Abstract
Amoebiasis is the third worldwide disease due to a parasite. The causative agent of this disease, the unicellular eukaryote Entamoeba histolytica, causes dysentery and liver abscesses associated with inflammation and human cell death. During liver invasion, before entering the parenchyma, E. histolytica trophozoites are in contact with liver sinusoidal endothelial cells (LSEC). We present data characterizing human LSEC responses to interaction with E. histolytica and identifying amoebic factors involved in the process of cell death in this cell culture model potentially relevant for early steps of hepatic amoebiasis. E. histolytica interferes with host cell adhesion signalling and leads to diminished adhesion and target cell death. Contact with parasites induces disruption of actin stress fibers and focal adhesion complexes. We conclude that interference with LSEC signalling may result from amoeba-triggered changes in the mechanical forces in the vicinity of cells in contact with parasites, sensed and transmitted by focal adhesion complexes. The study highlights for the first time the potential role in the onset of hepatic amoebiasis of the loss of liver endothelium integrity by disturbance of focal adhesion function and adhesion signalling. Among the amoebic factors required for changed LSEC adherence properties we identified the Gal/GalNAC lectin, cysteine proteases and KERP1.
Keywords: amoebiasis, cell death, Entamoeba, focal adhesions, integrins, KERP1, liver sinusoidal endothelial cells
The amoeba parasite Entamoeba histolytica causes amoebiasis in humans. Invasive trophozoites resident in the colon target the intestine, eventually generating dysentery. By haematogenous spread, amoebae may reach the liver where they form abscesses [1]. Multiple parasite factors are associated with pathogenicity and include markers for: adhesion, motility, extracellular matrix (ECM) degradation, cytotoxicity for and phagocytosis of human cells, induction of host cell death and inflammation. During intestinal invasive infection, E. histolytica degrades the colonic mucosa with amoebic proteolytic enzymes like Cysteine Proteinase (CP) A5 [2]. Trophozoites then interact with the intestinal epithelium, cross the basal lamina and disrupt the ECM. Invasion induces an acute inflammatory response characterised by the increase of Interleukin (IL)-1 and -8, Interferon (IFN)-γ and Tumour Necrosis Factor (TNF) [2, 3], which is chemo-attractant for amoebae in vitro [4]. Crossing the intestinal barrier allows subsequent E. histolytica dissemination, particularly to the liver via the portal vein. Hepatic sinusoids irrigate the organ and are the sites where E. histolytica interacts with endothelial cells and liver-resident macrophages (Kupffer cells), and crosses the endothelial barrier, prior to the penetration into the parenchyma. This leads to the formation of inflammatory loci by neutrophils and macrophages, and the establishment of abscesses (see [5] for review).
Tissue modifications during abscess establishment
Liver invasion by E. histolytica with production of abscesses is the most common extra-intestinal manifestation of amoebiasis. The hamster is a powerful model for hepatic amoebiasis. After intra-portal inoculation of trophozoites, histological features of infected livers are similar to those found in humans and allow to study amoebic liver abscess (ALA) development (Fig. 1). The ALA in humans and hamsters have a common characteristic structure: a central necrotic region containing inflammatory cells and lysed hepatocytes surrounded by a ring of motile trophozoites and few inflammatory cells that delimit the abscess from the apparently healthy hepatic tissue [6, 7]. E. histolytica infection of the liver has a fast temporal program during which parasites cross the liver sinusoidal endothelium, penetrate into the tissue and adapt to the new environment before starting division and successful establishment of the infection. Histological analysis revealed that at four hours post-inoculation small foci have already formed in the liver parenchyma containing E. histolytica, scattered inflammatory cells and some dead hepatocytes. Between six and twelve hours, parasites are massively destroyed by the innate immune system, as well as some host cells, a phenomenon that may be at least partially responsible for organ damage. Thus, early infection is critical for amoeba survival, host responses and consequently for subsequent ALA development [7]. Infected livers of severe combined immune deficiency (SCID) mice harbor immune cells and hepatocytes with positive TUNEL labelling, indicating that they undergo apoptosis [8]. Gene expression profiles of SCID mouse liver regions infested with virulent E. histolytica trophozoites for 4 h, 12 h and 24 h have been reported [9]. Profiles are supposedly composed of the response of several cell types of hepatic resident (mainly hepatocytes, but also Kupffer, stellate and endothelial cells) and circulating cells attracted to the sites of infection (neutrophils, macrophages, natural killer T (NKT) cells) and reflect the cross-talks between these cells. The gene expression changes indicate simultaneous activation of inflammatory, regenerative and apoptotic pathways with a bias towards cell death induction.
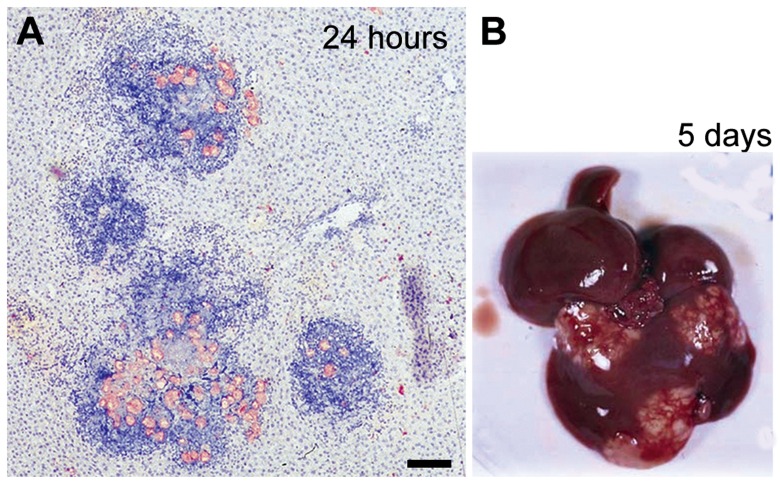
Fig. 1.
Amoebic liver abscess formation in the hamster model of hepatic amoebiasis Male Syrian golden hamsters (Mesocricetus auratus) were infected intraportally with virulent parasites (8×105 trophozoites per animal) according to our published protocol [7]. Livers were excised and fixed immediately after necropsy at 24 hours post-inoculation (A). Shown is a paraffin-embedded section of infected liver stained with hematoxylin to visualize the mammalian cells (in blue) and immuno-labelled with a human anti-E. histolytica antibody (in pink). Note the presence of massive infiltrates containing inflammatory cells. Over time, the inflammatory foci coalesce and form macroscopic abscesses (B). Scale bar, 100 μm
Trophozoites from ALA can be purified and adapted to in vitro culture, during which their ability to form abscesses in animals decreases. Recurrent passage into animals maintains parasite virulence [10]. These observations underline either adaptation of E. histolytica to the environment they encounter upon invasion, or selection of invasion-prone parasites during the pathology development.
Cell activation during abscess development
Liver presents a specific environment characterized by immunological tolerance to resident intestinal flora and innate and acquired immune responses against enteric pathogens. The first line of liver defence against E. histolytica invasion is composed of cells of the innate immunity that lead, upon recognition of pathogen-associated molecular patterns (PAMP) to the triggering of an inflammatory response. Integration of the immune response into the hepatic microenvironment can be achieved by interaction of antigen-presenting cells (APC) with various T lymphocyte subtypes. Among them, NKT cells are of major interest for their particular functions and for their abundance in the liver (approximately 30% of the lymphocytes). NKT cells have the ability to secrete various cytokines upon direct binding of PAMPs, cytokine stimulation or recognition of glycolipidic antigens presented by antigen-presenting glycoprotein CD1 family member d (CD1d) molecules on APC. The different NKT subtypes, which produce cytokines as diverse as IFN-γ, IL-4 or IL-17, determine diametrically opposite immune polarizations (see [11] for a complete review). Thus, NKT cells play crucial roles in the orientation of the hepatic immune response.
Differences in the early response to E. histolytica liver invasion were observed between female and male mice [12]. Females rapidly cleared the parasites, recruited higher numbers of NKTs to the site of infection, and IFN-γ was produced at higher levels. NKT-deficiency in females or IFN-γ neutralization led to increased parasite survival. IFN-γ is a key regulator of inflammation initiation, by activating macrophage TNF production, which in turn promotes nitric oxide (NO) synthesis by macrophages themselves and by polymorphonuclear neutrophils (PMN). Early in the host defence response, NKT cells are among the IFN-γ producing cell types, the so-called “innate” lymphocytes, which reside in tissues, and IFN-γ may also originate from macrophages. The IFN-γ effect can be bypassed in vitro by the recognition of E. histolytica surface proteophosphoglycan (LPPG) by Toll-like receptors 2 and 4, which results in direct production of cytokines such as TNF, IL-12p40 and IL-8 [13]. However, this model does not fully account for the interactions occurring in the hepatic microenvironment, since the cells used are non-immune Human Embryonic Kidney cells, transfected to overexpress either Toll-like receptor 2 or 4 (TLR2 or TLR4), and hepatic cell types that may modulate immune responses were not included in the system.
It has been proposed that either the inflammatory response or the death of immune cells recruited to inflammatory foci are responsible for liver damage, since leukopenic animals did not develop liver abscesses [14]. To produce ALA, E. histolytica may thus need leukocyte recruitment, their activation and eventually their death, triggering a delicate balance to create an environment in which host tissues are destroyed but not all the amoebae. This controlled state of inflammation may result from the integration of different proand anti-inflammatory as well as chemokine signals, starting from early times of infection. In this context, NKT cells may play an important role, since they can produce the different types of cytokines. Further characterization of the NKT subtypes during ALA will help to decipher their complementary, synergistic or eventually opposite roles in the host defence response to invading E. histolytica.
In hepatic sinusoids, amoebae enter in contact with liver sinusoidal endothelial cells (LSEC) which comprise 50% of the non-parenchymal cells of the liver [15]. The particular LSEC phenotype is adapted to the high metabolic and immuno-modulatory activity of the organ [16]. LSEC have open transcytoplasmic pores (fenestrations of ~100 nm diameter; [17]) clustered into sieve plates, are devoid of a basement membrane and of tight junctions and form a discontinuous sinusoid lining. The Disse’s space between LSEC and hepatocytes contains an attenuated ECM consisting mostly of fibronectin, tenascin and some collagen I and VII. This fenestrated sinusoidal endothelium allows direct exchange between the blood and the parenchymal compartment, and proteoglycans on the hepatocyte surface may reach through the Disse’s space into the sinusoid lumen.
LSEC potentially play a dual role in the establishment of liver infection by E. histolytica, as a barrier and as modulators of host defence responses. In particular, LSEC function as APC. They are characterized by a high endocytic activity, express scavenger, mannose and Fc-γ IIb2 receptor involved in the uptake of circulating proteins and they process and present antigens via major histocompatibility complex MHC I and II molecules. LSEC also participate in the modulation of the immune response by expressing surface receptors for leukocyte adhesion and their subsequent transendothelial migration, and by secreting cytokines and modulators upon stimulation (e.g. IL-1γ, IL-6, IL-10, NO) [16].
It was found that in humans suffering from amoebiasis liver endothelial cells produce pro-inflammatory factors following contact with E. histolytica [18]. At early stages post-infection, the pro-inflammatory factors intercellular adhesion molecules ICAM-1 and -2 lead to recruitment and activation of immune cells that are responsible for parasite killing. At early stages of ALA development in hamsters, amoeba-secreted material is deposited on the surface of LSEC [7] suggesting that these cells are rapidly aggressed by elements of the cytotoxic system of the parasites. This might be the initial trigger for the inflammatory response. Hamster sinusoidal endothelial cells undergo apoptosis in the presence of trophozoites [19]. The resulting disturbance of the endothelial barrier likely facilitates passage of trophozoites from the blood circulation into the hepatic tissue. E. histolytica then divides and induces abscess formation [7], dependent upon the expression of virulence factors like Gal/GalNAc lectin [20], amoebapore A [21], CP-A5 [22] and the highly positively-charged lysine- and glutamic acid-rich protein KERP1 [23].
Interaction between E. histolytica and human liver sinusoidal endothelial cells
Assuming that the initial stage of liver infection, when parasites arrive in the hepatic sinusoids, is crucial for the subsequent establishment of abscesses, the characterization of the interactions between amoebae and human hepatic target cells is relevant to understand the host defence and, in particular, the inflammatory response, as well as the changes in parasite phenotype required for adaptation and survival in the hepatic environment. To gain insight into early hepatic infection we have recently used a LSEC line established from immortalized cells of human liver endothelial cell primary cultures [24]. Cells are not tumorigenic, express phenotypic markers of the primary cultures [25] and respond to TNF [26]. Notably, the cells are sensitive to hypothermia/hypoxia-reoxygenation treatment inducing cell death by necrosis and apoptosis, causally related to an increase in matrix metalloprotease 2 release and NO production [27]. Using cultures of the human LSEC line incubated with virulent or virulence-attenuated (i.e. having lost their capacity to form ALA in the hamster model) E. histolytica, we documented cellular changes by confocal microscopy and video-microscopy (Figs 2 and 3), and cell death by FACS analysis [28]. We observed that E. histolytica induced retraction, apoptosis and death of the human cells. LSEC retraction was detected earlier than cell death suggesting that reduced spreading of LSEC could account in vivo for the physical changes in tissue architecture allowing E. histolytica to pass the endothelial barrier and to penetrate into the liver parenchyma, a process possibly facilitated by the absence of tight junctions and by inflammation-induced gap formation between neighbouring cells [29]. In the presence of virulent amoebae, the LSEC network of actin stress fibers was disrupted, and the subcellular localisation of paxillin and phosphorylated focal adhesion kinase (FAK), key com ponents of focal adhesion complexes formed upon activation of integrin receptors, was altered. These changes in the LSEC adhesion state could be a key event in the cascade leading to cell death induced by E. histolytica.
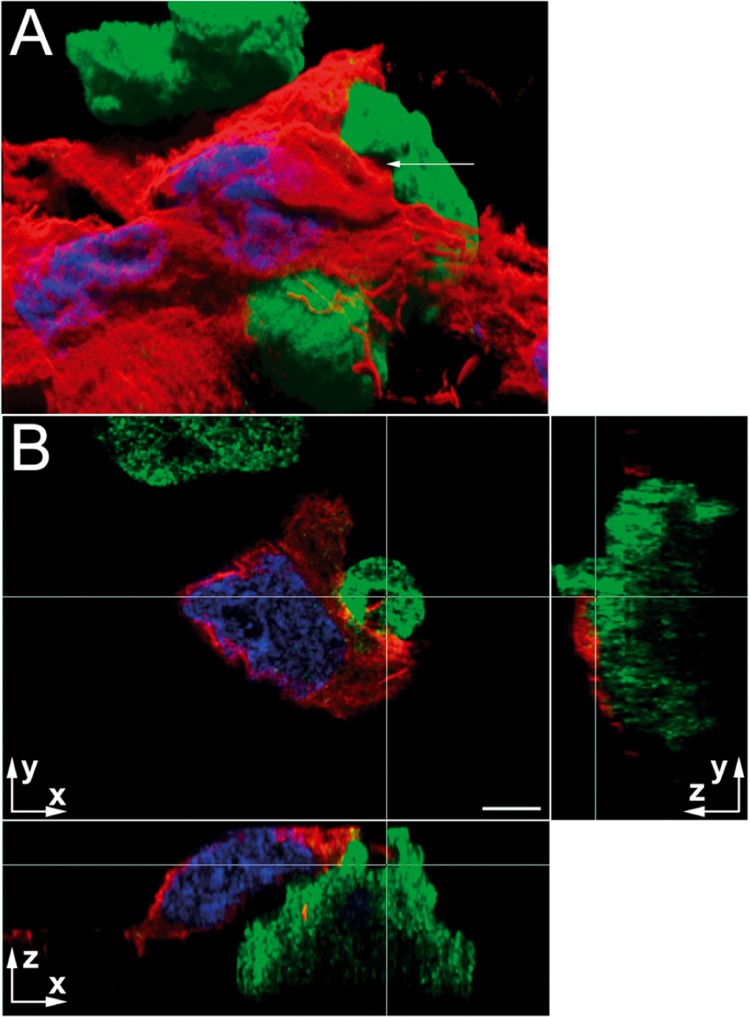
Fig. 2.
Immunolocalization of amoebic protein KERP1 during interaction of virulent trophozoites with LSEC. Confocal microscopy acquisitions showing immunolocalization of KERP1 (green) delimiting the parasite and detection of F-actin (red) the LSEC. Nuclei are stained by DAPI (blue). Scale bars (on axis labels), 5 µm. A. Virtual three-dimensional reconstruction of the stack. Blending and shadowing reveal the volumes on this two-dimensional representation obtained with Imaris software. The nuclei of two LSEC are detected. A trophozoite is seen in the upper part of the image and in the centre an amoeba is partially covered by a LSEC, which has retracted from the substrate and the neighbouring cells. A membrane protrusion emitted by the trophozoite engulfs a portion of the LSEC (arrow and intersection of the white lines in Panel B). B. The micrograph shows three orthogonal planar sections. The white lines correspond to the orthogonal projection of the planes presented aside and their intersection represents the point indicated by the arrow in Panel A. The arrows indicate the orientation of the plane and z = 0 corresponds to the focal plane acquired closest to substratum. The retracted LSEC is detected with its nucleus. The trophozoite surface in contact with the LSEC is not homogeneously enriched in KERP1, as detected at the top of the trophozoite in the xz section. The three orthogonal sections show actin from LSEC (at the intersection of the white lines) surrounded by membrane protrusions from the parasite that are strongly enriched in KERP1. Note that, in the yz section, the membrane invagination is also detected and corresponds to the area with the highest z coordinates (i.e. the most distant from the z = 0) and the greatest KERP1 density. The three-dimensional reconstruction allowed detection of this topological link
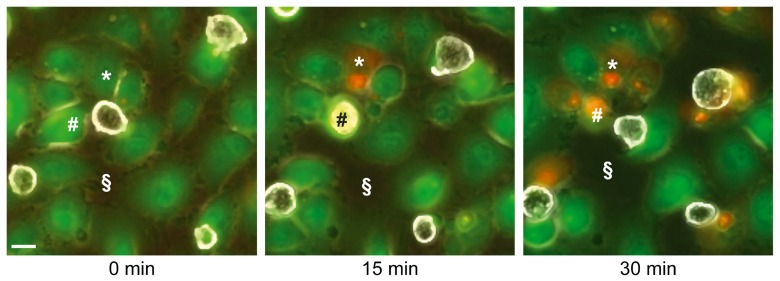
Fig. 3.
Retraction and cell death in LSEC cultures during incubation with E. histolytica. Micrographs of video-microscopy sequences show frames taken at 0, 15 and 30 min of incubation. LSEC were labelled with fluorescent CMFDA cell tracker (green). Trophozoites were then added (parasite to LSEC ratio 1:10) and observed by phase contrast microscopy, as non-fluorescent cells (white) with brightly reflecting plasma membranes. Dead cells were detected by incorporation of propidium iodide (red). Note that human cells (i.e. * and #) were in contact with an amoeba before dying. The space not occupied by cells (i.e. §) is increasing over time, indicating LSEC retraction. Scale bar, 10 µm
Endothelial cells are among the most sensitive cell types for apoptosis triggered by loss of adhesion, named anoikis and associated with decreased protein tyrosine phosphorylation levels [30]. By analysis of the LSEC transcriptome, we have shown that the integrin signalling pathway was modulated specifically by virulent trophozoites [28], suggesting that in addition to the cell retraction observed, virulent parasites rapidly trigger a signal transduction pathway not only determining cell adhesion properties, but also sensing the adhesion state and responding to its changes. Integrins are a family of cell surface receptor proteins mediating cell-to-cell and cell-to-ECM adhesion signalling. Integrin receptors are α/β subunit heterodimers, with ligand specificity dependent on the subunit combinations. Activation of the receptors occurs upon conformational conversion into the high affinity state for ligand binding and is regulated by intracellular signals (inside-out signalling), such as the concentration of free magnesium and calcium. Ligand binding promotes clustering of integrins, which do not have direct signalling functions, but serve as an anchoring point for many scaffold proteins and recruitment of numerous signalling molecules (outside-in-signalling). The resulting multiprotein complexes formed are named focal adhesions (FA). Key steps in their assembly are the initial association with talin, paxillin and FAK, leading to the activation of FAK by autophosphorylation (likely due to the increase in local concentration above critical threshold) and phosphorylation of paxillin. These tyrosine phosphorylations create binding sites for adaptor proteins like SHC1, signalling molecules such as Src kinase and for proteins involved in the organization of actin filaments (stress fibers). SHC1 is a substrate of FAK and is localized to FAs involved in mitogenic and survival signalling. Activation by tyrosine phosphorylation leads to downstream activity of Ras and MAPK pathways and SHC contributes also to cytoskeleton organization (see [31] for review). Furthermore, FAs and stress fibers play important roles in mechanotransduction and in line with this, endothelial cells which in vivo are exposed to mechanical forces induced by the blood flow, respond to fluid shear stress by changes in stress fiber and FA organization [32].
Most functions involved in the LSEC integrin/FA signalling pathway modified by E. histolytica were up-regulated and participate either in signalling (integrins, FAK and SHC1) or in actin cytoskeleton organization (β- and γ-actin, adducin 1, plectin 1, spectrin-α, talin1, vinexin, zyxin and regulators of Rho activity).
Amoebic factors involved in LSEC death
Amoebic factors responsible for LSEC retraction and death have been studied according to their abundance in lipidprotein clusters present in amoebic uropod-released frac tions, which allow E. histolytica to escape from the immune response and are thus relevant for parasite virulence. A proteomic analysis of uropod components in fact showed the abundance of factors such as the Gal/GalNAc lectin and several CPs [33]. Using amoebic strains silenced for CP-A5 gene expression and parasites blocked for Gal/GalNAc lectin activity, we have identified these functions as important factors involved in parasite adhesion, LSEC retraction and death. In contrast, amoebapores are not required for LSEC killing [28].
The pro-form of CP-A5 contains an RGD motif for which a role in target cell adherence and triggering of a proinflammatory host cell response has been described [34]. Thus, CP-A5 could play a role as a protease and as a RGD-motif ligand for integrins modifying target cell signalling. By flow cytometry analysis we observed that pre-treatment of trophozoites with an RGD-containing peptide reduced the fraction of dead LSEC, suggesting an involvement of amoebic RGD-binding proteins, such as the β1 integrin-like FN receptor (β1EhFNR), which shares a high degree of homology with the intermediate Gal/GalNAc lectin sub-units Igl1 and Igl2 ([35] and references therein). Amoebic RGD receptors could be involved in adherence to ECM components like fibronectin, produced by and present on LSEC, and serve as additional adhesion molecules.
Conclusion
Amoebiasis can be viewed as resulting from the balance between the “fitness” of the parasites (i.e. to express the phenotype required to resist host defence, to rapidly adapt to changes in the environment, to invade and to survive) and the “adequate” host defence response (e.g. the control of the parasite burden, of the degree of inflammation and immune responses, the type of cell death). Both are conditioned by the interactions between parasites, environmental factors and host target cells. The in vivo LSEC response to E. histolytica is likely composed of a reaction to contact with amoebae and to parasite-induced alterations of the microenvironment. For example, LSEC receive pro-inflammatory signals produced by Kupffer cells. A direct cytotoxic effect on LSEC of elevated NO levels has been demonstrated as well as a protective role of prostaglandin E2 [27]. Trophozoites of 10–50 µm in diameter with highly versatile morphology cause hindrance in sinusoids (diameter of 5–7 µm; [36]) particularly narrow and tortuous in periportal regions [15]. Obstruction may be reinforced by the recruitment of activated immune cells during the acute inflammatory response [29]. Amoebae may thus exert mechanical forces on the endothelium and the underlying Disse’s space and reduce the blood flow, and consequently the oxygen and nutrient supply of surrounding areas (Fig. 4). These changes probably create conditions similar to hypoxia/ischemia. Hypoxic conditions may reduce the oxidative stress for the trophozoites. Microcirculatory dysfunction has been proposed recently as the major cause of tissue necrosis during ALA formation [37]. LSEC respond to ischemia/reoxygenation stress by detaching from the sinusoids and undergo cell death by apoptosis, dependent on caspase as well as calpain protease activity [38, 39].
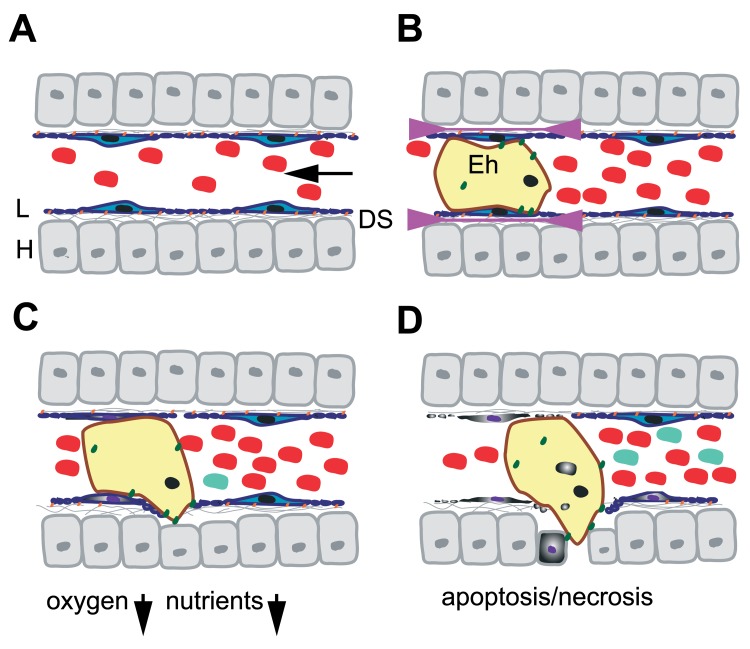
Fig. 4.
Model for the passage of E. histolytica through the liver sinusoidal barrier. A. Schematic representation of a liver sinusoid. Red blood cells circulate in the sinusoidal lumen lined by fenestrated LSEC (L; blue). LSEC adhere at FA plates (orange) to the ECM (gray lines) present in the Disse’s space (DS) and in contact which the hepatocytes (H). Stellate and Kupffer cells are not represented. The arrow indicates the direction of the blood flow. Note that the drawing is not in scale. B. During early stages of hepatic amoebiasis, E. histolytica trophozoites (yellow) obstruct hepatic sinusoid capillaries and induce LSEC retraction (indicated by arrowheads). The amoeba (10–50 µm) being bigger than the sinusoid diameter (5–7 µm), trophozoites exert mechanical forces on the endothelium. In addition, several virulence factors and adhesion molecules (green) facilitate the loss of FA complexes accelerating LSEC retraction. C. Obstruction caused by amoebae reduces the blood flow, locally creating ischemia and decreasing concentrations of oxygen and nutrients. As a consequence, the oxidative stress for the amoebae is diminished, LSEC death by apoptosis and necrosis (purple nucleus, grey cytoplasm) is induced and the inflammatory response initiated by immune cells (light blue). D. Retraction and cell death allow the amoeba to penetrate into the liver parenchyma in which it induces hepatocyte death. Phagocytosis of red blood cells, apoptotic bodies and necrotic debris provides nutrients and energy to the trophozoites. The immune response is developing
We propose that at early stages of liver infection and upon contact with the sinusoidal endothelium E. histolytica interferes with host cell adhesion signalling leading to diminished adhesion and induction of target cell death (Fig. 5). Disruption of FA complexes has been described as an early event in endothelial cell apoptosis, preceding caspase-dependent proteolysis of complex components and cell death [40]. For a variety of pathogens (bacterial, fungal, viral and parasitic) disseminating through the vascular system, interference with endothelial cell integrity/function is known as an important mechanism sustaining infection, immune response escape, propagation and dissemination to the underlying tissues. Interference results in changes in vascular permeability and/or in endothelial cell death, mainly suppression/delay or induction of apoptosis (e.g. [41–43]).
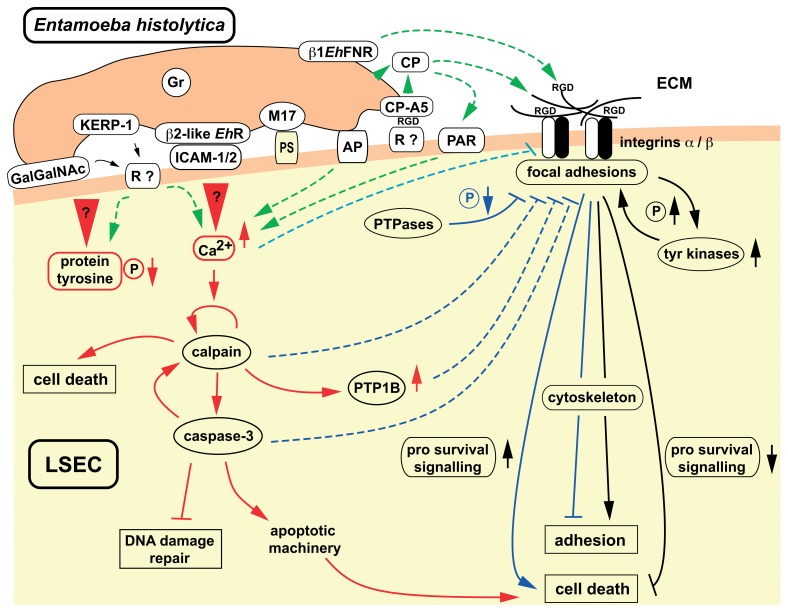
Fig. 5.
E. histolytica interference with cell adhesion signalling may induce cell death. The scheme summarizes the levels at which E. histolytica may interfere with target cell integrin-mediated cell adhesion signalling, here LSEC. Several relevant virulence factors are presented: Gal/GalNAc lectin and KERP-1 [44]. Host cell receptors for both amoebic molecules remain to be identified. Amoebic β2-integrin like molecule (β2-like EhR) shown to bind to intercellular adhesion molecules ICAM-1 and -2 [45] whose expression is induced in activated endothelial cells. RGD motif containing proteins, like the ECM component fibronectin expressed by LSEC, may serve as binding sites for β1 integrin-like fibronectin receptor β1EhFNR which shares a high degree of homology with the intermediate Gal/GalNAc lectin subunits Igl1 and Igl2 ([35] and references therein). Interaction may reinforce trophozoite adherence and eventually compete with integrins for RGD ligands, thus interfering with LSEC adhesion signalling. The immuno-dominant amoeba protein M17 [46] may be involved in recognition of phosphatidyl-serine (PS) exposed at the surface of apoptotic cells, which are preferentially phagocytosed by amoebae. Pore-forming amoebapore proteins (AP) though involved in lysis of other host cells and ALA, are not essential for LSEC apoptosis and death. The calcium-binding proteins Grainin-1 and -2 (Gr) are found in secreted granules, but their potential role in LSEC killing has not been elucidated so far. Cysteine proteases, among which CP-A5, present at the trophozoite surface or released into the extracellular medium, are implicated in ECM degradation. The pro-form of CP-A5, localized in secreted and surface-bound fractions, contains a RGD sequence that confers to this protein a protease activity-independent additional function in adherence and host cell inflammatory response induction; binding to integrin αvβ3 from colonic enterocyte cells has been found [34], potential receptors on LSEC are not characterized. Activation of vascular endothelial cell G-protein coupled receptors, like the protease-activated receptors (PAR), by pathogen-encoded CPs have been described for several microorganisms, including African trypanosome parasites. Anchorage-dependent survival of adherent cells relies on the activity of the integrin-mediated signal transduction pathway, regulating cell survival/death signalling activities and cytoskeleton organization. LSEC death results from abrogation of adhesion signalling leading to diminished adhesion, to inactivation of survival and to activity of cell death pathways. Loss of adhesion may in turn trigger apoptosis (anoikis). Elements intervening in E. histolytica contact-dependent apoptosis of LSEC were inferred from data obtained with the human lymphoma line Jurkat [47–49]. Upon contact with trophozoites, the protein tyrosine phosphorylation level rapidly decreases and the concentration of free intracellular calcium drastically rises. The initial trigger for both events remain yet unknown. Calcium-dependent activation of calpain in turn activates caspase-3 responsible for the execution of the apoptotic program. Calpain activity may also account for necrotic cell death and diminished protein phosphorylation levels, by cleavage-dependent activation of protein tyrosine phosphatase PTP1B. Amoeba-induced activity of calpain, caspase-3 and PTP1B and several other protein tyrosine phosphatases as well as increased calcium levels may negatively modulate integrin/FA pathway activity
Altogether, the in vivo and in vitro approaches to study hepatic amoebiasis and the molecular tools used to interfere with key factor functions will help to define the molecular and physical bases of ALA. These advances in the understanding of this neglected parasitic disease, used as a model, will help to understand better the physiology and the immunology of the liver in situations of health and in the context of other diseases.
Acknowledgments
Work in the BCP unit is supported by grants from ANR-MIE 08 (Intestinalamibe), ANR-GENOM-BTV (Genamibe), ANR-Blanc Inter SVSE3 (Paractin), the Application and Industry Transfer Department (DARRI) of the Pasteur Institute and the Pasteur Weizmann Research Council. The immuno-fluorecence and video-microscopy studies were performed thanks to the Imagopole of the Pasteur Institute. JMM and JSR were PhD fellows of the French Ministère de la Recherche et Technologie. JSR received a PhD supplemental fellowship from the “Régime Social des Indépendants” and JMM from the Pasteur-Weizmann Research Council.
Contributor Information
D. M. Faust, Institut Pasteur, Cell Biology of Parasitism Unit, Inserm U786, Paris, France
J. Marquay Markiewicz, Institut Pasteur, Cell Biology of Parasitism Unit, Inserm U786, Paris, France.
J. Santi-Rocca, Institut Pasteur, Cell Biology of Parasitism Unit, Inserm U786, Paris, France.
N. Guillen, Institut Pasteur, Cell Biology of Parasitism Unit, Inserm U786, Paris, France.
References
- 1.Haque R, Huston CD, Hughes M, Houpt E, Petri WA., Jr. Amebiasis. N Engl J Med. 2003 Apr 17;348(16):1565–1573. doi: 10.1056/NEJMra022710. [DOI] [PubMed] [Google Scholar]
- 2.Bansal D, Ave P, Kerneis S, Frileux P, Boché O, Baglin AC, Dubost G, Leguern AS, Prevost MC, Bracha R, Mirelman D, Guillén N, Labruyère E. An ex-vivo human intestinal model to study Entamoeba histolytica pathogenesis. PLoS Negl Trop Dis. 2009 Nov 17;3(11):e551. doi: 10.1371/journal.pntd.0000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanley SL, Jr., Reed SL. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VI. Entamoeba histolytica: parasite-host interactions. Am J Physiol Gastrointest Liver Physiol. 2001 Jun;280(6):G1049–G1054. doi: 10.1152/ajpgi.2001.280.6.G1049. [DOI] [PubMed] [Google Scholar]
- 4.Blazquez S, Zimmer C, Guigon G, Olivo-Marin JC, Guillén N, Labruyère E. Human tumor necrosis factor is a chemoattractant for the parasite Entamoeba histolytica. Infect Immun. 2006 Feb;74(2):1407–1411. doi: 10.1128/IAI.74.2.1407-1411.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santi-Rocca J, Rigothier MC, Guillén N. Host-microbe interactions and defense mechanisms in the development of amoebic liver abscesses. Clin Microbiol Rev. 2009 Jan;22(1):65–75. doi: 10.1128/CMR.00029-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsutsumi V, Mena-Lopez R, Anaya-Velazquez F, Martinez-Palomo A. Cellular bases of experimental amebic liver abscess formation. Am J Pathol. 1984 Oct;117(1):81–91. [PMC free article] [PubMed] [Google Scholar]
- 7.Rigothier MC, Khun H, Tavares P, Cardona A, Huerre M, Guillén N. Fate of Entamoeba histolytica during establishment of amoebic liver abscess analyzed by quantitative radioimaging and histology. Infect Immun. 2002 Jun;70(6):3208–3215. doi: 10.1128/IAI.70.6.3208-3215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seydel KB, Stanley SL., Jr. Entamoeba histolytica induces host cell death in amebic liver abscess by a non-Fas-dependent, non-tumor necrosis factor alpha-dependent pathway of apoptosis. Infect Immun. 1998 Jun;66(6):2980–2983. doi: 10.1128/iai.66.6.2980-2983.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelosof LC, Davis PH, Zhang Z, Zhang X, Stanley SL., Jr. Co-ordinate but disproportionate activation of apoptotic, regenerative and inflammatory pathways characterizes the liver response to acute amebic infection. Cell Microbiol. 2006 Mar;8(3):508–522. doi: 10.1111/j.1462-5822.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 10.Olivos A, Ramos E, Nequiz M, Barba C, Tello E, Castañón G, González A, Martínez RD, Montfort I, Pérez-Tamayo R. Entamoeba histolytica: mechanism of decrease of virulence of axenic cultures maintained for prolonged periods. Exp Parasitol. 2005 Jul;110(3):309–312. doi: 10.1016/j.exppara.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Swain MG. Natural killer T cells within the liver: conductors of the hepatic immune orchestra. Dig Dis. 2010;28(1):7–13. doi: 10.1159/000282059. [DOI] [PubMed] [Google Scholar]
- 12.Lotter H, Jacobs T, Gaworski I, Tannich E. Sexual dimorphism in the control of amebic liver abscess in a mouse model of disease. Infect Immun. 2006 Jan;74(1):118–124. doi: 10.1128/IAI.74.1.118-124.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maldonado-Bernal C, Kirschning CJ, Rosenstein Y, Rocha LM, Rios-Sarabia N, Espinosa-Cantellano M, Becker I, Estrada I, Salazar-González RM, López-Macías C, Wagner H, Sánchez J, Isibasi A. The innate immune response to Entamoeba histolytica lipopeptidophosphoglycan is mediated by toll-like receptors 2 and 4. Parasite Immunol. 2005 Apr;27(4):127–137. doi: 10.1111/j.1365-3024.2005.00754.x. [DOI] [PubMed] [Google Scholar]
- 14.Pérez-Tamayo R, Martínez RD, Montfort I, Becker I, Tello E, Pérez-Montfort R. Pathogenesis of acute experimental amebic liver abscess in hamsters. J Parasitol. 1991 Dec;77(6):982–988. [PubMed] [Google Scholar]
- 15.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007 Feb 2;100(2):174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 16.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 17.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007 Feb 2;100(2):158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 18.Ventura-Juárez J, Campos-Rodríguez R, Rodríguez-Martínez HA, Rodríguez-Reyes A, Martínez-Palomo A, Tsutsumi V. Human amebic liver abscess: expression of intercellular adhesion molecules 1 and 2 and of von Willebrand factor in endothelial cells. Parasitol Res. 1997;83(5):510–514. doi: 10.1007/s004360050289. [DOI] [PubMed] [Google Scholar]
- 19.Blazquez S, Rigothier MC, Huerre M, Guillén N. Initiation of inflammation and cell death during liver abscess formation by Entamoeba histolytica depends on activity of the galactose/N-acetyl-D-galactosamine lectin. Int J Parasitol. 2007 Mar;37(3-4):425–433. doi: 10.1016/j.ijpara.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Katz U, Ankri S, Stolarsky T, Nuchamowitz Y, Mirelman D. Entamoeba histolytica expressing a dominant negative N-truncated light subunit of its gal-lectin are less virulent. Mol Biol Cell. 2002 Dec;13(12):4256–4265. doi: 10.1091/mbc.E02-06-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bracha R, Nuchamowitz Y, Mirelman D. Transcriptional silencing of an amoebapore gene in Entamoeba histolytica: molecular analysis and effect on pathogenicity. Eukaryot Cell. 2003 Apr;2(2):295–305. doi: 10.1128/EC.2.2.295-305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ankri S, Padilla-Vaca F, Stolarsky T, Koole L, Katz U, Mirelman D. Antisense inhibition of expression of the light subunit (35 kDa) of the Gal/GalNac lectin complex inhibits Entamoeba histolytica virulence. Mol Microbiol. 1999 Jul;33(2):327–337. doi: 10.1046/j.1365-2958.1999.01476.x. [DOI] [PubMed] [Google Scholar]
- 23.Santi-Rocca J, Weber C, Guigon G, Sismeiro O, Coppée JY, Guillén N. The lysine- and glutamic acid-rich protein KERP1 plays a role in Entamoeba histolytica liver abscess pathogenesis. Cell Microbiol. 2008 Jan;10(1):202–217. doi: 10.1111/j.1462-5822.2007.01030.x. [DOI] [PubMed] [Google Scholar]
- 24.Salmon P, Oberholzer J, Occhiodoro T, Morel P, Lou J, Trono D. Reversible immortalization of human primary cells by lentivector-mediated transfer of specific genes. Mol Ther. 2000 Oct;2(4):404–414. doi: 10.1006/mthe.2000.0141. [DOI] [PubMed] [Google Scholar]
- 25.Zhang WJ, Ye LY, Wu LQ, Xin YL, Gu F, Niu JX, Yang ZH, Zhu GJ, Grau GE, Lou JN. Morphologic, phenotypic and functional characteristics of endothelial cells derived from human hepatic cavernous hemangioma. J Vasc Res. 2006;43(6):522–532. doi: 10.1159/000095965. [DOI] [PubMed] [Google Scholar]
- 26.Wu LQ, Zhang WJ, Niu JX, Ye LY, Yang ZH, Grau GE, Lou JN. Phenotypic and functional differences between human liver cancer endothelial cells and liver sinusoidal endothelial cells. J Vasc Res. 2008;45(1):78–86. doi: 10.1159/000109079. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Majno P, Morel P, Toso C, Triponez F, Oberholzer J, Mentha G, Lou J. Prostaglandin E(1) protects human liver sinusoidal endothelial cell from apoptosis induced by hypoxia reoxygenation. Microvasc Res. 2002 Jul;64(1):94–103. doi: 10.1006/mvre.2002.2404. [DOI] [PubMed] [Google Scholar]
- 28.Faust DM, Marquay Markiewicz J, Danckaert A, Soubigou G, Guillen N. Human liver sinusoidal endothelial cells respond to interaction with Entamoeba histolytica by changes in morphology, integrin signalling and cell death. Cell Microbiol. 2011 Jul;13(7):1091–1106. doi: 10.1111/j.1462-5822.2011.01604.x. [DOI] [PubMed] [Google Scholar]
- 29.Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009 Oct;89(4):1269–1339. doi: 10.1152/physrev.00027.2008. [DOI] [PubMed] [Google Scholar]
- 30.Meredith JE, Jr., Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993 Sep;4(9):953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravichandran KS. Signaling via Shc family adapter proteins. Oncogene. 2001 Oct 1;20(44):6322–6330. doi: 10.1038/sj.onc.1204776. [DOI] [PubMed] [Google Scholar]
- 32.Katoh K, Kano Y, Ookawara S. Role of stress fibers and focal adhesions as a mediator for mechano-signal transduction in endothelial cells in situ. Vasc Health Risk Manag. 2008;4(6):1273–1282. doi: 10.2147/vhrm.s3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marquay Markiewicz J, Syan S, Hon CC, Weber C, Faust D, Guillen N. A proteomic and cellular analysis of uropods in the pathogen Entamoeba histolytica. PLoS Negl Trop Dis. 2011 Apr 5;5(4):e1002. doi: 10.1371/journal.pntd.0001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou Y, Mortimer L, Chadee K. Entamoeba histolytica cysteine proteinase 5 binds integrin on colonic cells and stimulates NFkappaB-mediated pro-inflammatory responses. J Biol Chem. 2010 Nov 12;285(46):35497–35504. doi: 10.1074/jbc.M109.066035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sengupta K, Hernández-Ramírez VI, Rosales-Encina JL, Mondragón R, Garibay-Cerdenares OL, Flores-Robles D, Javier-Reyna R, Pertuz S, Talamás-Rohana P. Physical, structural, and functional properties of the beta1 integrin-like fibronectin receptor (beta1EhFNR) in Entamoeba histolytica. Infect Genet Evol. 2009 Sep;9(5):962–970. doi: 10.1016/j.meegid.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002 Aug 23;1(1):1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campos-Rodríguez R, Jarillo-Luna RA, Larsen BA, Rivera-Aguilar V, Ventura-Juárez J. Invasive amebiasis: a microcirculatory disorder? Med Hypotheses. 2009 Nov;73(5):687–697. doi: 10.1016/j.mehy.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Sindram D, Kohli V, Madden JF, Clavien PA. Calpain inhibition prevents sinusoidal endothelial cell apoptosis in the cold ischemic rat liver. Transplantation. 1999 Jul 15;68(1):136–140. doi: 10.1097/00007890-199907150-00025. [DOI] [PubMed] [Google Scholar]
- 39.Natori S, Selzner M, Valentino KL, Fritz LC, Srinivasan A, Clavien PA, Gores GJ. Apoptosis of sinusoidal endothelial cells occurs during liver preservation injury by a caspase-dependent mechanism. Transplantation. 1999 Jul 15;68(1):89–96. doi: 10.1097/00007890-199907150-00018. [DOI] [PubMed] [Google Scholar]
- 40.Harrington EO, Smeglin A, Newton J, Ballard G, Rounds S. Protein tyrosine phosphatase-dependent proteolysis of focal adhesion complexes in endothelial cell apoptosis. Am J Physiol Lung Cell Mol Physiol. 2001 Feb;280(2):L342–L353. doi: 10.1152/ajplung.2001.280.2.L342. [DOI] [PubMed] [Google Scholar]
- 41.Kim KS. Microbial translocation of the blood-brain barrier. Int J Parasitol. 2006 May 1;36(5):607–614. doi: 10.1016/j.ijpara.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Grab DJ, Garcia-Garcia JC, Nikolskaia OV, Kim YV, Brown A, Pardo CA, Zhang Y, Becker KG, Wilson BA, de A Lima AP, Scharfstein J, Dumler JS. Protease activated receptor signaling is required for African trypanosome traversal of human brain microvascular endothelial cells. PLoS Negl Trop Dis. 2009 Jul 21;3(7):e479. doi: 10.1371/journal.pntd.0000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu-Hsieh BA, Yen YT, Chen HC. Dengue hemorrhage in a mouse model. Ann N Y Acad Sci. 2009 Sep;1171(Suppl 1):E42–E47. doi: 10.1111/j.1749-6632.2009.05053.x. [DOI] [PubMed] [Google Scholar]
- 44.Seigneur M, Mounier J, Prevost MC, Guillén N. A lysine- and glutamic acid-rich protein, KERP1, from Entamoeba histolytica binds to human enterocytes. Cell Microbiol. 2005 Apr;7(4):569–579. doi: 10.1111/j.1462-5822.2005.00487.x. [DOI] [PubMed] [Google Scholar]
- 45.Pillai DR, Kain KC. Entamoeba histolytica: identification of a distinct beta2 integrin-like molecule with a potential role in cellular adherence. Exp Parasitol. 2005 Mar;109(3):135–142. doi: 10.1016/j.exppara.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Marion S, Guillén N. Genomic and proteomic approaches highlight phagocytosis of living and apoptotic human cells by the parasite Entamoeba histolytica. Int J Parasitol. 2006 Feb;36(2):131–139. doi: 10.1016/j.ijpara.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Teixeira JE, Mann BJ. Entamoeba histolytica-induced dephosphorylation in host cells. Infect Immun. 2002 Apr;70(4):1816–1823. doi: 10.1128/IAI.70.4.1816-1823.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim KA, Lee YA, Shin MH. Calpain-dependent calpastatin cleavage regulates caspase-3 activation during apoptosis of Jurkat T cells induced by Entamoeba histolytica. Int J Parasitol. 2007 Sep;37(11):1209–1219. doi: 10.1016/j.ijpara.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Kim KA, Lee YA, Shin MH. Calpain-dependent cleavage of SHP-1 and SHP-2 is involved in the dephosphorylation of Jurkat T cells induced by Entamoeba histolytica. Parasite Immunol. 2010 Mar;32(3):176–183. doi: 10.1111/j.1365-3024.2009.01175.x. [DOI] [PubMed] [Google Scholar]