Abstract
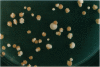
In wild-type diploid cells of Saccharomyces cerevisiae, an HO endonuclease-induced double-strand break (DSB) at the MAT locus can be efficiently repaired by gene conversion using the homologous chromosome sequences. Repair of the broken chromosome was nearly eliminated in rad52delta diploids; 99% lost the broken chromosome. However, in rad51delta diploids, the broken chromosomes were repaired approximately 35% of the time. None of these repair events were simple gene conversions or gene conversions with an associated crossover, instead, they created diploids homozygous for the MAT locus and all markers in the 100-kb region distal to the site of the DSB. In rad51delta diploids, the broken chromosome can apparently be inherited for several generations, as many of these repair events are found as sectored colonies, with one part being repaired and the other part being lost the broken chromosome. Similar events occur in about 2% of wild-type cells. We propose that a broken chromosome end can invade a homologous template in the absence of RAD51 and initiate DNA replication that may extend to the telomere, 100 or more kb away. Such break-induced replication appears to be similar to recombination-initiated replication in bacteria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboussekhra A., Chanet R., Adjiri A., Fabre F. Semidominant suppressors of Srs2 helicase mutations of Saccharomyces cerevisiae map in the RAD51 gene, whose sequence predicts a protein with similarities to procaryotic RecA proteins. Mol Cell Biol. 1992 Jul;12(7):3224–3234. doi: 10.1128/mcb.12.7.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T., Sommer S., Bailone A., Kogoma T. Homologous recombination-dependent initiation of DNA replication from DNA damage-inducible origins in Escherichia coli. EMBO J. 1993 Aug;12(8):3287–3295. doi: 10.1002/j.1460-2075.1993.tb05998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile G., Aker M., Mortimer R. K. Nucleotide sequence and transcriptional regulation of the yeast recombinational repair gene RAD51. Mol Cell Biol. 1992 Jul;12(7):3235–3246. doi: 10.1128/mcb.12.7.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke B. D., Golin J. Long-tract mitotic gene conversion in yeast: evidence for a triparental contribution during spontaneous recombination. Genetics. 1994 Jun;137(2):439–453. doi: 10.1093/genetics/137.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B., Szauter P., Pardue M. L., Szostak J. W. Transfer of yeast telomeres to linear plasmids by recombination. Cell. 1984 Nov;39(1):191–201. doi: 10.1016/0092-8674(84)90205-8. [DOI] [PubMed] [Google Scholar]
- Esposito M. S. Evidence that spontaneous mitotic recombination occurs at the two-strand stage. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4436–4440. doi: 10.1073/pnas.75.9.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fishman-Lobell J., Rudin N., Haber J. E. Two alternative pathways of double-strand break repair that are kinetically separable and independently modulated. Mol Cell Biol. 1992 Mar;12(3):1292–1303. doi: 10.1128/mcb.12.3.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formosa T., Alberts B. M. DNA synthesis dependent on genetic recombination: characterization of a reaction catalyzed by purified bacteriophage T4 proteins. Cell. 1986 Dec 5;47(5):793–806. doi: 10.1016/0092-8674(86)90522-2. [DOI] [PubMed] [Google Scholar]
- Haber J. E., Hearn M. Rad52-independent mitotic gene conversion in Saccharomyces cerevisiae frequently results in chromosomal loss. Genetics. 1985 Sep;111(1):7–22. doi: 10.1093/genetics/111.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E. In vivo biochemistry: physical monitoring of recombination induced by site-specific endonucleases. Bioessays. 1995 Jul;17(7):609–620. doi: 10.1002/bies.950170707. [DOI] [PubMed] [Google Scholar]
- Hadfield C., Harikrishna J. A., Wilson J. A. Determination of chromosome copy numbers in Saccharomyces cerevisiae strains via integrative probe and blot hybridization techniques. Curr Genet. 1995 Feb;27(3):217–228. doi: 10.1007/BF00326152. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov E. L., Sugawara N., Fishman-Lobell J., Haber J. E. Genetic requirements for the single-strand annealing pathway of double-strand break repair in Saccharomyces cerevisiae. Genetics. 1996 Mar;142(3):693–704. doi: 10.1093/genetics/142.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer K. M., Brock J. A., Bloom K., Moore J. K., Haber J. E. Two different types of double-strand breaks in Saccharomyces cerevisiae are repaired by similar RAD52-independent, nonhomologous recombination events. Mol Cell Biol. 1994 Feb;14(2):1293–1301. doi: 10.1128/mcb.14.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer K. M., Haber J. E. New telomeres in yeast are initiated with a highly selected subset of TG1-3 repeats. Genes Dev. 1993 Dec;7(12A):2345–2356. doi: 10.1101/gad.7.12a.2345. [DOI] [PubMed] [Google Scholar]
- Liefshitz B., Parket A., Maya R., Kupiec M. The role of DNA repair genes in recombination between repeated sequences in yeast. Genetics. 1995 Aug;140(4):1199–1211. doi: 10.1093/genetics/140.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne G. T., Weaver D. T. Dominant negative alleles of RAD52 reveal a DNA repair/recombination complex including Rad51 and Rad52. Genes Dev. 1993 Sep;7(9):1755–1765. doi: 10.1101/gad.7.9.1755. [DOI] [PubMed] [Google Scholar]
- Moore J. K., Haber J. E. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol. 1996 May;16(5):2164–2173. doi: 10.1128/mcb.16.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushnova E. A., Bulat S. A., Korolev V. G. Comparative analysis of spontaneous mitotic recombination in [cir0] and [cir+] strains of the yeast Saccharomyces cerevisiae. Curr Genet. 1992 Oct;22(4):259–265. doi: 10.1007/BF00317918. [DOI] [PubMed] [Google Scholar]
- Rattray A. J., Symington L. S. Use of a chromosomal inverted repeat to demonstrate that the RAD51 and RAD52 genes of Saccharomyces cerevisiae have different roles in mitotic recombination. Genetics. 1994 Nov;138(3):587–595. doi: 10.1093/genetics/138.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A. The repair of double-strand breaks in DNA; a model involving recombination. J Theor Biol. 1976 Jun;59(1):97–106. doi: 10.1016/s0022-5193(76)80025-2. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Rudin N., Haber J. E. Efficient repair of HO-induced chromosomal breaks in Saccharomyces cerevisiae by recombination between flanking homologous sequences. Mol Cell Biol. 1988 Sep;8(9):3918–3928. doi: 10.1128/mcb.8.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell L. L., Zakian V. A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993 Nov 19;75(4):729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- Shinohara A., Ogawa H., Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992 May 1;69(3):457–470. doi: 10.1016/0092-8674(92)90447-k. [DOI] [PubMed] [Google Scholar]
- Sugawara N., Ivanov E. L., Fishman-Lobell J., Ray B. L., Wu X., Haber J. E. DNA structure-dependent requirements for yeast RAD genes in gene conversion. Nature. 1995 Jan 5;373(6509):84–86. doi: 10.1038/373084a0. [DOI] [PubMed] [Google Scholar]
- Sung P. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science. 1994 Aug 26;265(5176):1241–1243. doi: 10.1126/science.8066464. [DOI] [PubMed] [Google Scholar]
- Sung P., Robberson D. L. DNA strand exchange mediated by a RAD51-ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell. 1995 Aug 11;82(3):453–461. doi: 10.1016/0092-8674(95)90434-4. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Voelkel-Meiman K., Roeder G. S. Gene conversion tracts stimulated by HOT1-promoted transcription are long and continuous. Genetics. 1990 Dec;126(4):851–867. doi: 10.1093/genetics/126.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath D., Davis R. W., Connelly C., Hieter P. Physical mapping of large DNA by chromosome fragmentation. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6027–6031. doi: 10.1073/pnas.85.16.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiffenbach B., Haber J. E. Homothallic mating type switching generates lethal chromosome breaks in rad52 strains of Saccharomyces cerevisiae. Mol Cell Biol. 1981 Jun;1(6):522–534. doi: 10.1128/mcb.1.6.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. I., Haber J. E. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J. 1990 Mar;9(3):663–673. doi: 10.1002/j.1460-2075.1990.tb08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Haber J. E. MATa donor preference in yeast mating-type switching: activation of a large chromosomal region for recombination. Genes Dev. 1995 Aug 1;9(15):1922–1932. doi: 10.1101/gad.9.15.1922. [DOI] [PubMed] [Google Scholar]