Abstract
Purpose.
We followed cone and rod development in the pig and we correlated development with the potential for cone and rod precursor integration and differentiation following subretinal transplantation.
Methods.
Rod and cone precursors were identified during development by their position in the outer retina and by immunostaining for markers of differentiation. Embryonic retinal cells from green fluorescent protein (GFP)+ transgenic pigs at different developmental stages were transplanted into adult retinas and integration and differentiation was followed and quantified by immunostaining for markers of cone and rod differentiation.
Results.
Pig cones and rods are spatially segregated, allowing us to follow rod and cone development in situ. Gestation in the pig is 114 days. By embryonic day (E) 50, postmitotic cone progenitors had formed the outer two rows of the retina. These cone progenitors are marked by expression of Islet1 (ISL1) and Recoverin (RCVRN) (at this embryonic stage, RCVRN exclusively marks these cone precursors). By contrast, postmitotic neural retina leucine zipper (NRL)+ rod precursors, located interior to the cone precursors, did not appear until E65. At E50, before NRL+ rod precursors are evident, transplanted cells gave rise almost exclusively to cones. At, E57, transplanted cells gave rise to equal numbers of rods and cones, but by E65, transplanted cells gave rise almost exclusively to rods. Transplantation of cells at E85 or E105, as precursors initiate opsin expression, led to few integrated cells.
Conclusions.
Consistent with their sequential appearances in embryonic retina, these results demonstrate sequential and surprisingly narrow developmental windows for integration/differentiation of cone and rod precursors following transplantation.
Keywords: retinal transplantation, cones, precursors
We demonstrate sequential and surprisingly narrow windows for differentiation of embryonic rod and cone progenitors into rods and cones following subretinal transplantation.
Introduction
Rod photoreceptors are more sensitive to light and thus are critical for low-light vision, whereas cone photoreceptors are important for color vision and visual acuity.1 In humans, cones are concentrated into a pit-like structure in the central retina known as the fovea, whereas rods predominate in the peripheral retina.2 Rods express rhodopsin (RHO), whereas cones can express S opsin (sensitive to blue light, or UV in the mouse), M opsin (sensitive to green light), or L opsin (sensitive to red light).3–5 Interestingly, in the mouse, all cones are thought to express M opsin, and a subset of these M opsin+ cones also coexpress S opsin.6 As in the human fovea, cones in the pig are concentrated within the posterior retina in a region dorsal to the optic disk known as the area centralis or visual streak.3 Because of anatomical and physiological similarity between the pig and human eye, there is interest in using the pig as a model of photoreceptor development and retinal disease.
Rod and cone precursors are derived from proliferating retinal progenitors that express eye field transcription factors, including paired box protein (PAX)6, which diminish as the cells become postmitotic precursors.7–12 In the mouse, neural retina leucine zipper (NRL) marks rod precursors, but there is a paucity of early cone precursor markers, and it is still unclear when early photoreceptor precursors become fully committed to a cone precursor fate.
Cone and rod nuclei are completely segregated in the outer nuclear layer (ONL) of the swine retina, with cones comprising the outer two rows and rods confined to the inner rows of the ONL. This segregation allowed us to follow cell populations giving rise to cones and rods during embryonic retinal development. We show that postmitotic cone-committed precursors are in place in the outer retina before midgestation at embryonic day (E) 50 (gestation in the pig is ∼114 days), and they are marked by loss of PAX6, transient expression of the Islet1 (ISL1) transcription factor and Recoverin (RCVRN) expression. By contrast, postmitotic neural retina leucine zipper (NRL)+ rod precursors do not appear until E65. We transplanted embryonic retinal cells at different embryonic stages into adult retina to evaluate their potential to integrate and differentiate into cones and rods. At E50 where postmitotic PAX6−, ISL1+, RCVRN+ cone precursors have formed but NRL+ rod precursors are not yet evident, transplanted cells gave rise to cones. By contrast, cells at E57 gave rise to rods and cones in equal numbers, but by E65, transplanted cells gave rise to rods. These results demonstrate a sequential, transient integration/differentiation potential that coincides with the sequential appearance of cones and then rod precursors in the embryonic retina. By, E85 and E105, as photoreceptor precursors began to express opsins, few integrated cells were seen following transplantation (the cells remained in the subretinal space and did not integrate into the neural retina), demonstrating that the maturing photoreceptors do not integrate into the retina.
Materials and Methods
Tissue Processing and Immunohistochemistry
All methods were approved by the University of Louisville Institutional Animal Care and Use Committee and adhered to the ARVO Statement for Use of Animals in Ophthalmic and Vision Research. Domestic pigs were obtained from the National Swine Research Resource Center, University of Missouri. Pregnant sows were euthanized by ear vein injection of beuthanasia (a mixture of sodium pentobarbital sodium and sodium phenytoin, 0.1 mL/lb) through an ear vein catheter after sedation with ketamine/dexmedetomidine/atropine, and embryos were removed by cesarean section. Retinas from E50, E55, E65, E75, E85, and E105 were used in this study. Adult swine eyes were obtained from the local slaughterhouse. Both eyes from at least three pigs were used for each developmental time point. Eyes were enucleated and immediately immersed in the CO2-independent media on ice. Retinas for cryosections were then dissected and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 20 minutes followed by three washes with 0.1 M phosphate buffer. Tissues were then cryoprotected through 5%, 10%, 15% sucrose for 1 hour sequentially and 20% sucrose overnight. Each retina was bisected along the horizontal plane through the dorsal margin of the optic disc and vertically cut through the optic disc. Each of the four pieces was notched on its dorsal edge to preserve orientation. After that, the retinas were embedded with polyvinyl alcohol, polyethylene glycol-based optimal cutting temperature cutting reagent (OCT): 20% sucrose (2:1). Serial sectioning was performed at 18 μm on a cryostat and tissues were mounted on Super-Frost glass slides (Fisher Scientific, Pittsburgh, PA). Retinas for paraffin sections were fixed with 10% formalin for 48 hours. The retinas were then imbedded with 3% agar gel in 5% formalin and reoriented transversely, then dehydrated in 70% ethanol for paraffin embedding. The tissues were cut at 5 μm and hematoxylin-eosin (H&E) staining was performed on every fifth slide.
Frozen sections of swine retina were dried at 37°C for 15 minutes followed by a rinse through PBS for 5 minutes. The samples were then blocked with 2% bovine serum albumin, 5% serum, and 0.1% Triton X-100 at 25°C for 1 hour, then incubated with primary antibody at 4°C overnight. After primary antibodies had been removed and the samples washed, secondary antibodies were applied for 1 hour at 25°C. The primary antibodies used were rabbit anti-PAX6 (1:300; Millipore, Billerica, MA), rabbit anti-RCVRN (1:1500; Millipore), mouse anti-Rho (1:300; Millipore), rabbit anti-NRL (1:1000; a generous gift from Anand Swaroop, National Eye Institute, Bethesda, MD), mouse anti-ISL1 (1:20; generated by T. Jessell, University of Iowa hybridoma bank 39.3F7, Iowa City, IA), chicken anti-JH492 and JH455 to L/M and S opsins (1:5000; gifts from Jeremy Nathans, Johns Hopkins Medicine, Baltimore, MD),13 rabbit anti-PKCa (1:10,000; Sigma-Aldrich, St. Louis, MO), rabbit anti-calbindin (1:500; Thermo Scientific, Pittsburgh, PA). CHX10 antibodies used were polyclonal X1179p (Exalpha Biologicals, Shirley, MA) monoclonal Sc-365519, polyclonal N-terminal SC-21690, and polyclonal C-terminal SC-21692 (Santa Cruz Biotechnology, Santa Cruz, CA). Bound antibodies were visualized with either Alexa Fluor 488- (1:500; Invitrogen, Grand Island, NY) or Alexa Fluor 568- (1:500; Invitrogen) conjugated secondary antibodies. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI).
Central retinas (1 mm above the dorsal margin of the optic disc) and peripheral retinas (1 mm from the edge of the retina) were imaged on an Olympus FV300 confocal microscope (Olympus Confocal America, Inc., Center Valley, PA), using a ×60 oil objective (1.42 numerical aperature). Images shown are maximum projections of confocal stacks, adjusted for contrast and brightness with Adobe Photoshop Elements v9.0.2 (Adobe Systems, Inc., San Jose, CA). As a negative control, no immunostaining was evident in the absence of primary antibodies.
Embryonic Retinal Cell Isolation for Green Fluorescent Protein (GFP) Transgenic Pigs and Transplantation
For creation of GFP transgenic pigs, fibroblasts were transduced with a retrovirus expressing GFP under control of the viral cytomegalovirus promoter.4 Fibroblast nuclei were then transferred to oocytes for creation of transgenic pigs. The eyes from GFP+ pig embryos were enucleated and retinas were isolated and dissected in Mg2+/Ca2+-free Hanks' balanced salt solution (HBSS) and dissociated by incubating for 1 hour at 37°C in HBSS containing 20 units/mL papin and 0.005% DNase (Worthington Biochemicals, Lakewood, NJ). Retinal cells were then transferred to a solution with the papain inhibitor 1% ovomucoid (Worthington Biochemicals) and triturated. After centrifugation, the cells were washed and resuspended in Dulbecco's modified Eagle's medium high glucose without Ca and Mg on ice for transplantation.
Domestic pigs at 6 weeks of age were obtained from Oak Hill Genetics (Ewing, IL) and weighed before each experimental procedure (range, 12–16 kg). Iodoacetic acid (IAA) was dissolved in normal saline, and pigs were administered 12.5 mg/kg IAA intravenously via a catheter placed in the ear vein as we have described previously.5,14
Four days after IAA treatment, embryonic retinal cells were transplanted, as we have described.14 Pigs were sedated with tiletamine HCl and zolazepam HCl (2.0–8.8 mg/kg, Telazol; Zoetis, Inc., Florham Park, NJ) and intubated, and they were further sedated by intubated anesthesia with 1.5% to 2.0% isoflurane mixed with oxygen. Intravenous access was achieved by placement of a 21-gauge catheter in an ear vein Pupils were dilated and accommodation relaxed with topical applications of 2.5% phenylephrine hydrochloride and 1% tropicamide. A three-port pars plana vitrectomy was performed using instruments and surgical techniques used for vitreous surgery in humans. A core pars plana vitrectomy was performed using a suction of 100 to 150 mm Hg and a cutting rate of 800 oscillations per minute. Posterior vitreous detachments were made, and a bleb of neurosensory retinal detachment was created by injecting approximately 50 μL BSS Plus (Alcon, Fort Worth, TX) into the subretinal space using a 39-gauge needle; 20 μL cells at a concentration of 50,000 cells/μL were injected into two blebs created in the superior quadrant, as described previously.14 Retinas were harvested at 5 or 12 weeks. Results are shown at 12 weeks, but the 5-week time point gave similar results. Four eyes were transplanted with E50 and E57 retinal cells, eight eyes with E65 cells, and 10 eyes with E85 and E105 cells. Ten eyes were used for sham transplant controls.
Pigs were euthanized 12 weeks after cell transplantation with beuthanasia (1 mL per 5 kg) administered through an ear vein catheter. Eyes were enucleated, and retinas were isolated and fixed by immersion in 4% paraformaldehyde for 30 minutes. Retinal flat mounts were observed under low power with a fluorescence microscope to identify GFP+ cells at sites of cell transplantation area. Then strips of retina that extended from the dorsal to the ventral margin of the eyecup (including the surgery bleb area) were bisected and dehydrated by sucrose and embedded in OCT, and 20-μm frozen sections were cut for immunostaining and cell counting across the bleb area.
Results
Transition From a Single Neuroblastic Layer to Separate ONL and INL Layers Occurs in a Central-to-Peripheral Fashion in the Swine Retina at E75
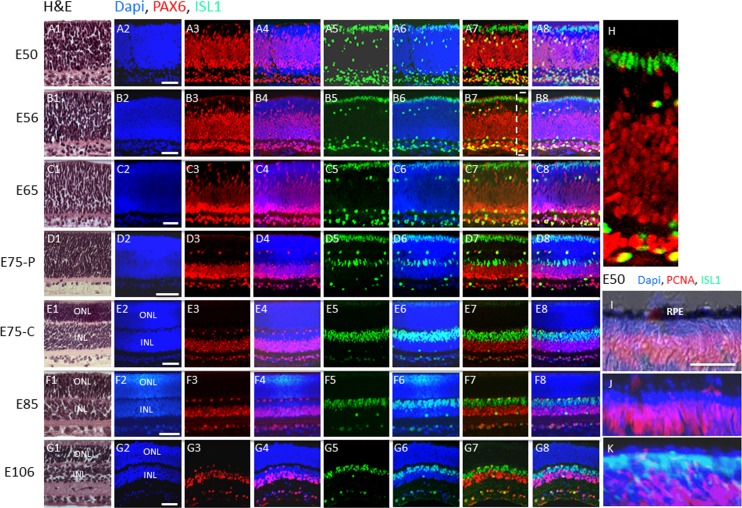
Hematoxylin-eosin staining of sections of swine retinas at various stages of development are shown in Figures 1A1 through 1G1. Separation of the single neuroblastic layer into the ONL and inner nuclear layer (INL) is first evident with appearance of eosin staining of synapses in the outer plexiform layer in the central retina (see Materials and Methods) at E75 (Fig. 1E1). At this developmental time, the peripheral retina (see Materials and Methods) is still composed of a single neuroblastic layer consistent with the notion that retinal development progresses in a central-to-peripheral fashion in the swine retina. Separation into ONL and INL is evident at E85 in the peripheral retina (Fig. 1F1).
Figure 1.
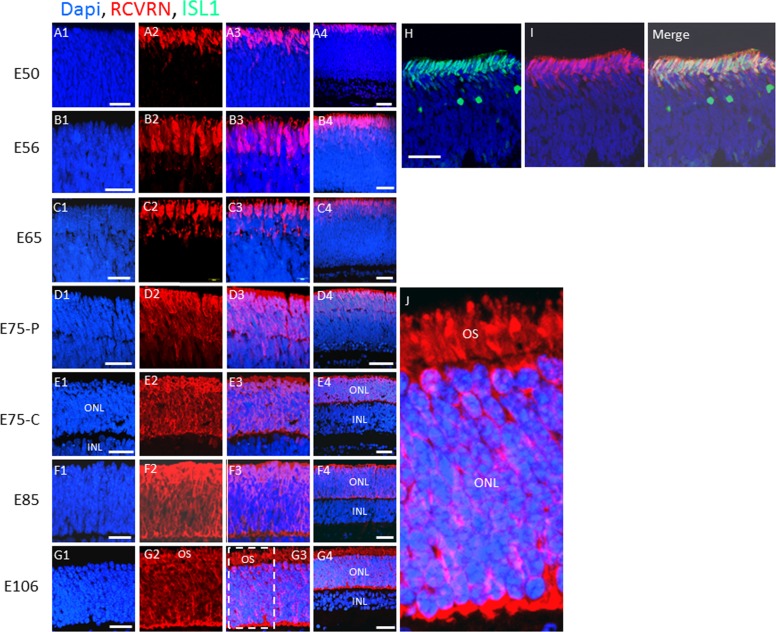
Postmitotic PAX6−, ISL1+ cells comprise the outer rows of the retina before midgestation in the pig. (A–G) H&E and immunostaining of swine retinal sections from the indicated embryonic days. The boxed region in (B7) is shown in (H). (I–K) Co-immunostaining for PCNA and ISL1 at E50. (I) Nomarski image showing immunostaining for PCNA. (J) DAPI and PCNA immunostaining. (K) Double immunostaining for PCNA and ISL1 along with DAPI. C, central retina; P, peripheral retina (see Materials and Methods). Scale bars: 50 μm.
Mitotic PAX6+, ISL1− Retinal Progenitors
To identify progenitors in the neuroblastic layer at E50 in the pig retina, we double immunostained for PAX6 and CHX10.8–12 We did not detect CHX10 immunostaining of retinal progenitors (Materials and Methods), suggesting that the antibodies might not cross react with the pig (we did observe immunostaining of the embryonic mouse retina). Nevertheless, the neuroblastic layer was uniformly positive for PAX6 at this developmental stage, with the noted exception of the outer two rows of cells (Fig. 1A). PAX6+ progenitors can be distinguished from differentiated cells based on nuclear shape: the progenitors have elongated nuclei, whereas the differentiated cells have rounded nuclei.15 Indeed, PAX6+ progenitors with elongated nuclei were evident in the central and outer regions of the neuroblastic layer, whereas PAX6+ cells with rounded nuclei, presumably representing forming amacrine cells, were evident in the inner region of the neuroblastic layer, and PAX6+ ganglion cells with rounded nuclei were evident near the inner retinal surface (Figs. 1A–G). We co-immunostained these sections for the transcription factor Islet1 (ISL1) (the closely related lSL2 family member has been reported previously to mark cells in the outer rows of the embryonic chick retina16) and PAX6. Like PAX6, ISL1 is expressed in horizontal, amacrine, and ganglion cells (as well as bipolar cells), and ISL1+ putative amacrine and ganglion cells were evident in the inner region of the neuroblastic layer, but PAX6+ retinal progenitor cells with elongated nuclei in the neuroblastic layer were ISL1− (Fig. 1A; Ref. 15). But, the two rows of PAX6− cells in the outer retina were ISL1+ (Figs. 1A–C, 1H). Between E56 and E65, PAX6 expression began to diminish in the neuroblastic layer in an outer-to-inner retinal gradient (Figs. 1B, 1C). During this period, ISL1 was maintained in the outer two rows of the retina (Figs. 1B, 1C). By E75, ISL1 began to diminish in these two outer rows in a central-to-peripheral fashion (Figs. 1D–F). As expected, ISL1 expression was maintained in putative horizontal, amacrine, and ganglion cells, and it was also evident in bipolar cells that appeared at E85 (Figs. 1D–G; results not shown).
PCNA marks proliferating cells, and at E50, retinal progenitors expressed PCNA and thus were proliferating, but all of the PAX6−, ISL1+ cells in the outer two rows were PCNA− and thus postmitotic (Figs. 1I–K). Taken together, these results demonstrated the presence of proliferating PAX6+ retinal progenitor cells comprising the neuroblast layer before midgestation, and PAX6 expression diminished on the cells in an outer-to-inner retinal gradient as development progressed. However, the identity of the postmitotic PAX6−, ISL1+ cells comprising the outer two rows of the retina at E50 was still unknown at this point.
Formation of Rod Precursors
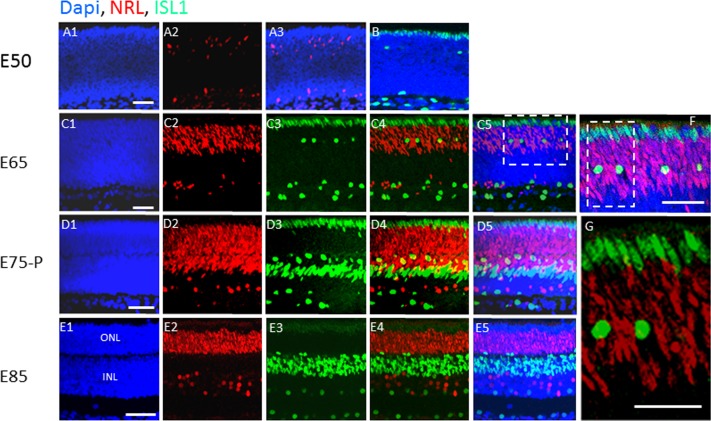
At E50, when proliferating PAX6+ retinal progenitors comprise the neuroblast layer (with the exception of the outer two postmitotic PAX6−, ISL1+ rows), NRL was evident only in a few scattered cells, and none of these NRL+ cells were in the outer two PAX6−, ISL1+ rows (Figs. 2A, 2B). By E65, when PAX6 was diminishing on retinal progenitor cells in the outer retina, NRL expression was induced in the nuclei of cells in this region, but it was still excluded from the outer two ISL1+ rows (Figs. 2C, 2F, 2G). The nuclei in these two ISL1+ outer rows were elongated, whereas ISL1+ cells with rounded nuclei corresponding to putative horizontal cells were evident among the NRL+ cells (Figs. 2C, 2D, 2F, 2G). By E85, ISL1 had diminished in the two outer rows, but it was still evident on putative horizontal, bipolar, amacrine, and ganglion cells located interior to the NRL+-forming rods (Fig. 2E). Even though ISL1 diminished on the outer two rows in the retina, NRL was still excluded from these two rows, and this exclusion was maintained in the adult retina (Fig. 2E; also see Figs. 4, 5 below).
Figure 2.
Islet1 appears before NRL in the developing outer retina, and it is confined to the outermost rows, whereas NRL marks the inner rows. (A–E) Immunostaining for ISL1 and NRL in the swine retina at the indicated embryonic days. The boxed region in (C5) is shown in (F), and the boxed region in (F) is shown in (G). Scale bars: 50 μm.
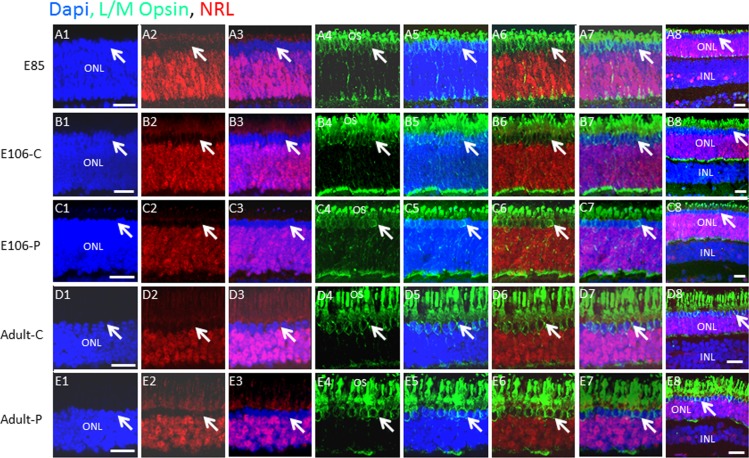
Figure 4.
L/M opsin+ cones are confined to the outer rows of the ONL, whereas NRL+ rods make up the inner rows. (A–E) Swine retinal sections at the indicated developmental ages were co-immunostained for L/M cone opsin and NRL. Lower-power views are shown in panel 8 of each row. Arrows indicate a similar position in each row. Scale bars: 50 μm.
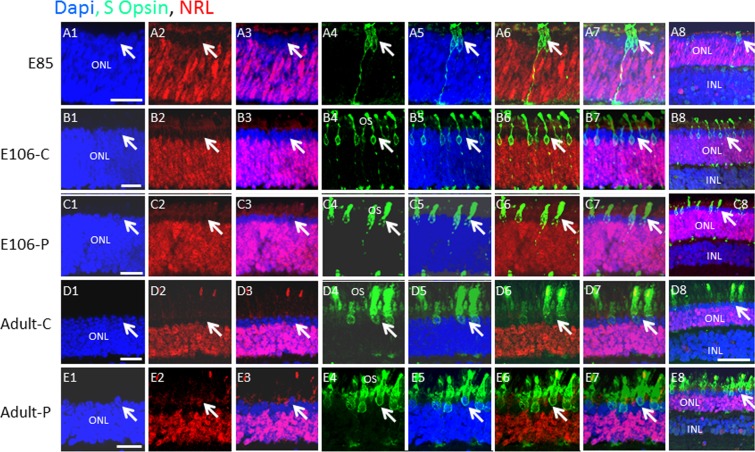
Figure 5.
S opsin is expressed after L/M opsin, and both are confined to cells in the outer two rows of the embryonic ONL. (A–E) Swine retinal sections at the indicated developmental ages were co-immunostained for S cone opsin and NRL. Lower-power views are shown in panel 8 of each row. Arrows indicate a similar position in each row. Scale bars: 50 μm.
RCVRN Marks the Postmitotic ISL1+ Cells in the Early Outer Retina
RCVRN marks both rods and cones.17 When we immunostained for RCVRN, we noted that the outer two ISL1+ rows were uniformly RCVRN+ at E50, and RCVRN was not expressed on any other cells in the retina at this stage (Figs. 3A, 3H, 3I). Exclusive expression of RCVRN on the ISL1+ outer two rows was maintained until E65, when it first became evident on a subset of inner retinal cells (Fig. 3C). An outer-to-inner gradient of RCVRN expression continued, in a central-to-peripheral fashion, until RCVRN was evident uniformly in cells in the newly forming ONL by E85 (Figs. 3D–F, 3J). These results demonstrate that transient expression of ISL1 and early expression of RCVRN mark the outer two rows of postmitotic PAX6−, NRL− retinal cells at E50. Recoverin expression subsequently extends to NRL+ rod precursors located interior to the ISL1+ cells, but induction of RCVRN in these rod precursors does not occur until after NRL is expressed.
Figure 3.
Recoverin and ISL1 are coexpressed on the outermost rows of the retina before midgestation at E50, then RCVRN expands to all cells in the ONL by E106. (A–G) Immunostaining for RCVRN in swine retinal sections at the indicated embryonic days. (H, I) A section at E50 was double immunostained for RCVRN and ISL1. “Merge” indicates the overlay of (H) and (I). The boxed region in (G3) is shown in (J). OS, outer segments. Scale bars: 50 μm.
The Postmitotic PAX6−, NRL−, ISL1+, and RCVRN+ Cells Forming the Outer Two Rows of the Early Retina Are Cone Precursors
Immunostaining of late-stage embryonic and adult swine retinas for NRL demonstrated that NRL expression continued to be excluded from the outer two rows of the ONL in the adult (Fig. 2E; also see Figs. 4, 5 below). We concluded that these two outer rows of postmitotic PAX6−, NRL− cells that are marked before midgestation by ISL1 and RCVRN must be cone precursors. In the mouse, S cone precursors induce M opsin, and a subset of these M opsin+ cones continue to coexpress S opsin.6 The balance between S and M opsin expression in cones is regulated by NeuroD1, RXRγ, and Trb2.18,19 We co-immunostained retinal sections for NRL and cone red/green opsin (L/M opsin). Few L/M opsin+ cells were evident at E75 (results not shown), but expression was evident at E85 on both OS and cell bodies, and the number of opsin+ cells was similar in the adult indicating that L/M opsin is fully expressed by E85 (Figs. 4A–E). Immunostaining was confined to the two outer NRL− rows demonstrating that nuclei of rods and L/M opsin cones are completely segregated at E85 and this pattern continues in the adult.
Next, we co-immunostained for S opsin and NRL. By contrast to L/M opsin, very few cells were S opsin+ at E85 (Fig. 5A), but S opsin+ cells increased at E106 and in the adult (Figs. 5B–E), although the overall number of S opsin+ cells was fewer than L/M opsin+ cells. As with L/M opsin, S opsin+ cells were also confined to the outer two layers of the ONL. Together, these results demonstrate that the outer two retinal rows are composed exclusively of L/M and S cones, and that L/M opsin is expressed before S opsin in the developing pig retina. Additionally, there is a 35-day delay between the appearance of postmitotic, ISL1+, RCVRN+ cone precursors in the outer rows of the retina at E50, and the first appearance of opsin+ cones at E85. And, there is a delay of at least 20 days from the point of NRL expression at E65 and the earliest evidence of S opsin expression at E85.
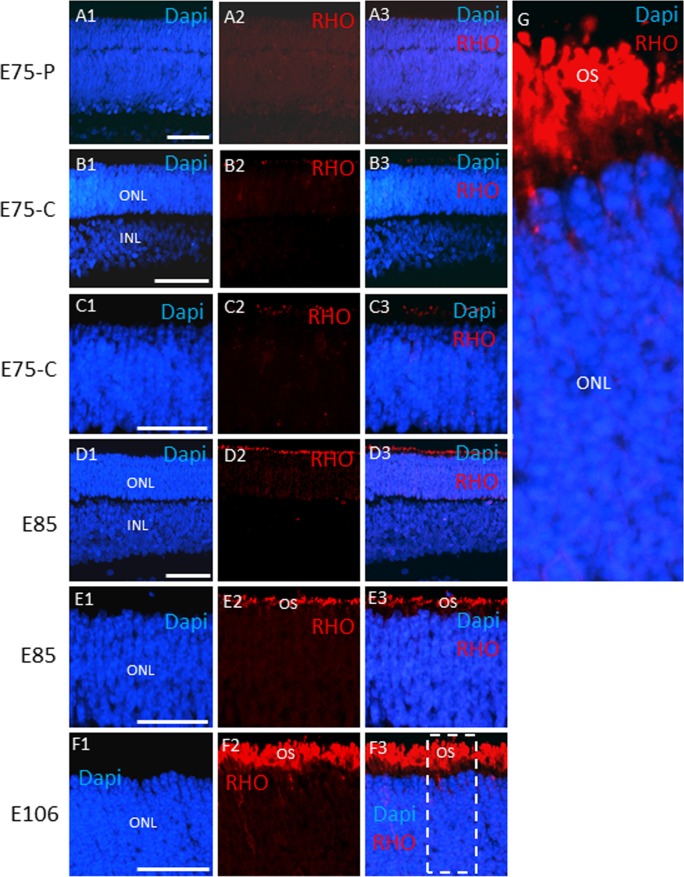
Rhodopsin was first evident at E85, and as it became concentrated in outer segments at E106 (Figs. 6A–G), as the rod bipolar cell marker protein kinase C-α was induced (Supplementary Fig. S1). Thus, as with cone precursors, there is a substantial delay between expression of NRL, as the first evidence of rod precursors, and onset of RHO expression. But this 20-day delay (NRL appears at E65 and RHO at E85) is shorter than the 35-day delay between appearance of cone precursors and cone opsin expression.
Figure 6.
Rhodopsin expression in the developing swine retina. (A–F) Rhodopsin immunostaining is shown of retinal sections at the indicated embryonic days. The boxed region in (F3) is shown in (G). Scale bars: 50 μm.
Rod and Cone Precursors With the Potential to Integrate and Differentiate Following Transplantation Appear Transiently and Sequentially During Embryogenesis
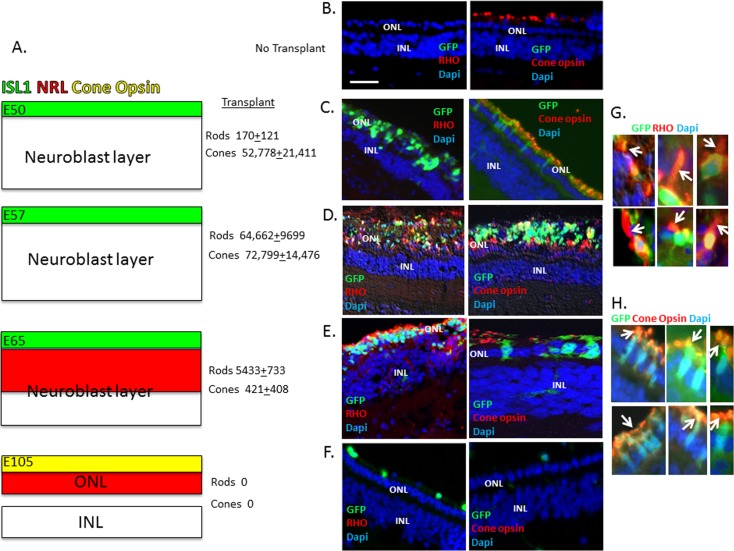
To functionally assess the regeneration potential of embryonic retinal cells, we harvested embryonic retinal cells from GFP+ pig embryos4 at different embryonic stages: E50 and E57, where cone precursors are evident but NRL+ rod precursors are not yet present; E65, where NRL+ rod precursors are evident; and E85 and E105 where both rod and cone precursors are undergoing terminal differentiation to express opsins (Fig. 7A). We then transplanted the cells into the subretinal space of 6-week-old pigs, but we did not observe integration of the transplanted cells into the host retina (results not shown). Previously, we demonstrated that pig-induced pluripotent stem cells, exposed to a photoreceptor differentiation protocol in culture, could efficiently integrate into the swine retina following photoreceptor damage induced by IAA.14 Iodoacetic acid covalently inactivates glyceraldehyde 3-phosphate dehydrogenase, thereby blocking glycolysis in photoreceptors, which depend on this pathway for ATP generation. Iodoacetic acid treatment leads to loss of rod photoreceptors, and it diminishes cone photoreceptors to a single row14 (Fig. 7B). GFP+ retinal cells from E50, E65, E85, or E105 were then transplanted into the subretinal space 4 days after Iodoacetic acid treatment, and retinas were isolated and examined for integration and differentiation of GFP+ transplanted cells 12 weeks later, as we have described.5,14 Transplanted E50 cells gave rise to cone opsin+ cells but few RHO+ cells (Fig. 7C). The cells did not express cone opsin before transplantation, thus their differentiation occurred within the host retina. Transplanted E57 cells gave rise to both rods and cones in similar numbers, and the integration/differentiation efficiency for cones was similar to that seen with E50 cells (Fig. 7). Interestingly, despite the fact that ISL1+/RCVRN+ cone precursors were still evident in the outer two rows of the retina at E65, transplantation of these cells gave almost exclusively RHO+ rods (Fig. 7). But, the efficiency of E65 cell integration/differentiation to give RHO+ rods was reduced compared with E57 (Fig. 7). Similarly, transplanted E85 cells gave only RHO+ rods, but the number of rods was further reduced compared with E65 cells (with E85 cells, only 362 ± 101 GFP/RHO+ integrated cells were seen per 1,000,000 injected cells, and no cones were observed) (Fig. 7). Few integrated cells were seen when E105 cells were transplanted, and none of the integrated cells expressed RHO or cone opsin (Fig. 7). Taken together, these results demonstrate a sequential and surprisingly transient potential for embryonic rod and cone precursor integration/differentiation following transplantation.
Figure 7.
Evaluation of integration/differentiation following transplantation of embryonic retinal cells into the subretinal space of adult pigs. (A) Summary of the time course of appearance of cone precursors (green; marked by transient expression of ISL1 and early expression of RCVRN) and rod precursors (red; marked by NRL) in the developing pig retina. (B) No transplant shows loss of RHO expression and diminished expression of cone L/M opsin in retinas after IAA treatment (Materials and Methods; Ref. 16). (C–F) Representative examples of immunostaining of GFP+ transplanted cells for cone opsin and RHO is shown corresponding to embryonic ages in (A). The average number ± SEM of integrated GFP/RHO+ (rods) and GFP/cone opsin+ (cones) in at least three independent injections of 1,000,000 cells is shown. GFP+ integrated cells were counted in 20-μm frozen sections across the injection site, which corresponded in size to approximately 2 optic disc diameters. (G, H) High-power views showing colocalization of GFP and RHO or cone opsin. Arrows indicate OS. Scale bar: 50 μm. P < 0.01 for rod number at E50, E57, and E65, and for cone number at E50 and E57 versus E65.
Discussion
Cone and rod nuclei are spatially segregated in the pig ONL, and we have used their position to follow their formation in situ. Cone precursors form initially and are marked by both ISL1 and RCVRN. Then, 15 days later, NRL+ rod precursors appear and form the inner rows of the ONL. In the mouse, photoreceptors are thought to arise from a common S-opsin+ precursor; a subset of these precursors go on to express NRL and form rods and others go on to induce M opsin and become M cones.12 In the pig, we did not observe an early S-opsin+ precursor; in fact, S-opsin appeared after L/M opsin in the developing retina. Based on position in the developing retina, we provide evidence that ISL1 and early RCVRN expression mark the initial postmitotic precursors appearing in the embryonic pig retina, and these precursors are committed to the cone lineage. We suggest that proliferating PAX6+ progenitors located more centrally in the developing retina later induce NRL to form rod precursors. Importantly, these findings provide evidence of a set of photoreceptor precursors that are committed to the cone lineage and marked by ISL1. And, such sequential appearance of cone and then rod progenitors in the pig may have important consequences for the selection of markers and developmental stages for transplantation of embryonic cells or precursors derived from iPSC or embryonic stem cells as experiments move toward large animals where cones play a major role in functional visual acuity.
To assess integration/differentiation potential, we transplanted embryonic retinal cells into the adult pig retina following damage with IAA. Once photoreceptor precursors initiated expression of RHO or cone opsin, the transplanted cells failed to be integrated into the retina. This finding is consistent with previous results in the mouse showing that NRL+ or Crx+ rod and cone precursors effectively integrate on cell transplantation, whereas mature rods do not.20,21 But, these findings do not preclude the possibility that differentiated rods or cones might be capable of integration in another model of retinal degeneration or if cell viability were enhanced. Although experiments transplanting embryonic retinal cells measure both integration and differentiation potential in the host retina, it is of note that transplantation of E50 cells yielded only cones. Rod precursors that appeared later in development showed similar integration as these earlier-forming cone precursors, implying that the difference in cone and rod integration following transplantation might be due primarily to a lack of rod precursors at early developmental time points leading to a lack of rod differentiation. Consistent with this notion, in addition to cells integrating into the neural retina, we also observed clumps of viable cells persisting in the subretinal space. Cells in the subretinal space were not counted in Figure 7, but it is of note that, as with integrated cells, we observed only cone opsin expression in such E50 subretinal cells, similar E57 cells expressed both RHO and cone opsin, and similar E65 cells expressed primarily RHO. Crx marks both rod and cone precursors in the mouse, and using Crx-GFP precursors for transplantation has led to integration of both cones and rods.21 However, analogous to our findings of developmental differences in cone versus rod integration following transplantation into the pig, Crx-GFP photoreceptor precursors from embryonic mice gave rise primarily to cones following transplantation, whereas similar cells from mice at postnatal day 2 gave rise primarily to rods. The authors concluded that Crx+ photoreceptor precursors at different developmental stages have different integration potential. Although, as noted above, photoreceptor precursor formation might be somewhat different in the pig, it is conceivable that these results in the mouse with Crx-GFP cells at different developmental stages also reflect a component of precursor differentiation potential in addition to integration potential. Nevertheless, these transplantation results in the mouse and pig point to an early population of precursors capable of efficiently generating cones following transplantation. These findings are consistent with our developmental studies in the pig identifying early cone precursors and thus they further emphasize the importance of carefully assessing markers of cone precursor formation for cell transplants.
Acknowledgments
We thank Giurong Liu for histological sections and Anand Swaroop for the NRL antibody.
Supported in part by American Health Assistance Foundation, National Institutes of Health (P20 RR018733 and EY015636), Research to Prevent Blindness, and The Commonwealth of Kentucky Research Challenge.
Disclosure: W. Wang, None; L. Zhou, None; S.J. Lee, None; Y. Liu, None; J. Fernandez de Castro, None; D. Emery, None; E. Vukmanic, None; H.J. Kaplan, None; Douglas C. Dean, None
References
- 1. Sung CH, Chuang JZ. The cell biology of vision. J Cell Biol. 2010; 190: 953–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mustafi D, Engel AH, Palczewski K. Structure of cone photoreceptors. Prog Retin Eye Res. 2009; 28: 289–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hendrickson A, Hicks D. Distribution and density of medium- and short-wavelength selective cones in the domestic pig retina. Exp Eye Res. 2002; 74: 435–444 [DOI] [PubMed] [Google Scholar]
- 4. Lai L, Park KW, Cheong HT, et al. Transgenic pig expressing the enhanced green fluorescent protein produced by nuclear transfer using colchicine-treated fibroblasts as donor cells. Mol Reprod Dev. 2002; 62: 300–306 [DOI] [PubMed] [Google Scholar]
- 5. Wang W, Fernandez de Castro J, Vukmanic E, et al. Selective rod degeneration and partial cone inactivation characterize an iodoacetic acid model of swine retinal degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 7917–7923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Applebury ML, Antoch MP, Baxter LC, et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000; 27: 513–523 [DOI] [PubMed] [Google Scholar]
- 7. Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001; 105: 43–55 [DOI] [PubMed] [Google Scholar]
- 8. Belecky-Adams T, Tomarev S, Li HS, et al. Pax-6, Prox 1, and Chx10 homeobox gene expression correlates with phenotypic fate of retinal precursor cells. Invest Ophthalmol Vis Sci. 1997; 38: 1293–1303 [PubMed] [Google Scholar]
- 9. Baumer N, Marquardt T, Stoykova A, Ashery-Padan R, Chowdhury K, Gruss P. Pax6 is required for establishing naso-temporal and dorsal characteristics of the optic vesicle. Development. 2002; 129: 4535–4545 [DOI] [PubMed] [Google Scholar]
- 10. Ashery-Padan R, Gruss P. Pax6 lights-up the way for eye development. Curr Opin Cell Biol. 2001; 13: 706–714 [DOI] [PubMed] [Google Scholar]
- 11. Hsieh YW, Yang XJ. Dynamic Pax6 expression during the neurogenic cell cycle influences proliferation and cell fate choices of retinal progenitors. Neural Dev. 2009; 4: 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swaroop A, Kim D, Forrest D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci. 2010; 11: 563–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Macke JP, Merbs SL, et al. A locus control region adjacent to the human red and green visual pigment genes. Neuron. 1992; 9: 429–440 [DOI] [PubMed] [Google Scholar]
- 14. Zhou L, Wang W, Liu Y, et al. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cells. 2011; 29: 972–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guduric-Fuchs J, Ringland LJ, Gu P, Dellett M, Archer DB, Cogliati T. Immunohistochemical study of pig retinal development. Mol Vis. 2009; 15: 1915–1928 [PMC free article] [PubMed] [Google Scholar]
- 16. Fischer AJ, Foster S, Scott MA, Sherwood P. Transient expression of LIM-domain transcription factors is coincident with delayed maturation of photoreceptors in the chicken retina. J Comp Neurol. 2008; 506: 584–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sharma RK, O'Leary TE, Fields CM, Johnson DA. Development of the outer retina in the mouse. Brain Res Dev Brain Res. 2003; 145: 93–105 [DOI] [PubMed] [Google Scholar]
- 18. Liu H, Etter P, Hayes S, et al. NeuroD1 regulates expression of thyroid hormone receptor 2 and cone opsins in the developing mouse retina. J Neurosci. 2008; 28: 749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci U S A. 2006; 103: 6218–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006; 444: 203–207 [DOI] [PubMed] [Google Scholar]
- 21. Lakowski J, Baron M, Brainbridge J, et al. Cone and rod photoreceptor transplantation in models of the childhood retinopathy leber congenital amaurosis using flow-sorted Crx-positive donor cells. Human Mol Genet. 2010; 19: 4545–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]