Abstract
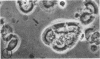
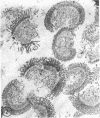
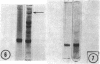
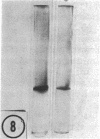
The major soluble protein of the isolated brush-border of the intestinal epithelium has a molecular weight and net charge indistinguishable from those of skeletal-muscle actin, as determined by polyacrylamide gel electrophoresis. Furthermore, this protein, isolated from acetone powders of the purified brush-border, undergoes a G to F transformation in the presence of Mg++. The filaments have a substructure indistinguishable from muscle actin, as seen by the negative-staining technique, and bind heavy meromyosin with the arrowhead configuration characteristic of actin. The filaments in the microvilli of the intact bruch-border also bind heavy meromyosin. Thus, actin seems to be present in intestinal epithelial cells.
Keywords: electron microscopy, gel electrophoresis, G to F transformation, heavy meromyosin binding
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold J. M. Cleavage furrow formation in a telolecithal egg (Loligo pealii). I. Filaments in early furrow formation. J Cell Biol. 1969 Jun;41(3):894–904. doi: 10.1083/jcb.41.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. C., Schroeder T. E. Cytoplasmic filaments and morphogenetic movement in the amphibian neural tube. Dev Biol. 1967 May;15(5):432–450. doi: 10.1016/0012-1606(67)90036-x. [DOI] [PubMed] [Google Scholar]
- Cloney R. A. Cytoplasmic filaments and cell movements: epidermal cells during ascidian metamorphosis. J Ultrastruct Res. 1966 Feb;14(3):300–328. doi: 10.1016/s0022-5320(66)80051-5. [DOI] [PubMed] [Google Scholar]
- Cloney R. A. Cytoplasmic filaments and morphogenesis: the role of the notochord in ascidian metamorphosis. Z Zellforsch Mikrosk Anat. 1969;100(1):31–53. doi: 10.1007/BF00343819. [DOI] [PubMed] [Google Scholar]
- Forstner G. G., Sabesin S. M., Isselbacher K. J. Rat intestinal microvillus membranes. Purification and biochemical characterization. Biochem J. 1968 Jan;106(2):381–390. doi: 10.1042/bj1060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano S., Oosawa F. Isolation and characterization of plasmodium actin. Biochim Biophys Acta. 1966 Oct 31;127(2):488–498. doi: 10.1016/0304-4165(66)90402-8. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Nachmias V. T., Huxley H. E. Electron microscope observations on actomyosin and actin preparations from Physarum polycephalum, and on their interaction with heavy meromyosin subfragment I from muscle myosin. J Mol Biol. 1970 May 28;50(1):83–90. doi: 10.1016/0022-2836(70)90105-1. [DOI] [PubMed] [Google Scholar]
- Nagai R., Rebhun L. I. Cytoplasmic microfilaments in streaming Nitella cells. J Ultrastruct Res. 1966 Mar;14(5):571–589. doi: 10.1016/s0022-5320(66)80083-7. [DOI] [PubMed] [Google Scholar]
- Perry M. M., John H. A., Thomas N. S. Actin-like filaments in the cleavage furrow of newt egg. Exp Cell Res. 1971 Mar;65(1):249–253. doi: 10.1016/s0014-4827(71)80075-7. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Korn E. D. Filaments of Amoeba proteus. II. Binding of heavy meromyosin by thin filaments in motile cytoplasmic extracts. J Cell Biol. 1971 Jan;48(1):216–219. doi: 10.1083/jcb.48.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T. E. Cytokinesis: filaments in the cleavage furrow. Exp Cell Res. 1968 Oct;53(1):272–276. doi: 10.1016/0014-4827(68)90373-x. [DOI] [PubMed] [Google Scholar]
- Schroeder T. E. The contractile ring. I. Fine structure of dividing mammalian (HeLa) cells and the effects of cytochalasin B. Z Zellforsch Mikrosk Anat. 1970;109(4):431–449. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Szollosi D. Cortical cytoplasmic filaments of cleaving eggs: a structural element corresponding to the contractile ring. J Cell Biol. 1970 Jan;44(1):192–209. doi: 10.1083/jcb.44.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Gibbins J. R. Microtubules and filaments in the filopodia of the secondary mesenchyme cells of Arbacia punctulata and Echinarachnius parma. J Cell Sci. 1969 Jul;5(1):195–210. doi: 10.1242/jcs.5.1.195. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Marsland D. A fine structural analysis of cleavage induction and furrowing in the eggs of Arbacia punctulata. J Cell Biol. 1969 Jul;42(1):170–184. doi: 10.1083/jcb.42.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weihing R. R., Korn E. D. Ameba actin: the presence of 3-methylhistidine. Biochem Biophys Res Commun. 1969 Jun 27;35(6):906–912. doi: 10.1016/0006-291x(69)90710-4. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]