Introduction
Many pathogens are restricted to the site of colonization or have a distribution restricted to specific tissues. For others, the ability to disseminate from the initial point of infection and invade different niches is an integral part of their biology. For example, various protozoan and helminth parasites need to migrate through distinct host tissues to complete their life cycles. Thus, the success of Plasmodium is dependent on the ability of different developmental stages to migrate from the skin to the liver, and finally to the blood for transmission. Similarly, Schistosoma mansoni undergoes a protracted migration that starts in the skin and proceeds through multiple tissues, including the lungs, before adults pair in the mesenteric venules, allowing eggs to exit through the intestine. For these parasites, the clinical features and tissues affected are a consequence of the natural progression of the infection. For other parasites, inappropriate migration or dissemination forms the basis for disease and this is illustrated by the ability of Entamoeba histolytica to cross from the intestine and cause the development of liver abscesses. Similarly, there are several helminthes and nematodes that, when in inappropriate hosts, fail to develop fully and continuously migrate through tissues such as the brain where they can cause extensive tissue damage.
For all of these cases, the ability of the parasites to cross biological barriers at either the point of entry or subsequently within the host is key to reproductive success or to the pathology that can accompany these diseases. Similar principles apply to Toxoplasma gondii, a major pathogen of public health importance that causes a chronic infection in approximately one third of the world population [1, 2]. The ability of T. gondii to invade many tissues is an important element that contributes to the spectrum of diseases that can be associated with this organism. Thus, following the ingestion of oocysts or tissue cysts of T. gondii, this organism infects its host in the small intestine and converts to the tachyzoite form, which then disseminates rapidly to almost all tissues, including muscle, brain, eyes, liver, placenta and lungs. Typically, this infection leads to a robust innate and adaptive response that is characterized by the production of IFN-γ by NK and T cells, which leads to the control of the acute phase (for review see [3]). These events are associated with immune pressure that drives the parasite to develop into a chronic, usually asymptomatic stage, where this organism persists as bradyzoites in cyst form within multiple tissues, most notably the brain, eye and muscle.
Associated with this process of dissemination are a wide variety of clinical manifestations. The first recognized example involved the identification of T. gondii in a human fetus [4]. Subsequently, it was recognized that, in the case of primary T. gondii infection during pregnancy, the invasion of the placenta allows the parasites to infect the fetus and can lead to abortion, malformation of the fetus and congenital toxoplasmosis. Despite the fact that Toxoplasma affects multiple tissues, the most common clinical manifestations of toxoplasmosis involve the brain and eye. Thus, even in adults, primary infection can present as chorioretinitis and, in chronically infected individuals who develop defects in T cell function, such as during HIV infection or following chemotherapy, the reactivation of cysts in the brain can lead to Toxoplasmic encephalitis (TE). The basis for this tropism is uncertain – but could be a consequence of the immune privileged nature of these sites. Alternatively, there are multiple instances of parasites that affect the nervous system of their hosts to alter behaviour and promote predation of intermediate hosts [5–7]. For example, Dicrocoelium dendriticum, a flatworm, alters the behaviour of its intermediate host, the ant, to improve the chances of parasite transmission to herbivores (for an entertaining review on parasites and behaviour see [8]). Indeed, behavioural studies using mice and rats indicated that chronic T. gondii infection results in a specific switch from an aversion to cat urine to an attraction, presumably a change in behaviour that would lead to increased predation of infected rodents by cats, the definitive host, allowing sexual reproduction in the cat intestine [9].
Regardless of the biological consequences of the dissemination of T. gondii, many questions remain about the cellular basis for these events. For many pathogens, it is clear that an effective localized immune response can limit replication and the ability of a micro-organism to disseminate out of these focal areas would decrease the likelihood of the infection being completely eliminated. While the motility of some extracellular pathogens may allow these organisms to avoid the cellular components of the immune system, there are also examples where immune populations are hijacked for the success of the pathogen. Unlike other organ systems, the immune system is largely motile: cells detect foreign agents and travel to lymphoid organs for the activation and generation of cell-mediated and humoral responses, primed effector cells can traffic into and out of sites of inflammation. The aim of this review is provide an overview of our current understanding the role of the immune system in these events that lead to the dissemination of T. gondii and how this impacts the pathogenesis of this infection.
Mechanisms of spread
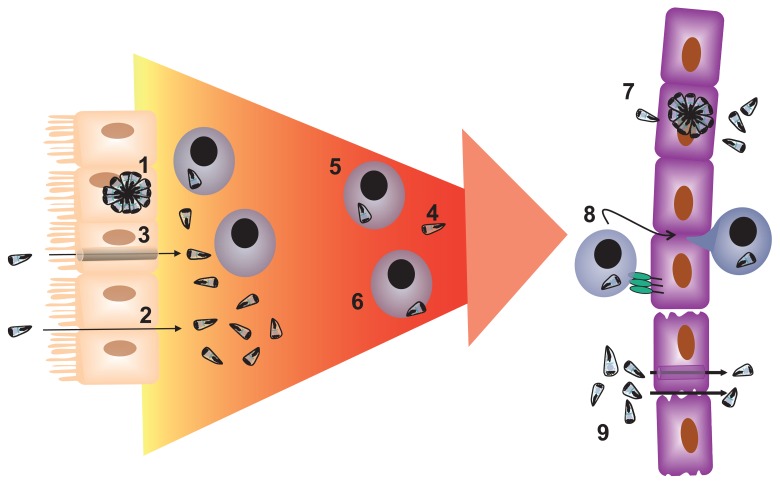
In general, dissemination of pathogens through the host may occur either extracellularly, with active or passive movement of the infectious agent via the blood or lymph, or host cell-dependent mechanisms whereby infected cells transport the micro-organism to distant sites. For T. gondii, there is evidence (reviewed below) that dissemination can occur via the migration of free parasites in blood and tissues, intracellular dissemination by hijacking migrating leukocytes, or extracellular migration with the parasite attached to the outside of the migrating leukocyte (Fig. 1).
Fig. 1.
Dissemination of Toxoplasma gondii in the intermediate host. Following ingestion T. gondii crosses the gastrointestinal tract via [1] infection of and replication within the gastrointestinal epithelial cells, [2] a paracellular route, and/or [3] by traversal of the gastrointestinal epithelial cell. Dissemination of T. gondii throughout the intermediate host may involve [4] the extracellular migration of the tachyzoite in blood or lymph, [5] intracellular migration, and/or [6] cell-dependent migration with adhesion to the host cell membrane. In order to penetrate tissues sites, such as the placenta, brain or eye, T. gondii may [7] infect the epithelial/endothelial or trophoblast cells that make up the tissue barrier, [8] employ a Trojan horse, and/or [9] enter the tissue via a paracellular or transcellular means. The parasite may also enter the tissue following increase permeability of the barrier in response to inflammatory signals
Extracellular migration
Toxoplasma tachyzoites are released from infected cells by either the mechanical rupture of the host cell due to the growing number of parasites within the host cell, or due to the induction of parasite egress in response to changes within the host cell [10, 11]. Free T. gondii tachyzoites are detected in the blood of infected mice [12]. Like other members of the Phylum apicomplexa, T. gondii tachyzoites employ an actinand myosin-dependent gliding motility that is required for the invasion of host cells, parasite egress and virulence [13–15] (for review see [16]). The gliding motion in Toxoplasma tachyzoites has been largely studied across substrates in vitro and it is not known whether free parasites in vivo use this process for extended movement across biological barriers or within a tissue [17–19]. However, in vivo imaging studies on another apicomplexan have shown that Plasmodium sporozoites move with gliding motility in the skin to reach a blood or a lymphatic vessel [20–22] (for review see [23]). Moreover, Plasmodium sporozoites can also migrate through cells, without the formation of a parasitophorous vacuole or rupturing these cells, in order to migrate from the skin, into the blood circulation and within the liver before eventually infecting Kupffer cells [20, 24–26]. It remains an open question whether T. gondii utilizes a similar mechanism to invade other tissues or cross biological barriers.
Host cell-dependent migration
The concept of a “Trojan horse” in microbiology refers broadly to any infectious agent that hides within a cell to gain access to a target tissue that would normally be difficult to enter. For Listeria monocytogenes, a subset of infected monocytes are thought to help this bacterium to enter the central nervous system [27]. The tracking of transferred T. gondii-infected dendritic cells and monocytes to the brain in mice provided experimental evidence that similar events may occur during toxoplasmosis [12, 28]. In addition, following colonization of the small intestine, the lamina propria (LP) and the mesenteric lymph nodes (MLN), a population of dendritic cells (CD11c+CD11b+/–) and monocytes (CD11b+CD11c) are preferentially infected and these can be detected in the blood and then in the brain, suggesting that these infected populations may allow T. gondii access to the central nervous system (CNS) [12]. Additional cells types and subsets, such as plasmacytoid dendritic cells, have also been implicated in dissemination of T. gondii to specific locations [29]. Interestingly, there is a population of cells that have tachyzoites attached to the external surface of the cell, suggesting that T. gondii can bind to surface receptors on the host cell or to the host cell’s plasma membrane itself and piggy-back it’s way to distant sites [12]. The mechanism involved in this attachment is unknown but since tachyzoites attached to the outside of the cell would be exposed to parasite-specific antibodies, this form of transport may only be relevant early in infection before production of parasite specific antibodies.
Crossing barriers
As highlighted earlier Toxoplasma must cross multiple biological barriers in order to infect, escape clearance and persist within the host. Here, we will focus on the main sites of pathology following primary T. gondii infection or reactivation, namely the gut, placenta, brain and eye. Each site has unique characteristics, and it should be noted that the latter three sites actively restrict access of immune cells. Regardless, T. gondii must cross a polarized cell layer made up of epithelial cells, endothelial cells or trophoblasts in order to access these sites and major questions remain about how these events occur.
The gut
Following ingestion, T. gondii must first cross a layer of mucus to access the gastrointestinal epithelium, below which lies the basement membrane, the lamina propria, populated by the host’s immune cells, and a thin layer of smooth muscle. Mucosal trapping has been linked to preventing invasion by helminth parasites but whether this represents a serious impediment to T. gondii is unknown [30]. In current models the infection of epithelial cells leads to an early amplification step and the subsequent release of tachyzoites into the lamina propria and the blood stream. However, T. gondii tachyzoites may also transmigrate across the gut epithelial layer by moving between epithelial cells [31]. Studies using polarized cell monolayers in vitro and mouse intestines ex vivo show parasites concentrated around intercellular junctions, suggesting that T. gondii uses a paracellular pathway that does not appear to disrupt the integrity of the cells or the gap junctions between the cells [32]. Interestingly, the parasite adhesin, MIC2, has an extracellular domain with two adhesive modules that can bind to intercellular adhesion molecule (ICAM)-1 on host cells and this binding is required for this migration [32]. Moreover, Barragan and Sybley [31] found that virulent type 1 strains of T. gondii have a greater capacity for transmigration across the gut epithelial layer, as well as penetration of lamina propria, submucosa and vascular endothelium, than the less virulent type II or type III strains. These observations suggest that the ability of different strains of T. gondii to cause fundamentally different courses of infection may be apparent at the very earliest points in the host–pathogen interaction.
Placental transmission
Once Toxoplasma has established infection, the process of dissemination occurs and, for vertical transmission, the parasite must cross the placenta. Materno-fetal transmission is generally restricted to cases where the mother experiences primary T. gondii infection during gestation [33]. Within the placenta, a single layer of fetal trophoblast cells is in contact with maternal blood for the metabolic exchange of gas and nutrients. This layer can also act as a barrier, protecting the fetus from infections occurring in the mother. However, there are parasitic, bacterial and viral pathogens, such as T. gondii, Neospora cananium, Plasmodium falciparum, L. monocytogenes and cytomegalovirus (CMV), that can harm the fetus by direct infection or by disrupting the placenta or fetal environment. Perhaps the best example of the latter process involves the maternal and fetal morbidity during malaria [34–36]. In this case, Plasmodium falciparum isolates expressing the variant surface antigen var2csa gene on the surface of the red blood cell specifically adhere to the receptor chondroitin sulfate A on the placenta [37–39]. The accumulation of infected red blood cells at the placenta allows the transport of infected red blood cells or Plasmodium antigens across the placenta, leading to severe complications that affect fetal development, as reviewed elsewhere (see [40]). For congenital CMV infection, which is considered the most frequent congenital infection in humans, transmission to the fetus is thought to occur by the direct infection of placental tissue, leading to the release of CMV into the amniotic fluid and ingestion by the fetus, as well as by trans-placental migration of infected leukocytes when the mother has an active infection (reviewed by [41]).
In the case of congenital Toxoplasma, although early infection of the fetus results in severe manifestations, transmission across the placenta is more common in the later stages of infection and long-term effects can include retinochoroiditis during childhood and adolescence [42]. Whether parasite strain influences tropism for the placenta has not been directly assessed but, in Europe, greater than 84% of congenital toxoplasmosis cases were found to be due to the avirulent type II strain [43]. In vitro studies have shown that
T. gondii infects human trophoblasts, suggesting that natural egress of parasites would allow the parasites to cross the placenta [44] but whether T. gondii tachyzoites make a paracellular transit across this barrier is unknown. In vitro studies show that human trophoblast cells up-regulate ICAM-1 and other adhesion molecules in the presence of T. gondii-infected cells and that ICAM-1 is required for the binding of these cells to the trophoblasts [46]. Given that T. gondii-infected cells may allow this parasite to cross biological barriers at other sites, intracellular traversal of the placenta may also take place. Further studies are required to determine whether Toxoplasma-infected maternal cells are found in cord blood, which would provide support for the Trojan horse model during congenital toxoplasmosis.
One other aspect of congenital toxoplasmosis that is poorly understood is how exposure of the fetus to parasite antigens impacts on the developing immune system. For Plasmodium, in utero exposure to malaria antigens may result in a tolerant phenotype in a subset of pre-exposed children, leading to increased susceptibility to malaria [34, 47]. T cell anergy to T. gondii antigen has been reported in some children with congenital Toxoplasmosis, suggesting that in utero exposure to T. gondii may also result in a tolerance to T. gondii [48–50]. This topic requires further investigation to determine whether congenital infection impacts on the long-term control of this persistent infection.
Blood-brain barrier
While the brain and eye are perhaps the two most clinically important sites, there is still remarkably little known about how T. gondii can access these unique anatomical locations. There are two main entry sites for motile cells or infectious agents into the brain, namely across the blood-brain barrier (BBB) or indirectly through the choroid plexus into the cerebrospinal fluid (CSF). The ependymal cells of the choroid plexus form a barrier between the blood capillaries of the choroid plexus and the CSF (reviewed in [51]). The endothelial cells of these capillaries are fenestrated, allowing easy leakage of blood components, while there are tight junctions between the ependymal cells, limiting blood-CSF mixing. Infection of the choroid plexus is observed amongst AIDS patients with acute cerebral toxoplasmosis, suggesting that the CSF may also be involved in the dissemination of Toxoplasma [52]. Indeed, Toxoplasma cysts and tachyzoites have been found in the CSF of mice and patients with Toxoplasmic encephalitis but there is little evidence for the dissemination of tachyzoites in the CSF during the acute stage of a primary infection [53–55].
The BBB separates the luminal contents of the blood vessel from the brain parenchyma, and consists of microvascular endothelial cells and pericytes surrounded by a layer by basement membrane, and astrocytic end feet (reviewed in [51]). The tight junctions between endothelial cells on the lumen side, as well as transporters, control the movement of ions, proteins and cells across the BBB into the brain parenchyma. This physical barrier limits direct access of many systemic pathogens to the CNS. However, many bacteria, viruses, fungi and parasites are thought to employ transcellular, paracellular and/or Trojan horse mechanisms to penetrate the BBB [27, 56–61]. It is not known whether the first tachyzoite to enter the brain does so in free tachyzoite form or within a host cell. Although brain endothelial cells can be infected by T. gondii in vitro, it is not known whether brain endothelial cells are infected during the acute stages of infection and whether this would facilitate the migration of tachyzoites into the brain [62]. Certainly, during toxoplasmosis, cell adhesion molecules ICAM-1 and VCAM-1 are up-regulated on the brain microvascular endothelial cells [63, 64] and these adhesion molecules are involved in the ability of T cells to crawl along the brain endothelium towards sites permissible for diapedesis [65, 66]. Chemokines, such as RANTES, monocyte chemoattractant protein-1 and CXCL10, which attract leukocytes into tissues, are expressed in the brain during chronic stage T. gondii infection and, together with adhesion molecule expression, may be involved in the recruitment of effector leukocytes to the brain [67, 68]. Similar events may aid in the movement of Toxoplasma, residing in leukocytes, over the BBB.
Blood retina barrier
T. gondii infection is responsible for considerable ocular diseases and T. gondii is the leading cause of infectious posterior uveitis [69, 70]. Ocular lesions are frequently a consequence of congenital infection; however, there are instances where postnatally acquired T. gondii can also cause ocular disease [71]. Indeed, like the brain, the eye is considered immune privileged – and the reactivation of this infection in this site, even in immune compromised patients, can cause severe tissue destruction that is the hallmark of this clinical disease. Human retinal epithelial cells are more susceptible to infection by T. gondii than human dermal epithelial cells in vitro, perhaps indicating that T. gondii may be specialized to infect retinal epithelial cells [72]. It has been proposed that tachyzoites can reach the retina by migration from the brain via the optic nerve, through passage of infected monocytes or dendritic cells across the retinal blood barrier, and by subsequent infection of the retinal vascular endothelium (summarized by [72]).
Future directions
Toxoplasma is a successful parasite due to its ability to navigate the host’s barriers, to disseminate widely throughout the host, and to persist in immune-privileged tissues, poised for further transmission. The dissemination of this organism, as well as the traversal of tissue barriers, could involve a combination of direct migration or utilization of host cells. These mechanisms, however, do not appear to be unique to T. gondii and similar mechanisms are exploited by other infectious agents. For example, the bacterium L. monocytogenes also crosses the intestinal, placental and blood-brain barriers, and it is at these sites that the pathology associated with this infectious agent occurs (reviewed in [73]). Like Toxoplasma, host adhesion molecules on the gastrointestinal epithelia are used by L. monocytogenes for entry into the host, and this pathogen employs monocytes as Trojan horses to penetrate the CNS [27, 74–76].
Despite similarities to other pathogens, there remain major questions about the precise mechanisms that allow Toxoplasma to access multiple immune privileged sites, but progress in understanding these events has been limited by technology. Bioluminescence imaging of mice infected with luciferase-expressing T. gondii has provided a more quantitative approach to follow dissemination and insights into the timing and the location of parasite spread in the same animal [77, 78]. However, bioluminescence imaging in its present state is not sufficiently sensitive to distinguish individual invasion events or track single infected cells over time. In recent years, multiphoton imaging of host immune cells has led to a better understanding of the behaviour of these cells within Toxoplasma infected mice, particularly within the brain during the chronic stage of infection [79–81]. Intravital imaging of single fluorescent Toxoplasma tachyzoites at barriers within the model system will allow many of the finer details of Toxoplasma dissemination to be elucidated. Given the genetic tractability of this parasite, understanding the cellular and molecular basis for these types of studies could be broadly applicable to other related parasites, like Neospora and Plasmodium, which also face similar challenges within the intermediate host.
Acknowledgments
The autors would like to thank Antonio Barragan, Henrik Larson, Beth Gregg for discussions on this topic, NIH and the State of Pennsylvania for providing support.
Contributor Information
L. M. Randall, Department of Pathobiology, School of Veterinary Medicine, University of Pennsylvania, Hill Pavilion, 380 South University Avenue, Philadelphia, PA 19146, USA
C. A. Hunter, Department of Pathobiology, School of Veterinary Medicine, University of Pennsylvania, Hill Pavilion, 380 South University Avenue, Philadelphia, PA 19146, USA
References
- 1.Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008 Sep;38(11):1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Dubey JP. The history of Toxoplasma gondii--the first 100 years. J Eukaryot Microbiol. 2008 Nov-Dec;55(6):467–475. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman LA, Hunter CA. The role of cytokines and their signaling pathways in the regulation of immunity to Toxoplasma gondii. Int Rev Immunol. 2002 Jul-Oct;21(4-5):373–403. doi: 10.1080/08830180213281. [DOI] [PubMed] [Google Scholar]
- 4.Wolf A, Cowen D, Paige BH. Toxoplasmic Encephalomyelitis: IV. Experimental Transmission of the Infection to Animals from a Human Infant. J Exp Med. 1940 Jan 31;71(2):187–214. doi: 10.1084/jem.71.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kavaliers M, Colwell DD. Decreased predator avoidance in parasitized mice: neuromodulatory correlates. Parasitology. 1995 Sep;111(Pt 3):257–263. doi: 10.1017/s0031182000081816. [DOI] [PubMed] [Google Scholar]
- 6.Holland CV, Cox DM. Toxocara in the mouse: a model for parasite-altered host behaviour? J Helminthol. 2001 Jun;75(2):125–135. [PubMed] [Google Scholar]
- 7.Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci. 2000 Aug 7;267(1452):1591–1594. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callahan GN. Madness. Emerg Infect Dis. 2002 Sep;8(9):998–1002. doi: 10.3201/eid0809.020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci U S A. 2007 Apr 10;104(15):6442–6447. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavine MD, Arrizabalaga G. Exit from host cells by the pathogenic parasite Toxoplasma gondii does not require motility. Eukaryot Cell. 2008 Jan;7(1):131–140. doi: 10.1128/EC.00301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moudy R, Manning TJ, Beckers CJ. The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J Biol Chem. 2001 Nov 2;276(44):41492–41501. doi: 10.1074/jbc.M106154200. [DOI] [PubMed] [Google Scholar]
- 12.Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gâtel D, Tardieux I. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006 Jan 1;107(1):309–316. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, Soldati-Favre D. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe. 2008 Feb 14;3(2):77–87. doi: 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Kucera K, Koblansky AA, Saunders LP, Frederick KB, De La Cruz EM, Ghosh S, Modis Y. Structure-based analysis of Toxoplasma gondii profilin: a parasite-specific motif is required for recognition by Toll-like receptor 11. J Mol Biol. 2010 Nov 5;403(4):616–629. doi: 10.1016/j.jmb.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meissner M, Schlüter D, Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002 Oct 25;298(5594):837–840. doi: 10.1126/science.1074553. [DOI] [PubMed] [Google Scholar]
- 16.Santos JM, Lebrun M, Daher W, Soldati D, Dubremetz JF. Apicomplexan cytoskeleton and motors: key regulators in morphogenesis, cell division, transport and motility. Int J Parasitol. 2009 Jan;39(2):153–162. doi: 10.1016/j.ijpara.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Dobrowolski JM, Sibley LD. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996 Mar 22;84(6):933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- 18.Dobrowolski JM, Carruthers VB, Sibley LD. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol Microbiol. 1997 Oct;26(1):163–173. doi: 10.1046/j.1365-2958.1997.5671913.x. [DOI] [PubMed] [Google Scholar]
- 19.Håkansson S, Morisaki H, Heuser J, Sibley LD. Time-lapse video microscopy of gliding motility in Toxoplasma gondii reveals a novel, biphasic mechanism of cell locomotion. Mol Biol Cell. 1999 Nov;10(11):3539–3547. doi: 10.1091/mbc.10.11.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amino R, Giovannini D, Thiberge S, Gueirard P, Boisson B, Dubremetz JF, Prévost MC, Ishino T, Yuda M, Ménard R. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe. 2008 Feb 14;3(2):88–96. doi: 10.1016/j.chom.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Amino R, Thiberge S, Blazquez S, Baldacci P, Renaud O, Shorte S, Ménard R. Imaging malaria sporozoites in the dermis of the mammalian host. Nat Protoc. 2007;2(7):1705–1712. doi: 10.1038/nprot.2007.120. [DOI] [PubMed] [Google Scholar]
- 22.Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004 Aug;34(9):991–996. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Vaughan AM, Aly AS, Kappe SH. Malaria parasite pre-erythrocytic stage infection: gliding and hiding. Cell Host Microbe. 2008 Sep 11;4(3):209–218. doi: 10.1016/j.chom.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mota MM, Hafalla JC, Rodriguez A. Migration through host cells activates Plasmodium sporozoites for infection. Nat Med. 2002 Nov;8(11):1318–1322. doi: 10.1038/nm785. [DOI] [PubMed] [Google Scholar]
- 25.Vanderberg JP, Stewart MJ. Plasmodium sporozoite-host cell interactions during sporozoite invasion. Bull World Health Organ. 1990;68(Suppl):74–79. [PMC free article] [PubMed] [Google Scholar]
- 26.Frevert U, Engelmann S, Zougbédé S, Stange J, Ng B, Matuschewski K, Liebes L, Yee H. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 2005 Jun;3(6):e192. doi: 10.1371/journal.pbio.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drevets DA, Dillon MJ, Schawang JS, Van Rooijen N, Ehrchen J, Sunderkötter C, Leenen PJ. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice. J Immunol. 2004 Apr 1;172(7):4418–4424. doi: 10.4049/jimmunol.172.7.4418. [DOI] [PubMed] [Google Scholar]
- 28.Unno A, Suzuki K, Xuan X, Nishikawa Y, Kitoh K, Takashima Y. Dissemination of extracellular and intracellular Toxoplasma gondii tachyzoites in the blood flow. Parasitol Int. 2008 Dec;57(4):515–518. doi: 10.1016/j.parint.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Bierly AL, Shufesky WJ, Sukhumavasi W, Morelli AE, Denkers EY. Dendritic cells expressing plasmacytoid marker PDCA-1 are Trojan horses during Toxoplasma gondii infection. J Immunol. 2008 Dec 15;181(12):8485–8491. doi: 10.4049/jimmunol.181.12.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller HR, Huntley JF, Wallace GR. Immune exclusion and mucus trapping during the rapid expulsion of Nippostrongylus brasiliensis from primed rats. Immunology. 1981 Oct;44(2):419–429. [PMC free article] [PubMed] [Google Scholar]
- 31.Barragan A, Sibley LD. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J Exp Med. 2002 Jun 17;195(12):1625–1633. doi: 10.1084/jem.20020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barragan A, Brossier F, Sibley LD. Transepithelial migration of Toxoplasma gondii involves an interaction of intercellular adhesion molecule 1 (ICAM-1) with the parasite adhesin MIC2. Cell Microbiol. 2005 Apr;7(4):561–568. doi: 10.1111/j.1462-5822.2005.00486.x. [DOI] [PubMed] [Google Scholar]
- 33.Roberts CW, Alexander J. Studies on a murine model of congenital toxoplasmosis: vertical disease transmission only occurs in BALB/c mice infected for the first time during pregnancy. Parasitology. 1992 Feb;104(Pt 1):19–23. doi: 10.1017/s0031182000060753. [DOI] [PubMed] [Google Scholar]
- 34.Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol. 2011 Mar 1;186(5):2780–2791. doi: 10.4049/jimmunol.1001188. [DOI] [PubMed] [Google Scholar]
- 35.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007 Feb;7(2):93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 36.Okoko BJ, Enwere G, Ota MO. The epidemiology and consequences of maternal malaria: a review of immunological basis. Acta Trop. 2003 Jul;87(2):193–205. doi: 10.1016/s0001-706x(03)00097-4. [DOI] [PubMed] [Google Scholar]
- 37.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996 Jun 7;272(5267):1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 38.Tuikue Ndam NG, Salanti A, Bertin G, Dahlbäck M, Fievet N, Turner L, Gaye A, Theander T, Deloron P. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J Infect Dis. 2005 Jul 15;192(2):331–335. doi: 10.1086/430933. [DOI] [PubMed] [Google Scholar]
- 39.Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, Hviid L, Theander TG. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. 2003 Jul;49(1):179–1791. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 40.Mens PF, Bojtor EC, Schallig HD. Molecular interactions in the placenta during malaria infection. Eur J Obstet Gynecol Reprod Biol. 2010 Oct;152(2):126–132. doi: 10.1016/j.ejogrb.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Syggelou A, Iacovidou N, Kloudas S, Christoni Z, Papaevangelou V. Congenital cytomegalovirus infection. Ann N Y Acad Sci. 2010 Sep;1205:144–147. doi: 10.1111/j.1749-6632.2010.05649.x. [DOI] [PubMed] [Google Scholar]
- 42.Dunn D, Wallon M, Peyron F, Petersen E, Peckham C, Gilbert R. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet. 1999 May 29;353(9167):1829–1833. doi: 10.1016/S0140-6736(98)08220-8. [DOI] [PubMed] [Google Scholar]
- 43.Ajzenberg D, Cogné N, Paris L, Bessières MH, Thulliez P, Filisetti D, Pelloux H, Marty P, Dardé ML. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J Infect Dis. 2002 Sep 1;186(5):684–689. doi: 10.1086/342663. [DOI] [PubMed] [Google Scholar]
- 44.Abbasi M, Kowalewska-Grochowska K, Bahar MA, Kilani RT, Winkler-Lowen B, Guilbert LJ. Infection of placental trophoblasts by Toxoplasma gondii. J Infect Dis. 2003 Aug 15;188(4):608–616. doi: 10.1086/377132. [DOI] [PubMed] [Google Scholar]
- 45.Abou-Bacar A, Pfaff AW, Georges S, Letscher-Bru V, Filisetti D, Villard O, Antoni E, Klein JP, Candolfi E. Role of NK cells and gamma interferon in transplacental passage of Toxoplasma gondii in a mouse model of primary infection. Infect Immun. 2004 Mar;72(3):1397–1401. doi: 10.1128/IAI.72.3.1397-1401.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfaff AW, Georges S, Abou-Bacar A, Letscher-Bru V, Klein JP, Mousli M, Candolfi E. Toxoplasma gondii regulates ICAM-1 mediated monocyte adhesion to trophoblasts. Immunol Cell Biol. 2005 Oct;83(5):483–489. doi: 10.1111/j.1440-1711.2005.01356.x. [DOI] [PubMed] [Google Scholar]
- 47.Malhotra I, Dent A, Mungai P, Wamachi A, Ouma JH, Narum DL, Muchiri E, Tisch DJ, King CL. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med. 2009 Jul;6(7):e1000116. doi: 10.1371/journal.pmed.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLeod R, Beem MO, Estes RG. Lymphocyte anergy specific to Toxoplasma gondii antigens in a baby with congenital toxoplasmosis. J Clin Lab Immunol. 1985 Jul;17(3):149–153. [PubMed] [Google Scholar]
- 49.Hara T, Ohashi S, Yamashita Y, Abe T, Hisaeda H, Himeno K, Good RA, Takeshita K. Human V delta 2+ gamma delta T-cell tolerance to foreign antigens of Toxoplasma gondii. Proc Natl Acad Sci U S A. 1996 May 14;93(10):5136–5140. doi: 10.1073/pnas.93.10.5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fatoohi AF, Cozon GJ, Wallon M, Kahi S, Gay-Andrieu F, Greenland T, Peyron F. Cellular immunity to Toxoplasma gondii in congenitally infected newborns and immunocompetent infected hosts. Eur J Clin Microbiol Infect Dis. 2003 Mar;22(3):181–184. doi: 10.1007/s10096-003-0903-9. [DOI] [PubMed] [Google Scholar]
- 51.Cipolla MJ. Colloquium Series on Integrated Systems Physiology: From Molecule to Function to Disease. San Rafael: Morgan & Claypool Publishers; 2009. The Cerebral Circulation. [Google Scholar]
- 52.Falangola MF, Petito CK. Choroid plexus infection in cerebral toxoplasmosis in AIDS patients. Neurology. 1993 Oct;43(10):2035–2040. doi: 10.1212/wnl.43.10.2035. [DOI] [PubMed] [Google Scholar]
- 53.DeMent SH, Cox MC, Gupta PK. Diagnosis of central nervous system Toxoplasma gondii from the cerebrospinal fluid in a patient with acquired immunodeficiency syndrome. Diagn Cytopathol. 1987 Jun;3(2):148–151. doi: 10.1002/dc.2840030211. [DOI] [PubMed] [Google Scholar]
- 54.Feldman HA. Toxoplasmosis. N Engl J Med. 1968 Dec 19;279(25):1370–1375. doi: 10.1056/NEJM196812192792505. (contd) [DOI] [PubMed] [Google Scholar]
- 55.Palm C, Tumani H, Pietzcker T, Bengel D. Diagnosis of cerebral toxoplasmosis by detection of Toxoplasma gondii tachyzoites in cerebrospinal fluid. J Neurol. 2008 Jun;255(6):939–941. doi: 10.1007/s00415-008-0691-3. [DOI] [PubMed] [Google Scholar]
- 56.Greiffenberg L, Goebel W, Kim KS, Weiglein I, Bubert A, Engelbrecht F, Stins M, Kuhn M. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect Immun. 1998 Nov;66(11):5260–5267. doi: 10.1128/iai.66.11.5260-5267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ring A, Weiser JN, Tuomanen EI. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Invest. 1998 Jul 15;102(2):347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain SK, Paul-Satyaseela M, Lamichhane G, Kim KS, Bishai WR. Mycobacterium tuberculosis invasion and traversal across an in vitro human blood-brain barrier as a pathogenic mechanism for central nervous system tuberculosis. J Infect Dis. 2006 May 1;193(9):1287–1295. doi: 10.1086/502631. [DOI] [PubMed] [Google Scholar]
- 59.Lossinsky AS, Jong A, Fiala M, Mukhtar M, Buttle KF, Ingram M. The histopathology of Candida albicans invasion in neonatal rat tissues and in the human blood-brain barrier in culture revealed by light, scanning, transmission and immunoelectron microscopy. Histol Histopathol. 2006 Oct;21(10):1029–1041. doi: 10.14670/HH-21.1029. [DOI] [PubMed] [Google Scholar]
- 60.Verma S, Lo Y, Chapagain M, Lum S, Kumar M, Gurjav U, Luo H, Nakatsuka A, Nerurkar VR. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: Transmigration across the in vitro blood-brain barrier. Virology. 2009 Mar 15;385(2):425–433. doi: 10.1016/j.virol.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grab DJ, Kennedy PG. Traversal of human and animal trypanosomes across the blood-brain barrier. J Neurovirol. 2008 Oct;14(5):344–351. doi: 10.1080/13550280802282934. [DOI] [PubMed] [Google Scholar]
- 62.Lachenmaier SM, Deli MA, Meissner M, Liesenfeld O. Intracellular transport of Toxoplasma gondii through the blood-brain barrier. J Neuroimmunol. 2011 Mar;232(1-2):119–130. doi: 10.1016/j.jneuroim.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deckert-Schlüter M, Schlüter D, Hof H, Wiestler OD, Lassmann H. Differential expression of ICAM-1, VCAM-1 and their ligands LFA-1, Mac-1, CD43, VLA-4, and MHC class II antigens in murine Toxoplasma encephalitis: a light microscopic and ultrastructural immunohistochemical study. J Neuropathol Exp Neurol. 1994 Sep;53(5):457–468. doi: 10.1097/00005072-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Michie SA, Xu B, Suzuki Y. Importance of IFN-gamma-mediated expression of endothelial VCAM-1 on recruitment of CD8+ T cells into the brain during chronic infection with Toxoplasma gondii. J Interferon Cytokine Res. 2007 Apr;27(4):329–338. doi: 10.1089/jir.2006.0154. [DOI] [PubMed] [Google Scholar]
- 65.Bartholomäus I, Kawakami N, Odoardi F, Schläger C, Miljkovic D, Ellwart JW, Klinkert WE, Flügel-Koch C, Issekutz TB, Wekerle H, Flügel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009 Nov 5;462(7269):94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 66.Steiner O, Coisne C, Cecchelli R, Boscacci R, Deutsch U, Engelhardt B, Lyck R. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood-brain barrier endothelium. J Immunol. 2010 Oct 15;185(8):4846–4855. doi: 10.4049/jimmunol.0903732. [DOI] [PubMed] [Google Scholar]
- 67.Aviles H, Stiles J, O'Donnell P, Orshal J, Leid J, Sonnenfeld G, Monroy F. Kinetics of systemic cytokine and brain chemokine gene expression in murine toxoplasma infection. J Parasitol. 2008 Dec;94(6):1282–1288. doi: 10.1645/GE-1309.1. [DOI] [PubMed] [Google Scholar]
- 68.Strack A, Asensio VC, Campbell IL, Schlüter D, Deckert M. Chemokines are differentially expressed by astrocytes, microglia and inflammatory leukocytes in Toxoplasma encephalitis and critically regulated by interferon-gamma. Acta Neuropathol. 2002 May;103(5):458–468. doi: 10.1007/s00401-001-0491-7. [DOI] [PubMed] [Google Scholar]
- 69.McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am J Ophthalmol. 1996 Jan;121(1):35–46. doi: 10.1016/s0002-9394(14)70532-x. [DOI] [PubMed] [Google Scholar]
- 70.Hooper C, McCluskey P. Intraocular inflammation: its causes and investigations. Curr Allergy Asthma Rep. 2008 Jul;8(4):331–338. doi: 10.1007/s11882-008-0053-3. [DOI] [PubMed] [Google Scholar]
- 71.Balasundaram MB, Andavar R, Palaniswamy M, Venkatapathy N. Outbreak of acquired ocular toxoplasmosis involving 248 patients. Arch Ophthalmol. 2010 Jan;128(1):28–32. doi: 10.1001/archophthalmol.2009.354. [DOI] [PubMed] [Google Scholar]
- 72.Zamora DO, Rosenbaum JT, Smith JR. Invasion of human retinal vascular endothelial cells by Toxoplasma gondii tachyzoites. Br J Ophthalmol. 2008 Jun;92(6):852–855. doi: 10.1136/bjo.2007.133314. [DOI] [PubMed] [Google Scholar]
- 73.Lecuit M. Understanding how Listeria monocytogenes targets and crosses host barriers. Clin Microbiol Infect. 2005 Jun;11(6):430–436. doi: 10.1111/j.1469-0691.2005.01146.x. [DOI] [PubMed] [Google Scholar]
- 74.Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 1999 Jul 15;18(14):3956–3963. doi: 10.1093/emboj/18.14.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996 Mar 22;84(6):923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 76.Lecuit M, Vandormael-Pournin S, Lefort J, Huerre M, Gounon P, Dupuy C, Babinet C, Cossart P. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science. 2001 Jun 1;292(5522):1722–1725. doi: 10.1126/science.1059852. [DOI] [PubMed] [Google Scholar]
- 77.Saeij JP, Boyle JP, Grigg ME, Arrizabalaga G, Boothroyd JC. Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infect Immun. 2005 Feb;73(2):695–702. doi: 10.1128/IAI.73.2.695-702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hitziger N, Dellacasa I, Albiger B, Barragan A. Dissemination of Toxoplasma gondii to immunoprivileged organs and role of Toll/interleukin-1 receptor signalling for host resistance assessed by in vivo bioluminescence imaging. Cell Microbiol. 2005 Jun;7(6):837–848. doi: 10.1111/j.1462-5822.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- 79.Wilson EH, Harris TH, Mrass P, John B, Tait ED, Wu GF, Pepper M, Wherry EJ, Dzierzinski F, Roos D, Haydon PG, Laufer TM, Weninger W, Hunter CA. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009 Feb 20;30(2):300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schaeffer M, Han SJ, Chtanova T, van Dooren GG, Herzmark P, Chen Y, Roysam B, Striepen B, Robey EA. Dynamic imaging of T cell-parasite interactions in the brains of mice chronically infected with Toxoplasma gondii. J Immunol. 2009 May 15;182(10):6379–6393. doi: 10.4049/jimmunol.0804307. [DOI] [PubMed] [Google Scholar]
- 81.John B, Harris TH, Tait ED, Wilson EH, Gregg B, Ng LG, Mrass P, Roos DS, Dzierszinski F, Weninger W, Hunter CA. Dynamic Imaging of CD8(+) T cells and dendritic cells during infection with Toxoplasma gondii. PLoS Pathog. 2009 Jul;5(7):e1000505. doi: 10.1371/journal.ppat.1000505. [DOI] [PMC free article] [PubMed] [Google Scholar]