Abstract
Campylobacter spp. is the most common bacterial pathogen of gastroenteritis worldwide. Poultry is the main reservoir and consequently the main origin of infections for humans. As a consequence of a primary Campylobacter infection which typically manifests as diarrhea, there is an increased risk to suffer from post-infectious complications such as reactive arthritis, neuropathia, myositis or a Guillain-Barré Syndrome. Usually the verification of acute campylobacteriosis is made by stool culture. In contrast, post-infectious complications can be diagnosed by serological assays. Since most of them are based on whole cell lysates, an insufficient specificity results from cross-reactions between related species. Therefore, the use of recombinant antigens becomes more and more favorable. Campylobacter is able to secrete a number of proteins, which are amongst others necessary for cell invasion and therefore play a crucial role for virulence. One of these, Cj0069, has a similar specificity and sensitivity in the detection of anti-Campylobacter jejuni IgG compared to the well-established antigens OMP18 and P39. This makes it a suitable antigen for diagnosing C. jejuni post-infectious complications.
Keywords: arthritis, Campylobacter jejuni, campylobacteriosis, Cj0069, Guillain-Barré syndrome, OMP18, P39, serodiagnostics
Introduction
Campylobacter jejuni, the most common germ causing bacterial foodborne enteritis worldwide, leads to numerous post-infectious complications [7, 12, 26, 35]. The frequency of Campylobacter-associated post-infectious diseases was estimated at 1:1000 [28]. One of the most serious Campylobacter-associated post-infectious complications is the Guillain-Barré syndrome (GBS) [2]. The symptoms of a GBS include paralysis, loss of neurological reflexes, electrocardiographic abnormalities (arrhythmia), electrolyte imbalance and respiratory failure [24]. Other pathogens, such as influenza virus, cytomegalovirus and Epstein-Barr virus have also been described to trigger GBS. However, since 80% of GBS cases are associated with Campylobacter seropositivity, it seems most likely that Campylobacter spp. are the main cause of this sequela [28]. Molecular mimicry between lipooligosaccharides (LOS) localized on the surface of the bacteria and CNS-gangliosides GM1 and GD1a induces cross-reacting antibodies during Campylobacter-infections. This cross-reactivity of anti-Campylobacter-LOS-antibodies is thought to be the leading pathomechanism of GBS. In particular, the sialic acid containing LOS classes A1, A2, B1, B2, C, M and R, are associated with GBS [19, 20]. Sialic acids are O- or N-substituted derivatives of neuraminic acid, a monosaccharide with a nine-carbon backbone. Especially N-substitutes can be found frequently in glycosylated gangliosides of the CNS [20]. In addition, other diseases such as reactive arthritis, reactive myositis and idiopathic peripheral neuropathy are associated with campylobacteriosis, as well [35].
The diagnosis of acute Campylobacter enteritis is established via stool culture, using selective media supplemented with trimethoprim, vancomycin and polymyxin B, in order to suppress the growth of the accompanying gut flora. Besides that, there is the possibility of Campylobacter-antigen detection from stool samples via ELISA.
In patients with reactive diseases, the period of acute gastroenteritis dates back, sometimes even several years in the past. Thus, cultivation of the pathogen from stool is no longer possible and therefore a causal link between the post-infectious disease and the causing agent cannot be traced back anymore. In such cases only serodiagnostics can give hints of a possible aetiopathology.
Based on agglutination reactions, the species C. jejuni can be subtyped into different serotypes. In Germany, the Lior serotype 4 is associated with campylobacteriosis in the majority of cases, whereas GBS is most often correlated with Lior serotype 11 [11]. Serotyping of Campylobacter isolates for epidemiological purposes has today been nearly completely replaced by biomolecular methods, such as multilocus sequence typing (MLST) [10] or the sequencing of the short variable region of the flagellin A gene (flaA-SVR sequencing) [16]. Thus, serodiagnosis of prior Campylobacter infections can be performed by using an immunoblot with whole cell lysates of the high prevalent C. jejuni serotype Lior 11 [11]. In general, the reactivity of the immunoglobulin classes IgA and IgG against C. jejuni is determined. The detection of IgM antibodies is also possible, but rarely used in routine diagnostics.
However, serological tests with whole cell lysates show partially an insufficient sensitivity and specificity for the detection of Campylobacter-specific antibodies. Strid and coworkers could obtain a 90% specificity and a combined sensitivity of 90% for IgM, IgA and IgG 90 days post-infection [29]. Reasons for a lower specificity could be a longer time interval from diarrhea to the post-infectious complication or serologic cross-reactivities with other Campylobacter species or phylogenetically related species such as Helicobacter spp. and Arcobacter spp. Cross-reactions with Salmonella spp., Legionella spp. [5] and Yersinia spp. were also reported [9]. In routine diagnostics, recombinant antigens such as MOMP [3, 4] and PEB1-4 [23] were increasingly used within commercial test kits.
The flagellar motility of the bacterium is an important factor for colonization of the intestine of its avian and mammalian hosts and especially for the invasion of intestinal epithelial cells and for overcoming the mucosal barrier [14, 17, 18, 22, 30, 31, 32]. Furthermore, bacterial secretion systems are used for the export of proteins including some virulence-associated factors. A total of five different secretion systems are known (T1-5SS). A type III secretion system (T3SS) is often found in pathogenic bacteria that export virulence factors in a Sec-signal independent way [1]. Campylobacter genome sequencing revealed that there are no elements encoding for a classic type III secretion system [21]. However, the amino acid sequences of some proteins and the structural composition of the flagellar apparatus are homologous to a T3SS. Therefore, one can speak of a flagellar type III secretion system [14]. The flagellar filament of motile bacteria is encoded by two genes: flaA and flaB. Knockout of the flaA-gene turns the bacterium immotile (Mot-), but keeps it still able to secrete the so-called Campylobacter invasion antigens (Cia). Knockout of both, the flaA and the flaB gene, leads to a combined loss of motility (Mot-) and the ability to secrete proteins (S-). Konkel and coworkers could show in a flaA/flaB-double-knockout mutant that at least one of the two genes has to be complemented in order to re-establish the secretion (S+) of the Cias [14]. These results show that the secretion of Cia depends on a functional flagellar apparatus. Thus, the Campylobacter flagellum has two major functions: the motility of the bacterium and the host cell invasion [13, 14]. The secretion of Cias is triggered by deoxycholate [15, 25] and contact with epithelial cells [25]. In addition, secretion of Cias could be induced by fetal bovine serum [25]. Christensen and coworkers examined genes by in silico analysis on properties characterizing potentially secretory Campylobacter proteins, such as transmembrane and periplasmic domains, as well as Secor twin-arginine translocation (Tat)-motifs [8]. All proteins secreted via a T3SS, or its flagellar homolog, have a specific sequence motif at their N-terminal end. This became evident through experiments with Yersinia enterocolitica, that owns a T3SS and that is able to export CiaB. The export of virulence factors via a T3SS homologous flagellar secretion system has been demonstrated for the first time in this bacterium [33]. A specific amino acid address-sequence as prerequisite for protein export through the T3SS is not species restricted. Thus, Y. enterocolitia proved to be a suitable model system to screen for C. jejuni secretory proteins and consequently brought 42 secretory proteins to light. The function of most of them is still unknown [8].
Schmidt-Ott et al. established in 2005 a Campylobacter immunoblot and an ELISA based on two recombinant proteins [27]. The first protein used for this purpose was P39, which is encoded by the gene cj0017c (dsbI). Sequence analysis suggested that P39 is an ATP/GTP-binding protein [21]. The second protein, OMP18, encoded by cj0113 (omp18) is a transmembrane outer membrane protein. It is homologous to the peptidoglycan-associated protein (Pal) of E. coli [6, 27] and is highly conserved in different C. jejuni strains. Due to its localization on the outer membrane, it has also highly immunogenic properties. The ELISA has a sensitivity of 63.9% for IgA, 58.3% for IgG and 80.6% for IgA + IgG and a specificity of 100% for patients with acute GBS. Flagellin A encoded by the cj1339 (flaA) gene was considered as another potential candidate antigen for establishing a recombinant Campylobacter ELISA but it was rejected, because of the low specificity and difficulties in purification of this antigen [27].
So far, only a few standardized test antigens with different sensitivities and specificities for the retrospective diagnosis of Campylobacter infections have been identified. In particular, the specificity suffers from the fact that there are cross-reactivities between Campylobacter spp., the high prevalent Helicobacter pylori and Corynebacterium spp.
The secreted proteins identified by Christensen and coworkers [8] have probably a high immunogenic potential due to their exposure to the human immune system and should therefore be capable of developing highly sensitive and specific serological tests.
Materials and methods
Bacterial strains and growth conditions
The E. coli strain DH5α (DSMZ, Braunschweig, Germany) was used to amplify all plasmids and to express the recombinant proteins. Bacteria were grown in Luria Bertani medium (LB medium), containing 10 g/L Bacto-tryptone (BD, Sparks, USA), 5 g/L Bacto Yeast Extract (BD, Sparks, USA) and 5–10 g/L NaCl (Merck, Darmstadt, Germany). For the preparation of LB agar plates, 15 g/L Bacto-Agar (BD, Sparks, USA) was added.
Cloning, transformation and induction of recombinant protein expression
Based on the published list of secreted proteins by Christensen and coworkers [8], three of them were selected randomly according to an easily distinguishable molecular weight. The complete sequenced and well-characterized strain C. jejuni NCTC 11168 was used as genomic DNA template for the amplification of the respective genes. The following primer pairs were used: cj0036 (forward: NNN-NNN-CCG-CGG-AGC-AAA-GCC-GTG-AAT-TTT-GGT-GT and reverse: NNN-NNN-CCA-TGG-TTA-GTG-ATG-GTG-ATG-GTG-ATG-ATC-CTC-ATC-TTT-TGC-TAA-ATT-TTC-AAG-AC), cj0069 (forward: NNN-NNN-CCG-CGG-ACC-GCC-TAG-AAG-GAC-AAA-CA and reverse: NNN-NNN-CCA-TGG-TTA-GTG-ATG-GTG-ATG-GTG-ATG-AGC-CTT-AGT-TTT-ACT-TAC-AA) and cj0125c (forward: NNN-NNN-CCG-CGG-ATG-CAT-CAT-CAC-CAT-CAC-CAC-CAA-CAA-CAA-TCA-CCC-TTA-TCA-C and reverse: NNN-NNN-CCA-TGG-AGA-TGC-CAA-GCA-AAG-GCA-AA). Bold characters indicate the artificially added hexa-histidine-tag, italic characters indicate the restriction enzyme cutting/cloning site. All primers were obtained from Sigma-Aldrich (Taufkirchen, Germany).
Amplicons of the respective genes were cloned into the pASK-IBA16 expression vector (IBA BioTAGnology, Göttingen, Germany) at the SacII and NcoI restriction sites. Plasmid isolation was performed with GenElute™ Plasmid Miniprep Kit (Sigma-Aldrich, Taufkirchen, Germany) according to the manufacturers’ instructions. Correct integration into the vector and the exclusion of mutations was confirmed by sequencing (Sequencing primers: forward: GAG-TTA-TTT-TAC-CAC-TCC-CT and reverse: CGC-AGT-AGC-GGT-AAA-CG) of the insertion site (SeqLab, Göttingen, Germany). Successfully transformed E. coli bacteria were screened on LB agar containing 100 µg/mL ampicillin (Sigma-Aldrich, Taufkirchen, Germany). The expression of the recombinant proteins was induced by anhydrotetracycline (IBA BioTAGnology, Göttingen, Germany) for 5 h at 37 °C with an end concentration of 200 µg/mL.
Test for bactericidal properties of the recombinant proteins
In order to test the recombinant proteins for bactericidal properties in the E. coli expression system, the Live/Dead BacLight Bacterial Viability Kit (Molecular Probes, Leiden, Netherlands) was applied according to the manufacturer’s instructions. Dead and live cells of ten visual fields per culture (three per transformant) were determined and counted at a ×100 magnification and averages were calculated.
Colony blot
The ability to express the recombinant proteins was tested by colony blot. Transformed E. coli clones were spotted in a grid on LB agar plates containing ampicillin. The E. coli ATCC-25922 (DSMZ, Braunschweig, Germany) strain served as negative control. Bacteria were incubated over night at 37 °C. A replica was made from each plate on a nitrocellulose membrane. For induction of protein expression, the membrane was applied with the colonies above on a LB agar plate containing 200 µg/L anhydrotetracycline and 50 µg/L ampicillin and incubated for 4 h at 37 °C. Proteins were fixed on the membrane and incubated with a primary anti-hexa-histidine-tag antibody (BD Pharmingen, San Jose, USA) in a 1:2000 dilution for one hour at room temperature. The secondary horseradish peroxidase-coupled anti-body, alkaline phosphatase-conjugated AffiniPure rabbit anti-human IgG, Fcγ (ImmunoResearch Inc., West Grove, USA), was incubated in a 1:10000 dilution for one hour at room temperature. Specific binding was detected by electrochemoluminescence (GE Healthcare, Munich, Germany; Camera: Fujifilm LAS-4000, Düsseldorf, Germany).
Isolation of recombinant proteins
The proteins were purified by Ni-NTA agarose (QIAGEN, Hilden, Germany) under denaturing conditions using an imidazole gradient. All fractions were analyzed by SDS-PAGE. Fractions with the highest concentrations of protein were pooled. The protein content was measured with the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, USA) according to the manufacturer’s instructions using a NanoDrop photometer (Thermo Scientific, Wilmington, USA). Bacterial cells were separated from the induction media by centrifugation at 4 °C for 15 min at 10000× g. Cells were washed and lysed for 15 to 60 min at 37 °C with a lysis buffer containing 100 µL EDTA-free proteinase inhibitor (PI) cocktail III (Calbiochem/Merck Chemicals Ltd., Nottingham, England), 10 U Benzonase (Novagen/Merck Chemicals Ltd., Nottingham, England) and a pinch lysozyme (Sigma, Deisenhofen, Germany). The crude lysate was centrifuged for 15 min at 4600× g. The supernatant was collected and the pellet was resuspended with 8 M urea solution containing 5 mM imidazole for 15 min at 57 °C, in order to dissolve inclusion bodies. The protein solution was centrifuged again with the conditions mentioned above. Molecular weights of recombinant proteins were confirmed by 15% SDS-PAGE.
Westernblot
For the production of the line blot stripes, 10 µg protein suspended in 150 µL elution buffer were mixed with 4x sample buffer and applied on a 15% gel, made without a comb. After the semi-dry blotting (Jancos, Copenhagen, Denmark) procedure, PVDF membranes (GE Healthcare, Munich, Germany) were stored in PBS-Tween sealed at –20 °C until use. As a quality control, both the SDS-PAGE gels and samples of each membrane were Coomassie and immunohisto-chemically stained, respectively. Membranes were incubated in blocking solution for 60 min at room temperature and membranes were incubated over night at 4 °C with the primary antibody mouse anti-hexa-histidine-tag (BD Pharmingen, San Jose, USA) at a 1:3000 dilution. After three washing steps, the membranes were incubated with the secondary horseradish-peroxidase coupled antibody HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Inc., West Grove, USA) diluted 1:2000 in washing buffer at room temperature for one hour. Membranes were washed three times for each 20 min in washing buffer. Reactive proteins were detected with electrochemoluminescence (ECL) according to manufacturer’s instructions.
Serological evaluation of recombinant C. jejuni antigens using human serum samples
Blood samples were drawn from patients with an acute campylobacteriosis confirmed by stool culture. As a negative control, sera from healthy blood donors were used. The sera were analyzed with the commercial test system recom-Line Campylobacter IgG und IgA (MIKROGEN Diagnostik, Neuried, Germany) according to the manufacturer’s instructions. Test stripes were evaluated manually, without considering the cut-off band (see discussion). The self-compounded Cj0069-line blot stripes (see above), were incubated with 20 µL serum diluted 1:100 in blocking buffer (3% milk powder in 1× PBS with 0.05% PBS-Tween 20). Alkaline phosphatase (AP)-conjugated secondary AffiniPure rabbit anti-human IgA-Fα and alkaline phosphatase-conjugated AffiniPure rabbit anti-human IgG-Fcγ (both obtained from ImmunoResearch Inc., West Grove, USA), were incubated in PBS-Tween in a 1:4000 dilution for one hour at room temperature. Antibody binding was detected with NBT/BCIP substrate solution. The reaction was stopped immediately by adding water after a color change was observed. The stripes were finally evaluated after 2 h of drying. As a positive control a purified mouse anti-hexa-histidine-tag antibody (BD Pharmingen, San Jose, USA) was used at a 1:2000 dilution.
Results
Demonstration of ubiquitary occurrence, expression and toxicity testing of C. jejuni secretory proteins
At first the ubiquitary occurrence of the chosen genes could be demonstrated by PCR using the same primer pairs as intended for cloning into the pASK-IBA16 expression vector. For cj0036, cj0069 and cj0125c, PCR analysis confirmed broad presence (results not shown) in genetic diverse C. jejuni isolates (the genetic diversity of these isolates was known from a preliminary study [34]).
A colony blot assay after successful cloning and transformation of the three genes with an anti-hexa-histidine-tag antibody (results not shown) revealed that the proteins Cj0036, Cj0069 and Cj0125c were expressed in most of the transformants.
Table 1.
Description of genes and proteins
| Gene | Gene length [bp] | Protein length [AA] | Molecular weight [kDa] | Protein function |
|---|---|---|---|---|
| cj0036 | 1365 | 455 | 50.5 | Hypothetical protein |
| cj0069 | 1047 | 349 | 38.7 | Hypothetical protein |
| cj0125c | 426 | 142 | 15.7 | Hypothetical protein |
To see if there is a possible toxic effect of the recombinant proteins on the E. coli transformants, a Live/Dead-assay was performed before and after induction with anhydrotetracycline. As shown in Table 2, the gene products of cj0036 and cj0125c are clearly toxic to E. coli DH5a. The number of viable bacteria after induction with anhydrotetracycline decreased significantly. In contrast, the gene products of cj0069, as well as cj0017c (dsbI) and cj00113c (omp18) [27], which were chosen as control genes, showed no significant toxic effects towards the E. coli cell survival. Furthermore, gene products of cj0036 and cj0125c were detected in mere minimal amounts, both in the bacterial lysate and the supernatant due to their toxicity to E. coli (results not shown). Thus, only Cj0069 was applicable for further purification and construction of a line blot assay.
Table 2.
Live/Dead-Assay before and after inductionof protein expression
| Gene | Percentage of dead bacteria
[%] |
|
|---|---|---|
| before induction | after induction | |
| cj0036 | ≈ 10 | ≈ 70 |
| cj0069 | ≈ 30 | ≈ 30 |
| cj0125c | <1 | 70–80 |
| cj0017c (dsbl) | <5 | = 10 |
| cj00113c (omp18) | <1 | 10–20 |
Cj0069 immunoblot
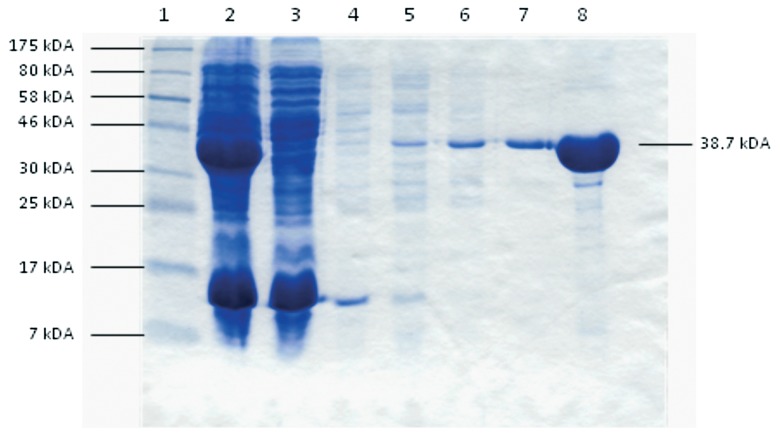
The imidazole gradient elution with 100 mM imidazol proved to be the best way to extract and purify the recombinant Cj0069 protein fused to a hexa-histidine-tag, which was predominantly found in the supernatant, probably due to a secretion signal in the protein sequence (Fig. 1). This protein was used to manufacture the line blot stripes.
Fig. 1.
Coomassie-stained gel: Imidazol gradient elution using a NaPO4-buffer system (pH 8.0). 1: standard, 2: unpurified supernatant, 3: flow through, 4: elution, using a NaPO4 buffersystem (pH 8.0) with 10 mM Imidazol, 5: ... with 20 mM imidazol 6: ... with 40 mM imidazol, 7: ... with 100 mM imidazol, 8: ... second elution with 100 mM imidazol (in a 1:10 dilution)
Testing of blood donor and patient sera with the Cj0069 line blot stripes and a commercial test kit from MIKROGEN Diagnostik
For evaluation of each antigen on the recomLine stripe the “cut-off band” was omitted, because this was not applicable to the “self-made” Cj0069 line blot stripes.
IgA reactivity
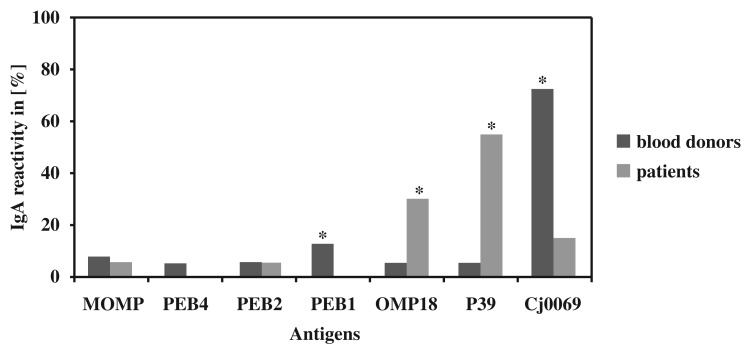
It was possible to recognize a significant difference (p-value ≤ 0.05) in the IgA reactivity for the antigens PEB1, OMP18, P39, and Cj0069 (Fig. 2) in comparison to blood donor sera (n = 40) and patient sera (n = 20). OMP18 (5.0% < 30.0%) and P39 (5.0% < 55.0%) were significantly more often reactive for IgA in patients’ sera. Contrary to expectations, a higher IgA reactivity for blood donor sera was found for PEB1 (12.5% > 0.0%) and Cj0069 (72.5% > 15.0%).
Fig. 2.
IgA reactivity of seven different Campylobacter jejuni antigens. Significant differences (p-value ≤ 0.05) are indicated with a *
IgG reactivity
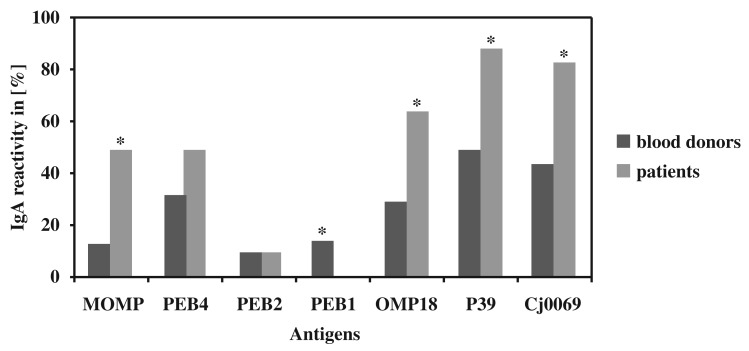
As shown in Fig. 3 significant differences (p-value ≤ 0.05) in the IgG reactivity between blood donors and patients were seen for MOMP (12.5% < 50.0%), PEB1 (32.5% < 50.0%), OMP18 (30.0% < 65.0%), P39 (50.0% < 90.0%) and Cj0069 (45.0% < 85.0%). As expected, IgG was more often reactive in patients’ sera for all antigens but PEB1 (15.0% > 0.0%) and PEB2 (10.0% = 10.0%).
Fig. 3.
IgG reactivity of seven different Campylobacter jejuni antigens. Significant differences (p-value ≤ 0.05) are indicated with a *
Discussion
The aim of this study was to analyze whether the secreted C. jejuni protein Cj0069 might be suitable as antigen in the serodiagnostics of post-infectious complications of campylobacteriosis. Presumably due to bacteriotoxic effects of Cj0036 and Cj0125c, it was not possible to produce line blot stripes for these two secretory proteins.
To rate the ability of Cj0069 to discriminate between healthy blood donors and campylobacteriosis patients, sensitivity and specificity as well as positive (PPV) and negative predictive values (NPV) were calculated and compared with the data for OMP18, P39, MOMP, PEB1, PEB2, PEB4 ascertained from testing with the recomLine test kit. Additionally the values estimated in preliminary studies were considered as well (Tables 3, 4, 5 and 6).
Table 3.
Comparison of IgA sensitivity and specificity sensitivity
| Protein | Sensitivity [%] |
Specificity [%] |
||
|---|---|---|---|---|
| this study | reference | this study | reference | |
| OMP18 | 30.0 | 40.71 | 95.0 | 1001 |
| P39 | 55.0 | 22.21 | 95.0 | 1001 |
| OMP18 + P39 | 55.0 | 40.71 | 90.0 | 1001 |
| Cj0069 | 15.0 | – | 27.5 | – |
| OMP18 + Cj0069 | 45.0 | – | 27.5 | – |
| P39 + Cj0069 | 60.0 | – | 27.5 | – |
| OMP18 + P39 + Cj0069 | 55.0 | – | 27.5 | – |
| MOMP | 5.0 | 41.82 | 92.5 | 81.42 |
| PEB1 | 0.0 | –3 | 87.5 | –3 |
| PEB2 | 5.0 | –3 | 100 | –3 |
| PEB4 | 0.0 | –3 | 95.0 | –3 |
| Whole cell lysate | – | 78.44 | – | 82.14 |
|
1 immunoblot of Schmidt-Ott et al. [27] 2 average value over all time points calculated from the ELISA of Blaser et al. [3] 3 no values given for IgA in Pei et al. [23] 4 calculated from Enders et al. (immunoblot), taking the bands of PEB1–4 into consideration [11] | ||||
Table 4.
Comparison of IgG sensitivity and specificity
| Protein | Sensitivity [%] |
Specificity [%] |
||
|---|---|---|---|---|
| this study | reference | this study | reference | |
| OMP18 | 65.0 | 74.11 | 70.0 | 79.21 |
| P39 | 90.0 | 70.31 | 50.0 | 87.51 |
| OMP18 + P39 | 90.0 | 85.21 | 50 | 72.91 |
| Cj0069 | 85.0 | – | 55.0 | – |
| OMP18 + Cj0069 | 95.0 | – | 45.0 | |
| P39 + Cj0069 | 100 | – | 32.5 | – |
| OMP18 + P39 + Cj0069 | 100 | – | 32.5 | – |
| MOMP | 50.0 | 45.62 | 87.5 | 73.62 |
| PEB1 | 0.0 | 78.93 | 85.0 | 1003 |
| PEB2 | 10.0 | 10.53 | 90.0 | 1003 |
| PEB4 | 50.0 | 31.63 | 67.5 | 1003 |
| Whole cell lysate | – | 90.24 | – | 79.54 |
|
1 immunoblot of Schmidt-Ott et al. [27] 2 average value over all time points calculated from the ELISA of Blaser et al. [3] 3 calculated from Pei et al. (ELISA) [23] 4 calculated from Enders et al. (immunoblot), taking the bands of PEB1–4 into consideration [11] | ||||
Table 5.
Positive and negative predictive values for IgA
| Protein | PPV [%] |
NPV [%] |
||
|---|---|---|---|---|
| this study | reference | this study | reference | |
| OMP18 | 75.0 | 1001 | 73.1 | 75.01 |
| P39 | 84.6 | 1001 | 80.9 | 69.61 |
| OMP18 + P39 | 73.3 | 1001 | 80.0 | 75.01 |
| Cj0069 | 9.4 | – | 29.0 | – |
| OMP18 + Cj0069 | 23.7 | – | 50.0 | – |
| P39 + Cj0069 | 29.3 | – | 57.9 | – |
| OMP18 + P39 + Cj0069 | 27.5 | – | 55.0 | – |
| MOMP | 25.0 | 70.82 | 66.1 | 56.42 |
| PEB1 | 0.0 | –3 | 63.6 | –3 |
| PEB2 | 100 | –3 | 67.8 | –3 |
| PEB4 | 0.0 | –3 | 65.5 | –3 |
| Whole cell lysate | - | 85.14 | – | 74.14 |
|
1 immunoblot of Schmidt-Ott et al. [27] 2 average value over all time points calculated from the ELISA of Blaser et al. [3] 3 no values given for IgA in Pei et al. [23] 4 calculated from Enders et al. (immunoblot), taking the bands of PEB1–4 into consideration [11] | ||||
Table 6.
Positive and negative predictive values for IgG
| Protein | PPV [%] | NPV [%] | ||
|---|---|---|---|---|
| this study | reference | this study | reference | |
| OMP18 | 50.0 | 66.71 | 80.0 | 84.41 |
| P39 | 47.4 | 76.01 | 91.0 | 84.01 |
| OMP18 + P39 | 47.4 | 63.91 | 90.1 | 89.71 |
| Cj0069 | 48.6 | – | 88.0 | – |
| OMP18 + Cj0069 | 46.3 | – | 94.7 | – |
| P39 + Cj0069 | 42.6 | – | 100 | – |
| OMP18 + P39 + Cj0069 | 42.6 | – | 100 | – |
| MOMP | 66.7 | 54.32 | 77.8 | 66.32 |
| PEB1 | 0 | 1003 | 63.0 | 71.43 |
| PEB2 | 33.3 | 1003 | 66.7 | 40.03 |
| PEB4 | 43.5 | 1003 | 73.0 | 43.53 |
| Whole cell lysate | – | 85.24 | – | 86.14 |
|
1 immunoblot of Schmidt-Ott et al. [27] 2 average value over all time points calculated from the ELISA of Blaser et al. [3] 3 calculated from Pei et al. (ELISA) [23] 4 calculated from Enders et al. (immunoblot), taking the bands of PEB1–4 into consideration [11] | ||||
It can be seen in Table 3 that Cj0069 has a significantly lower IgA sensitivity (15%) and specificity (27.5%) compared to the well-established antigens P39 (55% and 95%) and OMP18 (30% and 95%). Schmidt-Ott and coworkers demonstrated values of 22.2% and 100% for P39 and 40.7% and 100% for OMP18, respectively [27]. Similar specificity values could be reproduced with the MIKROGEN assay as well. The sensitivity of MOMP, PEB1, PEB2 and PEB4 was 5.0% or below. The higher sensitivity of P39 and other antigens in this study was obviously due to the non-consideration of the cut-off band on the recomLine test stripe, since it would lead to a systematic bias in the evaluation of the Cj0069 reactivity. Combination of P39 with OMP18 resulted not in a significant increase of sensitivity for IgA, because P39 and OMP18 were positive for the same corresponding sera. In contrast to that, combination of OMP18 or P39 with Cj0069 increases sensitivity and PPV. Because of its low sensitivity, the PPV and NPV of Cj0069 for IgA detection are very low (9.4% and 29.0%), compared to those of P39 (84.6% and 80.9%) and OMP18 (75.0% and 73.1%; Table 5). This leads to the conclusion that this antigen alone is not reliable for IgA testing.
In contrast to IgA, Cj0069 seems to be well suited for IgG detection. Sensitivity and specificity values of Cj0069 for IgG were 85% and 55% (Table 4). This is comparable to those values of P39 (90% and 50%) and OMP18 (65% and 70%). These values correspond with the values calculated in the original work of Schmidt-Ott and coworkers [27]. Combination of OMP18 or P39 with Cj0069 increases sensitivity and NPV up to 100% (P39+Cj0069) while decreasing the PPV only to a minor extend. In comparison to MOMP, PEB1, PEB2 and PEB4 the IgG sensitivity of Cj0069 is significantly higher (Table 4). Despite to IgA, the positive and negative predictive values of Cj0069 for IgG were similar to the ones calculated in this study for P39 and OMP18 (Table 6). This makes Cj0069 a good candidate to be used in addition to P39 and OMP18 in IgG testing.
Our study revealed that due to low IgA and IgG sensitivities (5.0%, 0.0%, 5.0%, 0.0% and 50.0%, 0.0%, 10%, 50%), MOMP, PEB1, PEB2 and PEB4 seem not to be capable to distinguish between campylobacteriosis patients and healthy persons. In contrast to that, their specificities for IgA and IgG reached in this study (92.5%, 87.5%, 100%, 95.0% and 87.5%, 85.0%, 90.0%, 67.5%) make them suitable antigens to use them as an exclusion criterion for a Campylobacter infection.
To draw a conclusion, it seems reasonable to use different antigens for IgA and IgG testing, respectively. In addition to that, a further screening for highly specific antigens only found in Campylobacter is necessary. To objectify the applicability of Cj0069 in the diagnostics of Campylobacter-associated post-infectious complications, further testing with larger numbers of sera from patients affected by campylobacteriosis, reactive arthritis or GBS is necessary.
Acknowledgments
This study was funded by the “Forschungsförderungsprogramm“ of the Universitätsmedizin Göttingen, Germany.
Contributor Information
J. Corso, Abteilung für Medizinische Mikrobiologie, Universitätsmedizin Göttingen, Kreuzbergring 57, D-37075 Göttingen, Germany
R. Lugert, Abteilung für Medizinische Mikrobiologie, Universitätsmedizin Göttingen, Kreuzbergring 57, D-37075 Göttingen, Germany
U. Groß, Abteilung für Medizinische Mikrobiologie, Universitätsmedizin Göttingen, Kreuzbergring 57, D-37075 Göttingen, Germany
A. E. Zautner, Abteilung für Medizinische Mikrobiologie, Universitätsmedizin Göttingen, Kreuzbergring 57, D-37075 Göttingen, Germany.
References
- 1.Aizawa SI. Bacterial flagella and type III secretion systems. FEMS Microbiol Lett. 2001 Aug 21;202(2):157–164. doi: 10.1111/j.1574-6968.2001.tb10797.x. [DOI] [PubMed] [Google Scholar]
- 2.Allos BM. Campylobacter jejuni Infections: update on emerging issues and trends. Clin Infect Dis. 2001 Apr 15;32(8):1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- 3.Blaser MJ, Duncan DJ. Human serum antibody response to Campylobacter jejuni infection as measured in an enzyme-linked immunosorbent assay. Infect Immun. 1984 May;44(2):292–298. doi: 10.1128/iai.44.2.292-298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser MJ, Hopkins JA, Vasil ML. Campylobacter jejuni outer membrane proteins are antigenic for humans. Infect Immun. 1984 Mar;43(3):986–993. doi: 10.1128/iai.43.3.986-993.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boswell TC, Kudesia G. Serological cross-reaction between Legionella pneumophila and campylobacter in the indirect fluorescent antibody test. Epidemiol Infect. 1992 Oct;109(2):291–295. doi: 10.1017/s095026880005024x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnens A, Stucki U, Nicolet J, Frey J. Identification and characterization of an immunogenic outer membrane protein of Campylobacter jejuni. J Clin Microbiol. 1995 Nov;33(11):2826–2832. doi: 10.1128/jcm.33.11.2826-2832.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect. 2004 Oct;10(10):868–876. doi: 10.1111/j.1469-0691.2004.00983.x. [DOI] [PubMed] [Google Scholar]
- 8.Christensen JE, Pacheco SA, Konkel ME. Identification of a Campylobacter jejuni-secreted protein required for maximal invasion of host cells. Mol Microbiol. 2009 Aug;73(4):650–662. doi: 10.1111/j.1365-2958.2009.06797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colmegna I, Cuchacovich R, Espinoza LR. HLA-B27-associated reactive arthritis: pathogenetic and clinical considerations. Clin Microbiol Rev. 2004 Apr;17(2):348–369. doi: 10.1128/CMR.17.2.348-369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingle KE, Colles FM, Wareing DR, Ure R, Fox AJ, Bolton FE, Bootsma HJ, Willems RJ, Urwin R, Maiden MC. Multilocus sequence typing system for Campylobacter jejuni. J Clin Microbiol. 2001 Jan;39(1):14–23. doi: 10.1128/JCM.39.1.14-23.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enders U, Karch H, Toyka KV, Michels M, Zielasek J, Pette M, Heesemann J, Hartung HP. The spectrum of immune responses to Campylobacter jejuni and glycoconjugates in Guillain-Barré syndrome and in other neuroimmunological disorders. Ann Neurol. 1993 Aug;34(2):136–144. doi: 10.1002/ana.410340208. [DOI] [PubMed] [Google Scholar]
- 12.European Food SafetyAuthority – EFSA. Campylobacter and Salmonella prevalence estimates. EFSA Journal. 2010;8:1503. [Google Scholar]
- 13.Konkel ME, Kim BJ, Rivera-Amill V, Garvis SG. Bacterial secreted proteins are required for the internaliztion of Campylobacter jejuni into cultured mammalian cells. Mol Microbiol. 1999 May;32(4):691–701. doi: 10.1046/j.1365-2958.1999.01376.x. [DOI] [PubMed] [Google Scholar]
- 14.Konkel ME, Klena JD, Rivera-Amill V, Monteville MR, Biswas D, Raphael B, Mickelson J. Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J Bacteriol. 2004 Jun;186(11):3296–3303. doi: 10.1128/JB.186.11.3296-3303.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik-Kale P, Parker CT, Konkel ME. Culture of Campylobacter jejuni with sodium deoxycholate induces virulence gene expression. J Bacteriol. 2008 Apr;190(7):2286–2297. doi: 10.1128/JB.01736-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meinersmann RJ, Helsel LO, Fields PI, Hiett KL. Discrimination of Campylobacter jejuni isolates by fla gene sequencing. J Clin Microbiol. 1997 Nov;35(11):2810–2814. doi: 10.1128/jcm.35.11.2810-2814.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morooka T, Umeda A, Amako K. Motility as an intestinal colonization factor for Campylobacter jejuni. J Gen Microbiol. 1985 Aug;131(8):1973–1980. doi: 10.1099/00221287-131-8-1973. [DOI] [PubMed] [Google Scholar]
- 18.Nachamkin I, Yang XH, Stern NJ. Role of Campylobacter jejuni flagella as colonization factors for three-day-old chicks: analysis with flagellar mutants. Appl Environ Microbiol. 1993 May;59(5):1269–1273. doi: 10.1128/aem.59.5.1269-1273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker CT, Gilbert M, Yuki N, Endtz HP, Mandrell RE. Characterization of lipooligosaccharide-biosynthetic loci of Campylobacter jejuni reveals new lipooligosaccharide classes: evidence of mosaic organizations. J Bacteriol. 2008 Aug;190(16):5681–5689. doi: 10.1128/JB.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker CT, Horn ST, Gilbert M, Miller WG, Woodward DL, Mandrell RE. Comparison of Campylobacter jejuni lipooligosaccharide biosynthesis loci from a variety of sources. J Clin Microbiol. 2005 Jun;43(6):2771–2781. doi: 10.1128/JCM.43.6.2771-2781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000 Feb 10;403(6770):665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 22.Pavlovskis OR, Rollins DM, Haberberger RL, Jr., Green AE, Habash L, Strocko S, Walker RI. Significance of flagella in colonization resistance of rabbits immunized with Campylobacter spp. Infect Immun. 1991 Jul;59(7):2259–2264. doi: 10.1128/iai.59.7.2259-2264.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pei ZH, Ellison RT, 3rd, Blaser MJ. Identification, purification, and characterization of major antigenic proteins of Campylobacter jejuni. J Biol Chem. 1991 Sep 5;266(25):16363–16369. [PubMed] [Google Scholar]
- 24.Pithadia AB, Kakadia N. Guillain-Barré syndrome (GBS) Pharmacol Rep. 2010 Mar-Apr;62(2):220–232. doi: 10.1016/s1734-1140(10)70261-9. [DOI] [PubMed] [Google Scholar]
- 25.Rivera-Amill V, Kim BJ, Seshu J, Konkel ME. Secretion of the virulence-associated Campylobacter invasion antigens from Campylobacter jejuni requires a stimulatory signal. J Infect Dis. 2001 Jun 1;183(11):1607–1616. doi: 10.1086/320704. [DOI] [PubMed] [Google Scholar]
- 26.Robert Koch Institut – RKI. Aktuelle Daten und Informationen zu Infektionskrankheiten und Puplic Health Epidemiologisches Bulletin Nr. 36. Robert Koch Institut; 2007. [Google Scholar]
- 27.Schmidt-Ott R, Brass F, Scholz C, Werner C, Gross U. Improved serodiagnosis of Campylobacter jejuni infections using recombinant antigens. J Med Microbiol. 2005 Aug;54(Pt 8):761–767. doi: 10.1099/jmm.0.46040-0. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt-Ott R, Schmidt H, Feldmann S, Brass F, Krone B, Gross U. Improved serological diagnosis stresses the major role of Campylobacter jejuni in triggering Guillain-Barré syndrome. Clin Vaccine Immunol. 2006 Jul;13(7):779–783. doi: 10.1128/CVI.00065-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strid MA, Engberg J, Larsen LB, Begtrup K, Mølbak K, Krogfelt KA. Antibody responses to Campylobacter infections determined by an enzyme-linked immunosorbent assay: 2-year follow-up study of 210 patients. Clin Diagn Lab Immunol. 2001 Mar;8(2):314–319. doi: 10.1128/CDLI.8.2.314-319.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szymanski CM, King M, Haardt M, Armstrong GD. Campylobacter jejuni motility and invasion of Caco-2 cells. Infect Immun. 1995 Nov;63(11):4295–4300. doi: 10.1128/iai.63.11.4295-4300.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wassenaar TM, Bleumink-Pluym NM, van der Zeijst BA. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991 Aug;10(8):2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao R, Burr DH, Doig P, Trust TJ, Niu H, Guerry P. Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells. Mol Microbiol. 1994 Dec;14(5):883–893. doi: 10.1111/j.1365-2958.1994.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 33.Young GM, Schmiel DH, Miller VL. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc Natl Acad Sci U S A. 1999 May 25;96(11):6456–6461. doi: 10.1073/pnas.96.11.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zautner AE, Herrmann S, Corso J, Tareen AM, Alter T, Gross U. Epidemiological association of different Campylobacter jejuni groups with metabolism-associated genetic markers. Appl Environ Microbiol. 2011 Apr;77(7):2359–2365. doi: 10.1128/AEM.02403-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zautner AE, Herrmann S, Groß U. [Campylobacter jejuni – The Search for virulence-associated factors] Arch für Lebensmittelhyg. 2010;61:91–101. [Article in German] [Google Scholar]