms is therefore critical for understanding the neurobiology of ASD (Kennedy and Courchesne, 2008; Menon, 2011; Minshew and Williams, 2007; Monk et al., 2009; Vissers et al., 2011). Despite the early developmental origins of this disorder and its variable developmental trajectory, almost all of the current literature on brain connectivity has focused on adolescents and adults with ASD, rather than children (Gotts et al., 2012; Kennedy and Courchesne, 2008).
Several previous studies in adults have reported that functional connectivity between brain areas engaged during cognitive tasks is weaker in ASD (Just et al., 2007; Kleinhans et al., 2008; Koshino et al., 2008), leading to the “under-connectivity theory” of autism (Just et al., 2012). Yet, empirical evidence in support of the under-connectivity theory comes primarily from analyses of a handful of regions of interest derived from task-based activation studies in adults, often with poor replication across studies because of variability in the choice of brain regions examined (Muller et al., 2011; Vissers et al., 2011).
Although very little is currently known about brain connectivity in childhood ASD, one of the earliest signs of autism is enlarged head circumference or macrocephaly (Lainhart et al., 1997). Infants and young children with ASD show signs of early brain overgrowth (Courchesne et al., 2003), and postmortem studies of children with ASD show that they have an overabundance or excess numbers of neurons in the prefrontal cortex (Courchesne et al., 2011). Animal models of autism have provided evidence for hyper-connectivity in intrinsic functional circuits at very early time points in development (Testa-Silva et al., 2011; Yizhar et al., 2011). These findings of macrocephaly and hyper-connectivity have yet to be reconciled with human neuroimaging studies. As a result, there is a profound inconsistency in the extant literature, arising both from the failure to adequately distinguish weak task-related modulation of functional connectivity from intrinsic functional brain connectivity, and from inadequate attention to childhood autism (Amaral, 2011). Additionally, a major weakness in the field has been limited sample sizes and, more importantly, the lack of replication of findings using identical analytic procedures (Vissers et al., 2011).
In this era of human brain connectomics, it is increasingly recognized that understanding complex brain function and dysfunction critically depends on accurate characterization of connections between brain regions (Sporns, 2011). Comprehensive descriptions of whole-brain functional connectivity profiles in clinical disorders have begun to provide greater insights into the functional consequences of altered brain connectivity (Fornito et al., 2012; Supekar et al., 2008). Yet, very little is known about whole-brain functional connectivity in neurodevelopmental disorders such as ASD during childhood, and a mechanistic understanding of neural processing in ASD is completely absent.
Here we address these critical gaps by using task-free functional magnetic resonance imaging (fMRI) (Greicius et al., 2003) to characterize whole-brain functional connectivity in three independent cohorts totaling 110 children aged 7–13 with ASD and age-, gender-, and IQ-matched typically developing (TD) children. We test the hypothesis that childhood ASD is associated with altered intrinsic functional connectivity patterns that impact brain systems critical for social cognition. Critically, we replicate our key findings across the three cohorts and provide the most robust evidence for widespread functional brain hyper-connectivity in children with autism demonstrating that at earlier ages, the brain in ASD is largely functionally hyper-connected. Extensive additional analyses confirmed our findings of intrinsic functional hyper-connectivity in childhood ASD. Finally, we demonstrate that this pattern of functional hyper-connectivity predicted autism symptoms such that children with greater functional connectivity exhibited more severe impairment in the social domain.
Results
One cohort of 40 children (ASD = 20; TD = 20) was recruited at Stanford University; a second cohort of 40 children (ASD = 20; TD = 20) was recruited at Georgetown University and Children’s National Medical Center (CNMC); a third cohort of 30 children (ASD = 15; TD = 15) was recruited at New York University (NYU) and obtained from the National Database of Autism Research (NDAR; ndar.nih.gov). Each cohort consisted of well-characterized children with ASD and a well-matched group of TD children. Task-free fMRI data were acquired from each child in the Stanford, Georgetown/CNMC and NYU/NDAR cohorts (demographic data, data acquisition protocols, and data preprocessing procedures are described in Experimental Procedures). Here we report findings from the Stanford cohort. Convergent findings from the Georgetown/CNMC and NYU/NDAR cohorts are described in Supplemental Information.
Functional brain hyper-connectivity in ASD children compared to TD children, at the whole-brain level
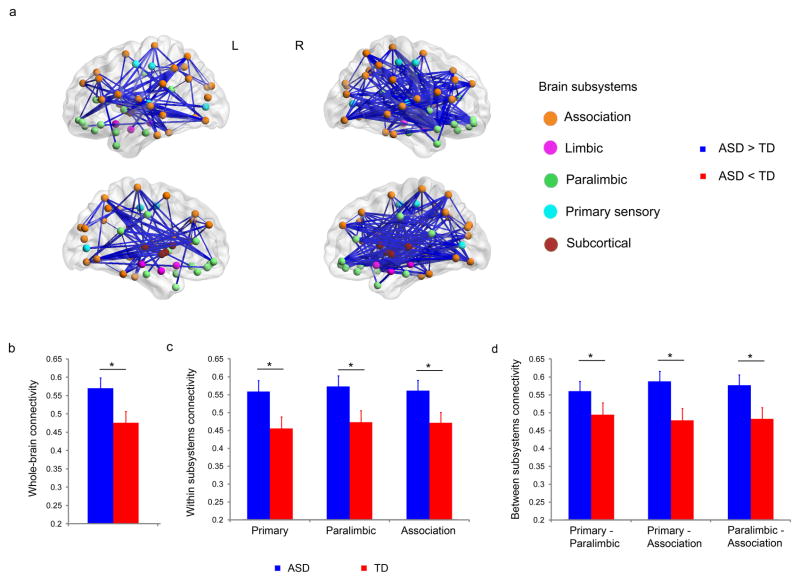
We first examined differences in whole-brain functional connectivity patterns between children with ASD and TD children. Preprocessed fMRI datasets were parcellated into 90 cortical and subcortical regions using anatomical templates (Tzourio-Mazoyer et al., 2002). A time series was computed for each of the 90 regions by averaging all voxels within each region. Wavelet analysis of the extracted regional fMRI time series (Supekar et al., 2009) was used to compute inter-regional functional connectivity across the whole brain. Mean global connectivity – the average of wavelet correlation values across all possible pair-wise functional connections – was higher in children with ASD, compared to TD children (p < 0.05, Cohen effect size d′ = 0.67, Figure 1b). Comparing wavelet correlation values of all possible pair-wise functional connections (n = 4005), we found that there were more instances of greater connectivity in children with ASD (Figure 1a). Specifically, 588 pairs (15%) of anatomical regions showed higher correlations in the ASD group than the TD group (p < 0.05, corrected for multiple comparisons (Zalesky et al., 2010)). No pairs of regions showed higher correlations in the TD, compared to the ASD group. Additional analyses were conducted to determine the robustness of these results. First, we examined the potential effects of alternate ROI specification strategies on our results. We repeated our entire analysis using two additional ROI specification strategies: voxel-based in which the regions of interest were cortical and subcortical voxels selected using the procedure described by van den Heuvel and colleagues (van den Heuvel et al., 2008) and functionally-defined in which the regions of interest were 264 putative functional brain areas selected using the procedure described by Power and colleagues (Power et al., 2011). These two strategies along with our original AAL-based ROI specification strategy capture a wide range of ROI specification strategies: from random-voxel based to anatomically-defined to functionally-defined. Results from these additional analyses were consistent with the results from the original analysis (which used AAL-based ROIs), namely: compared to TD children, children with ASD showed significantly higher functional connectivity between pairwise ROIs. Second, we examined the potential effect of global signal regression on our results. We regressed out the global signal and repeated our functional connectivity analyses on the regressed out fMRI data. Results from these additional analyses were consistent with the results from the original analysis (which did not include global signal regression), namely: compared to TD children, children with ASD showed significantly higher functional connectivity. Taken together, results from these additional analyses further confirm the robustness of our findings of hyper-connectivity in children with ASD (see Supplemental Information for details).
Figure 1. Functional brain hyper-connectivity in children with ASD.
(a) 588 pairs (15%) of anatomical regions showed higher correlations in children with ASD, compared to the TD group (p < 0.05, corrected for multiple comparisons). No pairs of regions showed higher correlations in the TD, compared to the ASD group. (b) Mean whole-brain connectivity was higher in children with ASD compared to TD children (p < 0.05, d′ = 0.67). (c) Within subsystems, mean connectivity was higher in children with ASD in primary sensory, paralimbic, and association areas (p < 0.05, d′ > 0.70). 25% of the total functional connections within primary sensory, 10% within paralimbic, and 19% within association areas showed greater functional connectivity in ASD than TD. (d) Across subsystems, mean functional connectivity between primary sensory and paralimbic, between primary sensory and association, and between paralimbic and association areas were greater in children with ASD (p < 0.05, d′ > 0.49). 18% of functional connections between primary sensory and paralimbic, 17% between primary sensory and association, and 17% between paralimbic and association areas were greater in children with ASD. No links, either within or between subsystems, showed greater connectivity in the TD, compared to the ASD, group. * p < 0.05. Error bars represent standard error of mean. See also Figure S1, S4.
Functional brain hyper-connectivity in ASD children compared to TD children, at the subsystems level
To investigate whether differences in functional brain connectivity span multiple functional subsystems, we used the parcellation scheme proposed by Mesulam (Mesulam, 1998) to examine functional connectivity in five functional subsystems: primary sensory, subcortical, limbic, paralimbic, and association areas. Each subsystem has a distinct cytoarchitectonic profile and subserves a unique set of functions, collectively mapping external sensory information to cognition (Mesulam, 1998). Higher mean functional connectivity in children with ASD, compared with TD children, was detected in the primary sensory, paralimbic and association areas (p < 0.05, d′ > 0.7, Figure 1c). Children with ASD also showed higher mean connectivity across subsystems: between primary sensory and paralimbic, primary sensory and association, and paralimbic and association (p < 0.05, d′ > 0.49, Figure 1d). No links between or within any of these subsystems showed greater connectivity in the TD group, compared with the ASD group. These results suggest that hyper-connectivity in ASD spans multiple functional subsystems of the human brain. The paralimbic subsystem consists of the insula, anterior cingulate cortex, posterior cingulate cortex and the orbitofrontal cortex, while association areas include the lateral frontal and parietal cortices.
Functional brain hyper-connectivity in ASD children between proximal as well as distant anatomical regions
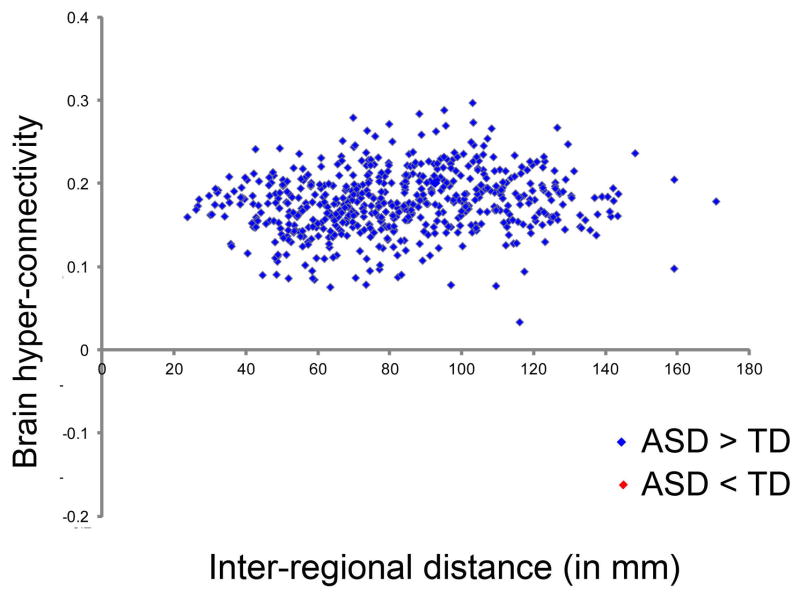
Reports in the literature suggest that short and long-range connections may be differentially affected in ASD (Courchesne and Pierce, 2005). To examine whether both short and long-range intrinsic functional connectivity is disrupted in children with ASD, we examined differences in regional functional connectivity in the two groups as a function of the interregional distance. The distance between two regions was computed by calculating the Euclidean distance between centroids of those regions (Fair et al., 2009; Supekar et al., 2009). We found that, compared to TD children, children with ASD showed higher functional connectivity across all distances examined (Figure 2). Thus, functional hyper-connectivity in ASD was observed between both proximal as well as distant anatomical regions.
Figure 2. Functional brain hyper-connectivity in children with ASD as a function of interregional distance.
Connections across all distances showed higher levels of hyper-connectivity in children with ASD, compared to TD children. Connectivity values that were stronger in children with ASD are shown in blue, connections that were stronger in TD children are in red (none in this cohort). Interregional distance d was computed by calculating the Euclidean distance between region centroids. See also Figure S2, S5.
Functional brain hyper-connectivity in ASD children is associated with high amplitude of low frequency fluctuations
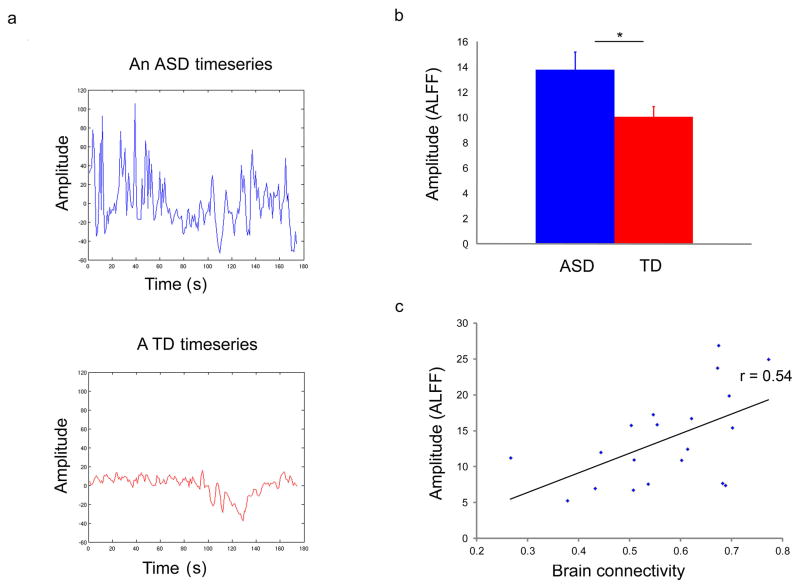
To investigate potential node-level abnormalities contributing to global hyper-connectivity in children with ASD, we examined the amplitude of low frequency fluctuations (ALFF) in the regional fMRI signal. Whereas functional connectivity provides an index of temporal synchrony between low frequency fluctuations in regional fMRI signals, ALFF is a measure of regional changes in signal level (Yang et al., 2007). We computed ALFF values for each of the 90 anatomical regions of interest for each participant (see Experimental Procedures for details). We found that the global mean ALFF values were greater in the ASD group than the TD group (p < 0.05, d′ = 0.68, Figure 3a,b). Furthermore, higher regional ALFF was associated with higher levels of whole-brain connectivity in children with ASD (r = 0.54, p = 0.01, Figure 3c). This empirical finding together with neurophysiological modeling of the underlying neural mechanisms (see below) suggests that both local circuit abnormalities and inter-regional hyper-connectivity may contribute to atypical brain function in ASD (Yizhar et al., 2011).
Figure 3. Higher levels of amplitude of BOLD oscillations are associated with functional brain hyper-connectivity in children with ASD.
(a) fMRI timeseries averaged across all gray-matter voxels in the brain from a representative child with ASD (blue) and a TD child (red), illustrating abnormally high amplitude fluctuations in children with ASD. (b) Mean amplitude of fMRI BOLD oscillations (ALFF) was greater in ASD than TD (p < 0.05, d′ = 0.68). (C) Higher regional ALFF was associated with higher levels of whole-brain connectivity in children with ASD (r = 0.54, p = 0.01). * p < 0.05. Error bars represent standard error of mean. See also Figure S3, S6.
Replication of results in two additional independent cohorts of children with ASD and TD children
We repeated our entire fMRI analysis on the second group of children from the Georgetown/CNMC cohort as well as the third group of children from the NYU/NDAR cohort (Table S1). In spite of differences in geographical location (Northern California vs. Washington DC vs. New York), scanner (GE vs. Siemens), fMRI pulse sequence (spiral in-out vs. echo planar imaging) and other data acquisition protocols, results from both the Georgetown/CNMC and NYU/NDAR cohorts entirely replicated the Stanford-cohort findings of widespread functional hyper-connectivity, enhanced ALFF as well as significant associations between ALFF and functional hyper-connectivity (see Supplemental Information for details).
Functional brain hyper-connectivity in ASD children predicts symptom severity
To investigate the extent to which widespread functional brain hyper-connectivity is associated with severity of symptoms in ASD, we examined the relationship between whole-brain functional connectivity and Autism Diagnostic Observation Schedule (ADOS) (Lord, 2000) and Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994) scores, using multivariate sparse regression analysis (Friedman et al., 2009) and nonparametric hypothesis testing (see Experimental Procedures for details). Functional brain hyper-connectivity predicted scores on the Social domain of the ADOS (p = 0.002) as well as the Social domain of the ADI-R (p = 0.04) such that children who showed greater levels of functional connectivity were more severely impaired in the social domain. This effect was independently replicated in the Georgetown/CNMC cohort for ADOS Social (p = 0.001) as well as ADI-R Social (p = 0.03) scores. Combining data from all 55 children with ASD across the three cohorts also replicated this effect for both the ADOS Social (p = 0.001) and the ADI-R Social (p = 0.001) domain scores, further demonstrating the robustness of our findings.
Discussion
To our knowledge this is the first study to examine functional connectivity at the whole-brain level in children with ASD. We analyzed data from 110 children collected across three different sites, providing the largest sample of pediatric brain imaging data to date. Our findings from multiple cohorts provide convergent, robust and replicable evidence for widespread functional brain hyper-connectivity in children with ASD. Our findings of functional hyper-connectivity are bolstered not only by the unprecedented triple-replication, but also by a link to clinical symptoms of ASD. Children with more severe impairment in the social domain exhibited greater functional connectivity. Our study is the first to report a significant brain-behavior relationship linking aberrant whole-brain functional connectivity to one of the core symptoms of ASD.
Widespread functional brain hyper-connectivity in childhood ASD
We examined functional whole-brain connectivity in children with ASD and observed widespread hyper-connectivity in these children compared to typically developing children. Extensive additional analyses were conducted to determine the robustness of our findings. Specifically, we examined the potential effect of alternate ROI specification strategies as well as global signal regression on our findings. Results from these additional analyses confirmed our findings of widespread functional brain hyper-connectivity in ASD children. More importantly, to address the recent concerns about the effect of subject motion on functional connectivity findings (Deen and Pelphrey, 2012), (1) we used stringent motion inclusion criteria and matched the ASD groups and TD groups in terms of motion parameters; (2) we examined functional connectivity within a narrow frequency range (0.01 to 0.05 Hz; Scale 3) further removing physiological and motion-related artifacts (Cordes et al., 2001; Cordes et al., 2000); (3) we computed correlations between movement parameters and brain connectivity values and found that there was no significant correlation between mean brain connectivity values and movement parameters; (4) we applied the revised data scrubbing procedure described by Power and colleagues (Power et al., 2012) and found that our results remained unchanged after applying the scrubbing procedures; (5) we performed imputation analysis that replaces volumes with relatively high motion proposed by Carp and colleagues (Carp, 2011) and found that our results remained unchanged after applying the imputation procedures. Critically, we replicated our main findings of brain hyper-connectivity in ASD across three independent cohorts further providing robust evidence for widespread functional brain hyper-connectivity in children with autism.
Very few studies have examined functional connectivity in young children with ASD. A previous study reported weaker inter-hemispheric functional connectivity between temporal and prefrontal language areas in sleeping toddlers with ASD after regressing out auditory stimulus processing (Dinstein et al., 2011). The differential influence of connectivity changes in ASD during sleep is currently not well understood. Another study of 7–13 year-olds found increased connectivity of striatal systems in children with ASD compared to TD children (Di Martino et al., 2011). This report of “ectopic” hyper-connectivity of a specific system in children with ASD is in line with our current findings, and extends them to the whole brain level for the first time. Importantly, our study provides novel evidence for whole-brain hyper-connectivity in awake, non-sedated children with ASD. Additionally, subsystem analyses revealed hyper-connectivity across multiple functional subsystems, including sensory and association cortices, in children with ASD. These findings point to aberrant patterns of functional connectivity in brain systems related to cognitive, social and affective processes (Mesulam, 1998). Examining the aberrant patterns of functional connectivity as a function of anatomical distance, we found functional hyper-connectivity in ASD between both proximal as well as distant anatomical regions, pointing to aberrant integration and segregation within both short- and long-range functional circuits in children with ASD (Sporns et al., 2000; Supekar et al., 2009). Taken together, these findings provide novel evidence for widespread functional brain hyper-connectivity in childhood ASD at both the whole-brain level and at the level of major functional subsystems, for both short- and long-range anatomical connections.
Mechanisms underlying functional brain hyper-connectivity in childhood ASD
To better characterize the neural mechanisms underlying functional hyper-connectivity in ASD, we then examined the amplitude of low frequency fluctuations (ALFF) in the regional fMRI signal. We found that abnormally high levels of fluctuations in regional fMRI signals were associated with higher levels of global functional hyper-connectivity in children with ASD. These results show that enhanced regional ALFF in children with ASD arising from aberrant balance of excitation and inhibition in local neural circuits (Testa-Silva et al., 2012; Tuchman and Cuccaro, 2011; Yizhar et al., 2011) may be an important factor that contributes to inter-regional hyper-connectivity observed in our fMRI data as well as related electrophysiological data (Léveillé et al., 2010). This potential mechanistic underpinning of our results is consistent with an increasingly promising theory of autism, which postulates that the neurophysiological substrate underlying the disorder is an imbalance between excitation and inhibition (Rubenstein, 2010; Rubenstein and Merzenich, 2003; Vattikuti and Chow, 2010; Yizhar et al., 2011). Specifically, the theory suggests that the imbalance of excitation and inhibition in the local brain circuits subserving sensory, social, and affective processes, possibly caused by either increased synaptic excitation or decreased synaptic inhibition, could engender cognitive and behavioral deficits observed in ASD. Importantly, the proposed theory unifies multiple lines of experimental findings in ASD. Molecular studies have indicated gene-, receptor- and enzyme-level deficits in inhibitory signaling pathways involving gamma-aminobutryic acid (GABA) in ASD (Baroncelli et al., 2011; Gatto and Broadie, 2010; Pizzarelli and Cherubini, 2011). Findings from imaging studies have suggested that ASD is associated with hyper-reactivity and high frequency cortical oscillations (Gomot et al., 2008; Orekhova et al., 2007). Notably, epidemiological studies have consistently reported high levels of epilepsy in children with ASD (Tuchman and Cuccaro, 2011). Indeed, over a third of children with ASD have comorbid epilepsy and half of them have epileptoform EEGs (Clarke et al., 2005; Matsuo et al., 2010). We postulate that such abnormal neuronal discharges arising from excitation-inhibition imbalance caused by atypical underlying cellular/molecular circuitry might contribute to high levels of intrinsic ALFF responses and global hyper-connectivity. Based on evidence from animal studies (Rubenstein and Merzenich, 2003), we further propose that this hyper-connected brain state may make it more difficult for children with ASD to modulate brain activity levels in response to cognitive demands.
Taken together, empirical findings from three different cohorts of children provide new insights into the mechanisms underlying widespread functional brain hyper-connectivity in childhood autism. More broadly, our findings provide novel brain-system level empirical support for the unifying theory that autism arises from an imbalance of excitation and inhibition in developing neural systems (Rubenstein and Merzenich, 2003), and further suggest that local circuit hyperexcitability as a result of this imbalance likely contributes to aberrations in global brain connectivity in autism.
Developmental account of functional brain hyper-connectivity in childhood ASD
The majority of published neuroimaging studies of ASD have focused on adolescents or adults with the disorder, leaving the question of brain connectivity in childhood ASD relatively open. The current results suggest that in children with ASD, unlike previously reported in adults with ASD (Assaf et al., 2010; Gotts et al., 2012; Kennedy and Courchesne, 2008; Monk et al., 2009; von dem Hagen et al., 2012), there are more instances of intrinsic functional hyper-connectivity than under-connectivity. This suggests that there may be a developmental trajectory in ASD that is altered from that of typical development. Structural neuroimaging studies of ASD provide evidence that brain volumetric differences observed in early childhood can be diminished or normalized or over-compensated with development (Amaral, 2011; Via et al., 2011).
Although the majority of studies of adolescent and adulthood ASD report intrinsic functional under-connectivity in the disorder (Assaf et al., 2010; Gotts et al., 2012; Kennedy and Courchesne, 2008; Monk et al., 2009; von dem Hagen et al., 2012), one of the few studies that has examined children below age 12 have reported functional hyper-connectivity of the striatum in children with ASD (Di Martino et al., 2011). Longitudinal studies are needed to fully characterize age-related changes in brain connectivity associated with ASD. However, the findings here suggest that there may be a critical developmental shift, perhaps during the time of puberty (Peper et al., 2011), that differentially affects the maturation of connections in ASD (Uddin et al., 2013).
Behavioral consequences of functional brain hyper-connectivity in childhood ASD
Our findings of widespread functional hyper-connectivity are strengthened not only by replication across the three cohorts, but also by a link to ASD symptoms. Brain hyper-connectivity predicted autism symptoms such that children with greater connectivity exhibited more severe impairment in the social domain. This brain-behavior relationship suggests that aberrant functional connectivity may underlie social deficits, which are the hallmark of ASD.
The relationships between functional brain hyper-connectivity and cognitive deficits in ASD we report here may provide a framework for understanding the complex behavioral manifestation of the disorder. Brain hyper-connectivity may result in isolation of neural systems involved in high-level cognitive processes, thus contributing to some of the core behavioral characteristics of the disorder including deficits in navigating real-world social scenarios. Brain hyper-connectivity may limit flexible resource allocation, resulting in the rigidity and need for sameness that is often observed in individuals with ASD. The current findings provide for the first time a neural processing account of cognitive deficits in childhood ASD. At the same time, such hyper-connectivity might also contribute to “islets” of spared ability in autism, as have been described in the domains of visual search (Keehn et al., 2012) and mathematics (Baron-Cohen et al., 2001).
Conclusions
Understanding the neurobiology of ASD requires a critical examination of brain connectivity in children (Belmonte et al., 2004; Minshew and Keller, 2010; Uddin and Menon, 2009). This study addresses a critical and controversial question regarding the nature and extent of brain connectivity alterations in childhood ASD. Our findings not only provide direct evidence for hyper-connectivity at the whole-brain level spanning multiple functional subsystems, but also demonstrate a link to core clinical symptoms in school age children with ASD. Our findings also provide novel insights into a link between enhanced local fluctuations and global aberrations in brain connectivity in school age children with the disorder. More generally, this work challenges the notion of under-connectivity as the central neurobiological feature of ASD. Furthermore, our study highlights the importance of studying neurodevelopmental disorders closer to their onset, rather than in adulthood when a lifetime of compensatory mechanisms may have already taken place (Amaral, 2011).
Experimental Procedures
I. Participants
Stanford Cohort
Twenty children with autism spectrum disorder (ASD) and 20 age-, gender-, and IQ-matched typically developing (TD) children participated in this study after giving written, informed consent. For those subjects who were unable to give informed consent, written, informed consent was obtained from their legal guardian. The study protocol was approved by the Stanford University Institutional Review Board. The children with ASD (16 males, 4 females) ranged in age from 7 to 13 years (mean age: 10.1) with an IQ range of 78 to 142 (mean IQ: 113); the TD children (16 males, 4 females) ranged in age from 7 to 13 years (mean age: 10) with an IQ range of 79 to 136 (mean IQ: 111) (Table S1). Participants were recruited locally, from schools and clinics near Stanford University. All children were required to have a Full Scale IQ≥70, as measured by the Wechsler Abbreviated Scale of Intelligence (WASI).
Children with ASD received a diagnosis based on scores from the Autism Diagnostic Interview-Revised (ADI-R) (Le Couteur et al., 1989; Lord et al., 1994) and/or the Autism Diagnostic Observation Schedule (ADOS) (Lord, 2000) following criteria established by the National Institute of Child Health & Human Development/National Institute of Deafness and Other Communication Disorders Collaborative Programs for Excellence in Autism (Lainhart, 2006).. Children with ASD were screened through a parent phone interview and excluded if they had any history of known genetic, psychiatric, or neurological disorders (e.g., Fragile X syndrome or Tourette’s syndrome), or were currently prescribed anti-psychotic medications. TD children were screened and excluded if they or a first-degree relative had developmental, language, learning, neurological, psychiatric disorders, or psychiatric medication usage, or if the child met the clinical criteria for a childhood disorder on the Child Symptom Inventory – Fourth Edition or Child and Adolescent Symptom Inventory. All participants underwent a battery of standardized neuropsychological assessments including WASI (Wechsler Intelligence Scale for Children–3rd Edition, Wechsler Intelligence Scale for Children–4th Edition, or Wechsler Abbreviated Scale of Intelligence (The Psychological, 1999)), and the Wechsler Individual Achievement Test (WIAT, 2nd edition). Full Scale IQ was determined from scores on the WASI.
Georgetown/CNMC Cohort
Twenty children with autism spectrum disorder (ASD) and 20 age-, and gender-matched typically developing (TD) children participated in this study after providing assent and parental consent according to guidelines of the Georgetown University Institutional Review Board. The children with ASD (15 males, 5 females) ranged in age from 8 to 13 years (mean age: 11.04) with an IQ range of 85 to 138 (mean IQ: 114); the TD children (12 males, 8 females) ranged in age from 8 to 13 years (mean age: 10.83) with an IQ range of 99 to 138 (mean IQ: 123) (Table S1). Children were recruited through the local community via advertisements and a hospital’s outpatient clinic specializing in ASD and neuropsychological assessment. All children were required to have a Full Scale IQ≥70, as measured by WASI.
Children with ASD received a clinical diagnosis using criteria similar to the Stanford cohort. Additionally, they also received a clinical diagnosis based on Diagnostic and Statistical Manual of Mental Disorders–Fourth Edition –Text Revised criteria (American Psychiatric Association., 2000). The participant inclusion and exclusion criteria were identical to those of the Stanford cohort. All participants underwent a battery of standardized neuropsychological assessments including the WASI. Full Scale IQ was determined from scores on the WASI.
NYU/NDAR Cohort
Fifteen children with autism spectrum disorder (ASD) and 15 age-, gender-, and IQ-matched typically developing (TD) children were included in this study. The subjects were identified from public domain research data repositories. Specifically, ASD subjects were identified by querying the National Database for Autism Research repository (NDAR; http://ndar.nih.gov). The query parameters were: age between 7 and 13, phenotype: ASD, task-free fMRI data: present. The age range was chosen to match that of the Stanford and Georgetown/CNMC cohorts. The query results yielded 15 children with ASD (11 males, 4 females) ranging in age from 7 to 13 years (mean age: 10.37) with an IQ range of 73 to 132 (mean IQ: 99), with task-free fMRI data. Notably, all of the subjects identified belong to one collection submitted by Francisco Castellanos at the New York University (NYU). This collection did not include data from TD children. To address this issue, we queried the ADHD200 dataset (http://fcon_1000.projects.nitrc.org/indi/adhd200/) which consists of task-free fMRI data from TD children and children with attention-deficit/hyperactivity disorder (ADHD) across 8 different sites including Dr. Castellanos’ lab at NYU. The query parameters were: site: NYU, age between 7 and 13, phenotype: typically developing, task-free fMRI data: present. The query results yielded 60 TD children. We used an in-house matching algorithm to select a subset of 15 typically developing children such that the mean age, mean IQ, and gender distribution was matched to the ASD group. The algorithm identified a well-matched subset of 15 TD children (11 males, 4 females) ranging in age from 7 to 13 years (mean age: 10.22) with an IQ range of 80 to 142 (mean IQ: 107) (Table S1).
II. Functional MRI
A. Data Acquisition & Preprocessing
For each subject in each of the three cohorts (Stanford, Georgetown/CNMC, and NYU/NDAR), a resting-state scan was acquired using protocols described in detail in Supplementary Information. The acquired data from the three cohorts underwent identical preprocessing, as described in Supplementary Information and analytical processing, as described below.
B. Analysis of Whole-Brain Functional Connectivity
Preprocessed fMRI datasets were parcellated into 90 cortical and subcortical regions using previously published AAL anatomical template. Wavelet analysis of the extracted regional fMRI time series was used to compute inter-regional functional connectivity across the whole brain. We first examined differences in whole-brain functional connectivity patterns between the two groups. To demonstrate the robustness of our findings, these analyses were repeated using two alternate ROI specification strategies: voxel-based (van den Heuvel et al., 2008) and functionally-defined (Power et al., 2011), which provided convergent results (see Supplementary Information for details).
Next, to investigate whether differences in functional brain connectivity spans multiple functional subsystems, we used the parcellation scheme of Mesulam.
The aforementioned procedures are described in detail in the Supplementary Information.
C. Analysis of Amplitude of Low-frequency Fluctuations
To further characterize functional connectivity, we computed amplitude of low frequency fluctuations (ALFF) in the regional fMRI signal. While functional connectivity provides an index of temporal synchrony between low frequency fluctuations in regional fMRI signals, ALFF is a measure of regional activity. ALFF has been previously used to quantify regional intrinsic activity (He et al., 2007; Zang et al., 2007) and has been suggested to reflect spontaneous neuronal activity (Zou et al., 2008). We computed ALFF values for each of the 90 anatomical regions of interest and the mean of these 90 ALFF values for each subject. For each anatomical region of interest, ALFF was computed by (1) transforming the regional timeseries to the frequency domain, (2) calculating the power spectra of this transformed signal, (3) averaging the square-root of amplitude at each frequency component across 0.01–0.08 Hz (Zou et al., 2008).
D. Analysis of Differences in Functional Connectivity as a Function of Anatomical Distance
We next examined the relationship between differences in regional correlation values (connectivity) in the two groups and the interregional distance. The distance between two regions was computed by calculating the Euclidean distance between centroids of those regions
E. Analysis of Functional Connectivity as a Function of Symptom Severity: Prediction Analysis
We next investigated whether regional connectivity in the ASD group predicted symptom severity. We used a novel multivariate sparse regression approach (Friedman et al., 2009) which models the relationship between the dependent variable (scores on ADOS or ADI-R Social domain) and the multiple independent variables (whole-brain inter-regional functional connectivity: 4005 wavelet correlation values). An advantage of using a multivariate sparse regression approach, as opposed to traditional univariate correlation, is that it examines patterns of connectivity across the whole-brain as opposed to a single average measure of whole-brain connectivity, and is thus more sensitive. More importantly, such sparse methods are particularly elegant when the number of possible predictor variables is large and the number of observations is small, which is the case in our analysis. We used GLMnet (http://www-stat.stanford.edu/~tibs/glmnet-matlab), a state-of-the-art sparse regression algorithm that is widely used to examine multivariate relationships in large-scale genomic data (Friedman et al., 2009). GLMnet computes the model in such a way that the coefficients of independent variables which do not contribute to the prediction of the dependent variable are set to zero, thus producing sparse-interpretable solutions. L1-norm regularization is used to produce this sparse model. Nonparametric testing was used to assess the performance of the regression algorithm in predicting symptom severity. We first estimated R2, the proportion of variance explained by the model, using a Leave-One-Out Cross Validation (LOOCV) procedure. In LOOCV, data are divided into N folds. A sparse regression model is built using N−1 folds leaving out one sample. The left out sample is then predicted using this model, and the predicted value is noted. The above procedure is repeated N times by leaving out one sample each time, and finally an R2 is computed based on the observed and predicted values. This cross-validation procedure avoids over-fitting that is likely to happen when the number of samples is low and the number of parameters in the models is large, which is the case in our analyses. Finally, the statistical significance of the sparse model was assessed using non-parametric analysis. The empirical null distribution of R2 was estimated by generating 10,000 surrogate datasets under the null hypothesis that there was no association between scores on ADOS or ADI-R Social domain and whole-brain inter-regional functional connectivity patterns. Each surrogate dataset Di of size equal to the observed dataset was generated by permuting the labels (scores on ADOS or ADI-R Social domain) on the observed data points. The sparse model computed on the observed data was used to predict labels of each surrogate dataset Di. Ri2 was computed using the actual labels of Di and predicted labels. This procedure produces a null distribution of R2 of the sparse model. The statistical significance (p value) of the sparse model was then determined by counting the number of Ri2 greater than R2 and then dividing that count by the number of Di (10,000 in our case).
F. Participant Motion Characterization
As reported in our manuscript, we address the critical issue related to motion in resting-state functional connectivity analyses, rigorously in several ways. First, we used stringent motion inclusion criteria, which is within the acceptable range of pediatric clinical neuroimaging studies. Second, the ASD group and TD groups at each of the three sites were very well-matched in motion parameters. Third, we examined functional connectivity within a narrow frequency range (0.01 to 0.05 Hz; Scale 3). Several studies have now shown unambiguously that narrow-band pass filtering in frequencies corresponding to Scale 3 is important for removing physiological and motion-related artifacts (Cordes et al., 2001; Cordes et al., 2000). Fourth, we computed correlations between movement parameters and brain connectivity values. We found that there was no significant correlation between mean brain connectivity values and movement parameters at each of three sites. Fifth, we applied the data scrubbing procedure the more stringent “revised data scrubbing” procedure by Power and colleagues (Power et al., 2012) and (ii) “imputation” procedure by Carp and colleagues (Carp, 2011), and found that our results remained unchanged after applying these correction procedures.
The details of these analyses and the ensuing results are described in detail in Supplementary Information. These results, and more importantly replication of our main findings of widespread brain hyper-connectivity in three independent cohorts comprised of children with varied movement parameters collected across three different scanners, confirm that our findings are robust against potential movement confounds.
II. Structural MRI
A. Data acquisition
For each subject in each of the three cohorts (Stanford, Georgetown/CNMC, and NYU/NDAR), a structural MRI scan was acquired using protocols described in detail in Supplementary Information.
Supplementary Material
Children with ASD show functional hyper-connectivity across multiple brain regions
Both long- and short-range connections were predominantly hyper-connected in ASD
Brain hyper-connectivity was associated with enhanced levels of local fluctuations
ASD children with greater hyper-connectivity exhibited more severe social deficits
Acknowledgments
This research was supported by grants from the Singer Foundation, the Isadore and Bertha Gudelsky Foundation, Stanford Institute for Neuro-Innovation & Translational Neurosciences, Children’s National Medical Center and the NIH (HD047520, HD059205, DC0111095, MH084164, MH084961, K01MH092288, K23MH086111 and P30HD40677). We greatly appreciate the contributions of the participants without which this work would not be possible.
Footnotes
Author contributions
K.S. and V.M. conceived and designed study; K.S. and V.M. analyzed data; K.S., L.Q.U. and V.M. wrote the paper; A.K. collected Stanford cohort imaging data; J.P. collected and analyzed Stanford cohort clinical data; W.D.G., L.K, B.E.Y and C.J.V. contributed Georgetown/CNMC cohort data and provided feedback on the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG. The promise and the pitfalls of autism research: an introductory note for new autism researchers. Brain research. 2011;1380:3–9. doi: 10.1016/j.brainres.2010.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, O’Boyle JG, Schultz RT, Pearlson GD. Abnormal functional connectivity of default mode subnetworks in autism spectrum disorder patients. NeuroImage. 2010;53:247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J. Prevalence of autism spectrum disorders - autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19. [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Baroncelli L, Braschi C, Spolidoro M, Begenisic T, Maffei L, Sale A. Brain plasticity and disease: a matter of inhibition. Neural plasticity. 2011;2011:286073. doi: 10.1155/2011/286073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. Journal of Neuroscience. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J. Optimizing the order of operations for movement scrubbing: Comment on Power et al. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.12.061. [DOI] [PubMed] [Google Scholar]
- Clarke DF, Roberts W, Daraksan M, Dupuis A, McCabe J, Wood H, Snead OC, 3rd, Weiss SK. The prevalence of autistic spectrum disorder in children surveyed in a tertiary care epilepsy clinic. Epilepsia. 2005;46:1970–1977. doi: 10.1111/j.1528-1167.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA: the journal of the American Medical Association. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K. Neuron number and size in prefrontal cortex of children with autism. JAMA: the journal of the American Medical Association. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Current opinion in neurobiology. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Deen B, Pelphrey K. Perspective: Brain scans need a rethink. Nature. 2012;491:S20. doi: 10.1038/491s20a. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, Lord C, Castellanos FX, Milham MP. Aberrant Striatal Functional Connectivity in Children with Autism. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, Courchesne E. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70:1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. NeuroImage. 2012;62:2296–2314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2009;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- Gatto CL, Broadie K. Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models. Frontiers in synaptic neuroscience. 2010;2:4. doi: 10.3389/fnsyn.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomot M, Belmonte MK, Bullmore ET, Bernard FA, Baron-Cohen S. Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain. 2008;131:2479–2488. doi: 10.1093/brain/awn172. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012 doi: 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M, Krasnow B, Reiss A, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T. Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. NeuroImage. 2007;35:488–500. doi: 10.1016/j.neuroimage.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev. 2012;36:1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Shih P, Brenner LA, Townsend J, Muller RA. Functional connectivity for an “Island of sparing” in autism spectrum disorder: An fMRI study of visual search. Human brain mapping. 2012 doi: 10.1002/hbm.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. NeuroImage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008 doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart JE. Advances in autism neuroimaging research for the clinician and geneticist. Am J Med Genet C Semin Med Genet. 2006;142C:33–39. doi: 10.1002/ajmg.c.30080. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Piven J, Wzorek M, Landa R, Santangelo SL, Coon H, Folstein SE. Macrocephaly in children and adults with autism. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:282–290. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J. Autism diagnostic interview: a standardized investigator-based instrument. J Autism Dev Disord. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Léveillé C, Barbeau EB, Bolduc C, Limoges E, Berthiaume C, Chevrier E, Mottron L, Godbout R. Enhanced connectivity between visual cortex and other regions of the brain in autism: A REM sleep EEG coherence study. Autism Research. 2010;3:280–285. doi: 10.1002/aur.155. [DOI] [PubMed] [Google Scholar]
- Lord C. Commentary: achievements and future directions for intervention research in communication and autism spectrum disorders. J Autism Dev Disord. 2000;30:393–398. doi: 10.1023/a:1005591205002. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Maeda T, Sasaki K, Ishii K, Hamasaki Y. Frequent association of autism spectrum disorder in patients with childhood onset epilepsy. Brain Dev. 2010;32:759–763. doi: 10.1016/j.braindev.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Keller TA. The nature of brain dysfunction in autism: functional brain imaging studies. Curr Opin Neurol. 2010;23:124–130. doi: 10.1097/WCO.0b013e32833782d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011;21:2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, Elam M. Excess of high frequency electroencephalogram oscillations in boys with autism. Biol Psychiatry. 2007;62:1022–1029. doi: 10.1016/j.biopsych.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Peper JS, van den Heuvel MP, Mandl RC, Hulshoff Pol HE, van Honk J. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology. 2011;36:1101–1113. doi: 10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Pizzarelli R, Cherubini E. Alterations of GABAergic signaling in autism spectrum disorders. Neural plasticity. 2011;2011:297153. doi: 10.1155/2011/297153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. NeuroImage. 2012 doi: 10.1016/j.neuroimage.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL. Three hypotheses for developmental defects that may underlie some forms of autism spectrum disorder. Curr Opin Neurol. 2010;23:118–123. doi: 10.1097/WCO.0b013e328336eb13. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. The human connectome: a complex network. Annals of the New York Academy of Sciences. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Edelman GM. Connectivity and complexity: the relationship between neuroanatomy and brain dynamics. Neural networks: the official journal of the International Neural Network Society. 2000;13:909–922. doi: 10.1016/s0893-6080(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa-Silva G, Loebel A, Giugliano M, de Kock CP, Mansvelder HD, Meredith RM. Hyperconnectivity and Slow Synapses during Early Development of Medial Prefrontal Cortex in a Mouse Model for Mental Retardation and Autism. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa-Silva G, Loebel A, Giugliano M, de Kock CP, Mansvelder HD, Meredith RM. Hyperconnectivity and slow synapses during early development of medial prefrontal cortex in a mouse model for mental retardation and autism. Cereb Cortex. 2012;22:1333–1342. doi: 10.1093/cercor/bhr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Psychological, C. Wechsler Abbreviated Scale of Intelligence. San Antonio: Harcourt Brace & Co; 1999. [Google Scholar]
- Tuchman R, Cuccaro M. Epilepsy and autism: neurodevelopmental perspective. Curr Neurol Neurosci Rep. 2011;11:428–434. doi: 10.1007/s11910-011-0195-x. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Menon V. The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev. 2009;33:1198–1203. doi: 10.1016/j.neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Frontiers in Human Neuroscience. 2013 doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Boersma M, Hulshoff Pol HE. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. NeuroImage. 2008;43:528–539. doi: 10.1016/j.neuroimage.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Vattikuti S, Chow CC. A computational model for cerebral cortical dysfunction in autism spectrum disorders. Biol Psychiatry. 2010;67:672–678. doi: 10.1016/j.biopsych.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via E, Radua J, Cardoner N, Happe F, Mataix-Cols D. Meta-analysis of gray matter abnormalities in autism spectrum disorder: should Asperger disorder be subsumed under a broader umbrella of autistic spectrum disorder? Archives of general psychiatry. 2011;68:409–418. doi: 10.1001/archgenpsychiatry.2011.27. [DOI] [PubMed] [Google Scholar]
- Vissers ME, MXC, Geurts HM. Brain connectivity and high functioning autism: A promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- von dem Hagen EA, Stoyanova RS, Baron-Cohen S, Calder AJ. Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Social cognitive and affective neuroscience. 2012 doi: 10.1093/scan/nss053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, Zang YF, Gong QY. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. NeuroImage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.