Abstract
Motivated behaviors, including sexual experience, activate the mesolimbic dopamine system and produce long-lasting molecular and structural changes in the nucleus accumbens. The transcription factor ΔFosB is hypothesized to partly mediate this experience-dependent plasticity. Previous research in our laboratory has demonstrated that overexpressing ΔFosB in the nucleus accumbens of female Syrian hamsters augments the ability of sexual experience to cause the formation of a conditioned place preference. It is unknown, however, whether ΔFosB-mediated transcription in the nucleus accumbens is required for the behavioral consequences of sexual reward. We therefore used an adeno-associated virus to overexpress ΔJunD, a dominant negative binding partner of ΔFosB that decreases ΔFosB-mediated transcription by competitively heterodimerizing with ΔFosB before binding at promotor regions on target genes, in the nucleus accumbens. We found that overexpression of ΔJunD prevented the formation of a conditioned place preference following repeated sexual experiences. These data, when coupled with our previous findings, suggest that ΔFosB is both necessary and sufficient for behavioral plasticity following sexual experience. Furthermore, these results contribute to an important and growing body of literature demonstrating the necessity of endogenous ΔFosB expression in the nucleus accumbens for adaptive responding to naturally rewarding stimuli.
Keywords: Delta FosB, Sex, Conditioned Place Preference, Adeno-Associated Virus, Plasticity
INTRODUCTION
Plasticity in the striatum, like many areas of the brain, is critical for adaptive responses to environmental stimuli at the molecular, cellular, and behavioral levels. Motivated behaviors, such as sex (Meisel & Mullins, 2006, Pitchers et al., 2010b, Wallace et al., 2008), aggression (Couppis & Kennedy, 2008, Staffend & Meisel, 2012), and wheel running (Greenwood et al., 2011, Vargas-Perez et al., 2003, Werme et al., 2002), as well as chronic exposure to drugs of abuse (Chen et al., 2010, Robinson & Kolb, 2004, Russo et al., 2010), result in activation of the mesolimbic dopamine system and long-lasting changes in the nucleus accumbens (NAc). Structural changes, particularly the formation of dendritic spines, are important components of this experience-based plasticity, which persists long after the motivated behavior or drug administration has ceased (Meisel & Mullins, 2006, Pitchers et al., 2010b, Robinson & Kolb, 2004).
Although the intervening molecular events that cause changes in dendritic morphology have not been fully described, the transcription factor ΔFosB appears to be at the heart of the molecular pathway that mediates the enduring structural and behavioral changes that occur following experience with motivated behaviors and/or drugs of abuse (Nestler, 2008). ΔFosB is an alternative splice product of the immediate early gene fosB and shares homology with other Fos family transcription factors, which heterodimerize with Jun family proteins to activate AP-1-mediated gene transcription (Robison & Nestler, 2011). Unlike other Fos family proteins that are highly unstable and return to basal levels within hours of stimulation, ΔFosB accumulates following repeated stimulation and remains elevated and stable for extended periods of time (Chen et al., 1997, Nestler, 2008, Robison & Nestler, 2011). This stability is a hypothesized mechanism by which changes in gene expression and dendritic morphology can persist in the absence of further stimulation.
We have used female sexual behavior in Syrian hamsters as a model of experience-based plasticity in the brain (Hedges et al., 2010, Meisel & Mullins, 2006). This is a valuable model because repeated sexual experience reliably produces changes analogous to chronic exposure to drugs of abuse (Bradley et al., 2005a, Bradley & Meisel, 2001, Kohlert & Meisel, 1999, Meisel & Joppa, 1994, Meisel et al., 1996, Meisel & Mullins, 2006). Furthermore, previous work in our laboratory has demonstrated that overexpression of ΔFosB in NAc using adeno-associated viral (AAV) vectors mimics the ability of sexual experience to cause the formation of a conditioned place preference (Hedges et al., 2009), suggesting that ΔFosB accumulation in NAc is sufficient to create the behavioral plasticity associated with sexual experience. It remains unknown, however, whether ΔFosB is also necessary for these behavioral consequences of sexual experience. The current study therefore used AAV vectors to overexpress ΔJunD, a dominant negative inhibitor of ΔFosB, in the NAc. We report here that overexpression of ΔJunD prevents the formation of a conditioned place preference following repeated sexual experiences. When coupled with our previous results, these results suggest that ΔFosB in NAc is both necessary and sufficient for behavioral plasticity following sexual experience.
MATERIALS AND METHODS
Subjects
Adult female and male Syrian hamsters (Mesocricetus auratus) were purchased from Charles River Laboratories (Wilmington, MA, USA) at approximately 60 days of age. Females were used as experimental subjects, whereas males were used as sex stimulus animals during conditioned place preference tests. Females were housed individually and males were housed in pairs in polycarbonate cages (females: 50.8 × 40.6 × 20.3 cm; males: 43.2 × 22.9 × 20.3 cm). All animals were maintained on a reversed 14 hr light/10 hour dark photoperiod (lights off between 1:00 and 11:00 PM) and all behavioral testing occurred during the dark phase. The animal room was maintained at a controlled temperature of 22°C and food and water were available ad libitum. All animal procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23; revised 1996) and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Viral Vectors
AAV is characterized by its ability to efficiently transfect neurons and maintain specific transgene expression for long periods of time (Chamberlin et al., 1998). AAV vectors exist in different serotypes based on the characterization of their capsid protein coat. This experiment used an AAV2 (serotype 2) from Stratagene with a titer of over 108/μl expressing green fluorescent protein (AAV-GFP) as well as an AAV vector that contained constructs for both GFP and ΔJunD (AAV-GFP-ΔJunD). ΔJunD decreases ΔFosB-mediated transcription by competitively heterodimerizing with ΔFosB before binding the AP-1 region within gene promoters. These AAV vectors mediate transgene expression in rats and mice that becomes maximal within 10 days of injection and then persists at this level for at least six months (Winstanley et al., 2007, Zachariou et al., 2006). Importantly, the vectors infect neurons only and produce no toxicity greater than vehicle infusions alone. Details for the production and use of these vectors are provided in earlier publications (Winstanley et al., 2007, Zachariou et al., 2006).
Surgical Procedures
Ovariectomy
Female hamsters were bilaterally ovariectomized under sodium pentobarbital anesthesia (Nembutal; 8.5 mg per 100 g body weight, i.p.). Subjects’ ovaries were removed through bilateral flank incisions via cauterization of the uterine horn and blood vessels. Chromic gut absorbable suture (Henry Schein, Melville, NY, USA) and wound clips were used to close the smooth muscle and skin incisions, respectively.
Viral Vector Delivery
Immediately following ovariectomy, females were given supplemental anesthesia if necessary, and then underwent stereotaxic delivery of either AAV-GFP or AAV-GFP-ΔJunD. Anesthetized subjects were secured in the stereotaxic apparatus such that their skull was level in the anterior-posterior (A-P) and medial-lateral (M-L) planes. Following a midline scalp incision, the skin and temporal muscles were retracted to expose the skull and a hand-operated drill was used to expose dura. All A-P and M-L measurements were taken in mm relative to bregma and all dorsal-ventral (D-V) measurements were taken in mm relative to dura. Bilateral stereotaxic injections were made by lowering a microinjection syringe (model number, Hamilton, Reno, NV, USA) under stereotaxic control (Microinjection Unit, Model 5002, David Kopf Instruments, Tujunga, CA, USA) into bilateral sites targeting NAc and injecting 0.7 μl of AAV. To minimize the flow of AAV up the needle tract, the syringe was left in place for 10 min after each injection. Viral vectors were injected into NAc at least 2 weeks prior to behavior testing to allow ΔJunD overexpression to develop.
Behavioral Testing
Sexual Experience
Ovariectomized hamsters were hormone-primed for weekly sexual experiences via subcutaneous injections of estradiol benzoate (10 μg in 0.1 ml of cottonseed oil) at approximately 48 and 24 hr prior to the sexual behavior test followed by a subcutaneous injection of progesterone (500 μg in 0.1 ml of cottonseed oil) 4–6 hr prior to the sexual behavior test. Females that received sexual experience were paired with a sexually-experienced male hamster for a 10 minute session. Measures of female copulatory behavior (lordosis latency and total lordosis duration) were scored live during each sexual experience. Each male and female was only paired once.
Conditioned Place Preference
Our conditioned place preference (CPP) apparatus consists of two lateral chambers (60 × 45 × 38 cm) connected by a clear central chamber (37 × 22 × 38 cm). The lateral chambers are differentiated by the color of the walls and the type of bedding on the chamber floor: one lateral chamber is gray and contains aspen bedding (Harlan Laboratories, IN, USA) while the other lateral chamber is white and contains corncob bedding (Harlan Laboratories, IN, USA). Female hamsters underwent three different types of behavioral tests in the CPP apparatus: a pre-test, 5 conditioning tests, and a post-test. During the pre-test, hormone-primed female hamsters were placed in the clear central chamber and allowed to freely explore the entire apparatus for 10 min in order to establish an initial preference for one of the lateral compartments. During conditioning tests, hormone-primed females were given sexual experience (see above) in their non-preferred chamber for 10 min. One hour after sexual conditioning in the non-preferred chamber, females were placed into the initially preferred chamber alone for 10 min. The order in which the conditioning stimuli (i.e., sexually-experienced male versus no stimulus) are presented does not impact the formation or strength of a subsequent conditioned place preference (Meisel & Joppa, 1994). Consequently, we chose a single order of presentation in which subjects were always given sexual experience in the non-preferred chamber before being placed alone in the preferred chamber during conditioning tests. After 5 consecutive weeks of conditioning tests, hormone-primed females were given a post-test during which they were again allowed to freely explore the entire apparatus to determine if their initial preference had changed. Previous research has demonstrated that five consecutive sexual experiences are sufficient to detect significant changes in place preference (Hedges et al., 2009, Meisel & Joppa, 1994, Meisel et al., 1996). For both AAV-GFP and AAV-GFP-ΔJunD groups, some animals did not receive sexual experience during the conditioning tests but were hormone-primed and placed alone in each chamber for 10 min. As such, there were four experimental groups in this experiment: A positive control group that received bilateral AAV-GFP injections and were given sexual experience during conditioning (n = 7); a negative control group that received bilateral AAV-GFP injections but were not given sexual experience during conditioning (n = 7); a second negative control group that received bilateral AAV-GFP-ΔJunD injections but were not given sexual experience during conditioning (n = 6); and an experimental group that received bilateral AAV-GFP-ΔJunD injections and were given sexual experience during conditioning (n = 8). All tests in the CPP apparatus were conducted and scored by an observer blind to the experimental group of the subjects.
Histology and Injection Verification
Following the last behavioral test, subjects were injected with an overdose of Sleepaway (0.2 ml i.p., Fort Dodge Laboratories, Fort Dodge, IA, USA), injected intracardially with 0.2 ml of heparin sulfate (1000 IU/ml, Sagent Pharmaceuticals, Schaumburg, IL, USA) and intracardially perfused with 25 mM PBS for 2 min (approximately 50 ml) followed by 4% paraformaldehyde in 25 mM PBS for 20 min (approximately 500 ml). The brains were removed and post-fixed for 1 hr in 4% paraformaldehyde then placed in a 10% sucrose solution in PBS overnight. Serial coronal sections (40-μm) of frozen brain tissue were sectioned on a microtome and every third section was processed for immunohistochemical localization of either GFP or JunD. Free-floating sections were rinsed in PBS with 0.1% bovine serum albumin (wash buffer) and then incubated in primary antibody (rabbit anti-GFP: 1:1,000, AB3080, Millipore, Billerca, MA, USA; rabbit anti-JunD: 1:5000, sc-74, Santa Cruz Biotechnology, Dallas, TX, USA) in wash buffer with 0.3% Triton-X at room temperature for 24 hr. After rinsing in wash buffer, sections were incubated in a biotinylated secondary antibody (1:200, Vector Laboratories, Burlingame, CA, USA) for 45 min at room temperature, rinsed in wash buffer, and then incubated in an avidin-biotin complex (Vectastain ABC Kit, Vector Laboratories) for 45 min at room temperature. Sections were then rinsed in wash buffer and reacted in a 3, 3′-diaminobenzidine (DAB) solution with 0.003% hydrogen peroxide. After 5 min, sections were rinsed in wash buffer to stop the chromagen reaction. Immunostained sections were mounted onto glass slides, coverslipped, and examined under a light microscope for the location and rostral-caudal spread of AAV injection as compared with published hamster neuroanatomical plates (Morin & Wood, 2001).
Statistical analysis
Female copulatory parameters during sexual conditioning tests were compared between AAV conditions (AAV-GFP-ΔJunD vs. AAV-GFP) using one-way repeated measures ANOVAs. Our operational definition for sexual reward was a significant increase in time spent in the conditioned chamber on the post-test compared to the pre-test. To test our hypothesis that ΔFosB in the nucleus accumbens is necessary for sexual reward, conditioned place preference data from each treatment group were analyzed individually with a repeated measures t-test comparing the amount of time spent in the initially non-preferred chamber between the pre-test and the post-test. Bonferroni corrections were applied to minimize the likelihood of Type I error.
RESULTS
Immunohistochemical Characterization of AAV Injections
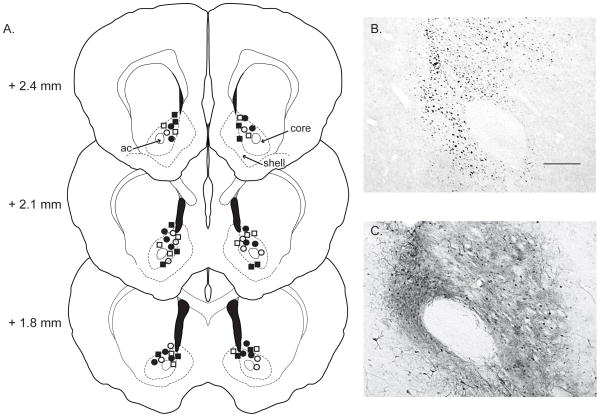
Brain tissue processed for immunohistochemical localization of either GFP or JunD was examined under a light microscope to determine the placement and extent of AAV injections. Injection placement was determined by tracing residual needle tracks. Bilateral AAV injections were consistently located in the dorsal NAc core (Figure 1A). Of the 29 animals analyzed, 12 animals had injection sites in the mid-caudal NAc core (Bregma + 2.1 mm), 9 animals had had injection sites located slightly more caudally (Bregma + 1.8 mm), and 8 animals had injection sites located slightly more rostrally (Bregma + 2.4 mm).
Figure 1. AAV injections.
A) AAV injections for the four experimental groups were localized to the dorsal nucleus accumbens core. Circles represent animals injected with AAV-GFP-ΔJunD, whereas squares represent animals injected with AAV-GFP. Filled symbols represent animals that received sexual experience, whereas open symbols represent animals that remained sexually-naïve. Numbers represent distance relative to bregma, ac, anterior commissure. B) Photomicrograph of representative nuclear immunostaining for JunD protein, scale bar = 200 μm. C) Photomicrograph of representative immunostaining for GFP in cell bodies and processes.
The extent of AAV infection was determined by examining JunD (Figure 1B) or GFP (Figure 1C) expression. All animals injected with either AAV-GFP (n = 15) or AAV-GFP-ΔJunD (n = 14) had significant transgene expression around the injection site that extended within the NAc core in both the rostral-caudal and dorsal-ventral planes. In most animals (n = 21), expression also extended into the NAc shell, albeit often to a lesser extent. In 6 animals, expression extended into the most rostral aspects of BNST, but labeling here was sparse. In one animal, the injection site was visible within the dorsal NAc core, but infection did not spread significantly outside of the region of the needle tip; as such, this animal was removed from the analysis. Although the exact shape of the spread of infection varied among individual animals, this variability was consistent between experimental groups.
Conditioned Place Preference
During the pre-test, 21 females demonstrated an initial preference for the white chamber and 8 females initially preferred the gray chamber. For those animals who received sexual experience, there was no difference in the average lordosis latency (Figure 2A) or lordosis duration (Figure 2B) between females injected with AAV-GFP and females injected with AAV-GFP-ΔJunD on any test day (all p > 0.05), suggesting that the overexpression of GFP or ΔJunD alone does not affect the receptive behavior of females.
Figure 2. Overexpression of ΔJunD in the NAc does not affect the expression of female sexual behavior.
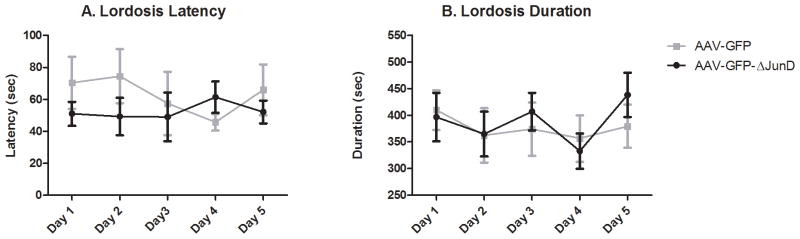
During sexual experience trials, females injected with AAV-GFP-ΔJunD did not differ from females injected with AAV-GFP in A) the latency to express lordosis or B) the total duration of lordosis on any test day. Data expressed as means ± standard errors.
For each group, the amount of time spent in the initially non-preferred chamber during the pre-test was compared to the time spent in the same chamber during the post-test (Figure 3). If sexual conditioning was successful, then the amount of time spent in the initially non-preferred chamber should increase significantly following sexual experience. Indeed, the positive control group that received AAV-GFP injections and was given sexual experience spent significantly more time in the initially non-preferred chamber during the post-test compared to during the pre-test (t(6)= 8.21, P < 0.05). In contrast, the amount of time spent in the initially non-preferred chamber did not differ between the pre- and post-tests for AAV-GFP-injected animals who did not receive sexual experience (t(6)= 0.58, P > 0.05). Similarly, the amount of time spent in the initially non-preferred chamber did not differ between the pre- and post-tests for AAV-GFP-ΔJunD-injected animals who did not receive sexual experience (t(5)= 0.92, P > 0.05). Interestingly, females in the AAV-GFP-ΔJunD group who received sexual experience also did not demonstrate a significant increase in the amount of time spent in the initially non-preferred chamber during the post-test (t(7)= 1.01, P > 0.05), suggesting that the overexpression of ΔJunD in NAc can prevent the formation of a conditioned place preference as a result of sexual experience.
Figure 3. Overexpression of ΔJunD in the NAc prevents the formation of conditioned place preference following sexual experience.
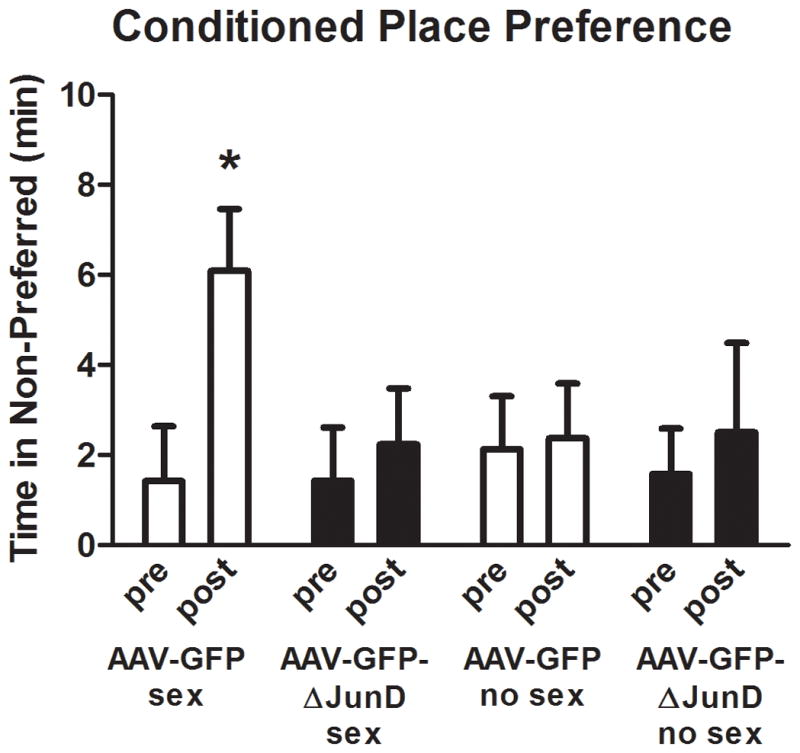
During the post-test, females who were injected with AAV-GFP and received sexual experience spent significantly more time in their initially non-preferred chamber (i.e., the chamber in which they received sexual experience) than they did during the pre-test. In contrast, the amount of time spent in the non-preferred chamber did not differ between the pre- and post-test for females injected with AAV-GFP-ΔJunD that received sexual experience. Likewise, females who were injected with either AAV-GFP or AAV-GFP-ΔJunD and did not receive sexual experience did not differ in the amount of time they spent in the non-preferred chamber during the pre- and post-tests. Data expressed as means ± standard errors. *significant difference between pre- and post-test (p < 0.05)
DISCUSSION
The hypothesized role of ΔFosB in mediating the neural and behavioral responses to natural rewards and drugs of abuse is based mainly on studies demonstrating that repeated experience with these stimuli correlates with the accumulation ΔFosB in the NAc (Meisel & Mullins, 2006, Pitchers et al., 2010c, Teegarden & Bale, 2007, Wallace et al., 2008, Werme et al., 2002, Zachariou et al., 2006) or that supraphysiological expression of ΔFosB in the NAc can augment the motivation for or response to these stimuli (Colby et al., 2003, Hedges et al., 2009, Pitchers et al., 2010c, Pitchers et al., 2013, Zachariou et al., 2006). Fewer studies, however, have attempted to experimentally manipulate the endogenous activity of ΔFosB in the brain to directly test the role of ΔFosB in reinforcement and reward. Our study therefore contributes to an important and growing body of literature demonstrating the necessity of endogenous ΔFosB expression in the NAc for adaptive responding to rewarding stimuli (Olausson et al., 2006, Peakman et al., 2003, Pitchers et al., 2010c, Pitchers et al., 2013, Werme et al., 2002).
In the current study, overexpression of ΔJunD was targeted to the NAc core and resulted in minimal spread to the NAc shell or other brain areas outside of the NAc. The ability of core-centered injections of ΔJunD to mitigate the rewarding consequences of sexual experience as measured in a conditioned place preference task is in agreement with previous research in our laboratory demonstrating that sexual experience increases c-Fos (Bradley & Meisel, 2001) and FosB expression (Meisel & Mullins, 2006) specifically within the NAc core. In addition, sexual experience (Meisel & Mullins, 2006) and another naturally rewarding behavior, aggressive experience (Staffend & Meisel, 2012), both increase dendritic spine density exclusively within the NAc core. Furthermore, the neural circuitry mediating the behavioral response to natural (e.g., sexual behavior) or synthetic (e.g., experience with drugs of abuse) rewards likely overlaps substantially, and recent evidence suggests that the NAc core may be particularly important for conditioning to social stimuli. In male rats, the acquisition of CPP for both social interaction and cocaine increases expression of the immediate early gene, Zif268, in the NAc shell and core (El Rawas et al., 2012). In the NAc core, however, CPP for social interaction increased Zif268 expression relative to CPP for cocaine, suggesting that the NAc core may be preferentially involved in the conditioning to naturally rewarding stimuli (El Rawas et al., 2012). It is also possible that separate populations of cells within both the shell and core may respond differentially to naturally rewarding stimuli versus drug stimuli, as neurons in both subregions of the NAc show similar neuronal activity during operant responding to two natural reinforcers (i.e., food and water), but different firing patterns during responding for a natural reinforcer versus cocaine (Carelli et al., 2000).
As in female hamsters, sexual experience in male rats produces long-lasting neural and behavioral changes that appear to depend on ΔFosB signaling in the NAc. Sexual experience in male rats results in the accumulation of FosB protein in the NAc (Pitchers et al., 2010c). Furthermore, viral overexpression of ΔFosB in the NAc facilitates copulatory efficiency in males, whereas viral overexpression of ΔJunD in the NAc inhibits experience-induced facilitation of male sexual behavior (Pitchers et al., 2010c, Pitchers et al., 2013). This ability of local manipulation of ΔFosB levels in the NAc to affect the overt expression of sexual behavior appears to be unique to males. Whereas overexpression of ΔJunD increases males’ latency to mount, intromit, and ejaculate during sexual experience sessions (Pitchers et al., 2013), overexpression of either ΔFosB in a previous study (Hedges et al., 2009) or ΔJunD in the current study affected the rewarding consequences of sexual behavior without affecting lordosis latency or duration in female hamsters. As our belief is that nucleus accumbens ΔFosB is a mediator of sexual motivation in both males and females, the apparent difference in the effects of ΔJunD on copulation in males and females may lie more in the fact that copulatory parameters in males includes both motivational and motoric components, whereas the lordosis response in females is primarily a “reflexive” behavior uncoupled from sexual motivation (Rivas & Mir, 1991).
Although the mechanisms underlying sexual experience-induced changes in ΔFosB signaling have not been completely defined, research in our laboratory and others suggests that elevations in NAc dopamine (DA) may drive this plasticity via D1 receptor activation. In female hamsters, sexual experience increases extracellular DA in the NAc (Kohlert & Meisel, 1999) and 6-hydroxydopamine lesions of the NAc block the experience-induced increase in female copulatory efficiency in future tests with males (Bradley et al., 2005b). Our assumption is that these effects are mediated by dopamine D1 neurotransmission, as sexual experience increases D1 receptor-mediated cyclic AMP production in the NAc compared with the response in sexually naïve female hamsters (Bradley et al., 2004). Further, our recent work demonstrates that sexual experience increases dendritic spine density specifically on D1-bearing medium spiny neurons in the NAc core of female hamsters (Staffend and Meisel, in preparation). Complementary evidence from male rats has demonstrated that sexual experience-induced increases in ΔFosB accumulation are dependent on mating-induced D1 receptor activation in the NAc as well. For example, ΔJunD overexpression blocks the sexual experience-induced increase in dendritic spine density in the NAc and pharmacological blockage of D1 receptors during sexual behavior prevents increases in dendritic spine density in males (Pitchers et al., 2013). Our working hypothesis is that sexual experience drives behavioral and structural plasticity through dopamine D1-mediated activation of signaling pathways that induce ΔFosB expression.
The model of experienced-based changes in ΔFosB signaling depending on D1 activation in the NAc is not limited to naturally rewarding experiences such as sexual behavior. Recent research by Grueter et al. (2013) has demonstrated that overexpression of ΔFosB in the NAc changes the synaptic properties of D1-expressing neurons in the NAc and increases dendritic spine density specifically on D1-bearing neurons in the NAc. Furthermore, selective overexpression of ΔFosB in D1 but not D2 neurons enhances locomotor sensitization to cocaine as well as CPP for cocaine (Grueter et al., 2013). Given the shared neural substrates for responding to natural rewards and drugs of abuse, and the knowledge that sexual experience can sensitize behavioral responses to drugs of abuse (Bradley & Meisel, 2001, Pitchers et al., 2010a, Pitchers et al., 2013), it is important to understand how experience with natural rewards can impact the vulnerability to drug addiction. By comparing the neural changes that result from naturally rewarding behaviors to those seen after exposure to drugs of abuse, we can separate the neural properties of drug addiction from the endogenous plasticity that underlie activities in everyday life.
Acknowledgments
The authors wish to thank Calyn Maske for her assistance collecting and scoring behavioral data. Research reported in this publication was supported by DA013680 to RLM and MH51399 to EJN. In addition, LEB was supported by The National Institutes on Drug Abuse under award number T32DA007234. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Bradley KC, Boulware MB, Jiang H, Doerge RW, Meisel RL, Mermelstein PG. Changes in gene expression within the nucleus accumbens and striatum following sexual experience. Genes Brain Behav. 2005a;4:31–44. doi: 10.1111/j.1601-183X.2004.00093.x. [DOI] [PubMed] [Google Scholar]
- Bradley KC, Haas AR, Meisel RL. 6-Hydroxydopamine lesions in female hamsters (Mesocricetus auratus) abolish the sensitized effects of sexual experience on copulatory interactions with males. Behav Neurosci. 2005b;119:224–232. doi: 10.1037/0735-7044.119.1.224. [DOI] [PubMed] [Google Scholar]
- Bradley KC, Meisel RL. Sexual behavior induction of c-Fos in the nucleus accumbens and amphetamine-stimulated locomotor activity are sensitized by previous sexual experience in female Syrian hamsters. J Neurosci. 2001;21:2123–2130. doi: 10.1523/JNEUROSCI.21-06-02123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KC, Mullins AJ, Meisel RL, Watts VJ. Sexual experience alters D1 receptor-mediated cyclic AMP production in the nucleus accumbens of female Syrian hamsters. Synapse. 2004;53:20–27. doi: 10.1002/syn.20030. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Du B, de Lacalle S, Saper CB. Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain Res. 1998;793:169–175. doi: 10.1016/s0006-8993(98)00169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Hopf FW, Bonci A. Synaptic plasticity in the mesolimbic system: therapeutic implications for substance abuse. Ann N Y Acad Sci. 2010;1187:129–139. doi: 10.1111/j.1749-6632.2009.05154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. J Neurosci. 2003;23:2488–2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couppis MH, Kennedy CH. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology. 2008;197:449–456. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- El Rawas R, Klement S, Kummer KK, Fritz M, Dechant G, Saria A, Zernig G. Brain regions associated with the acquisition of conditioned place preference for cocaine vs. social interaction. Front Behav Neurosci. 2012;6:63. doi: 10.3389/fnbeh.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217:354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. ΔFosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Nat Acad Sci. 2013;110:1923–1928. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges VL, Chakravarty S, Nestler EJ, Meisel RL. Delta FosB overexpression in the nucleus accumbens enhances sexual reward in female Syrian hamsters. Genes Brain Behav. 2009;8:442–449. doi: 10.1111/j.1601-183X.2009.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges VL, Staffend NA, Meisel RL. Neural mechanisms of reproduction in females as a predisposing factor for drug addiction. Front Neuroendocrinol. 2010;31:217–231. doi: 10.1016/j.yfrne.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlert JG, Meisel RL. Sexual experience sensitizes mating-related nucleus accumbens dopamine responses of female Syrian hamsters. Behav Brain Res. 1999;99:45–52. doi: 10.1016/s0166-4328(98)00068-0. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Joppa MA. Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiol Behav. 1994;56:1115–1118. doi: 10.1016/0031-9384(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Joppa MA, Rowe RK. Dopamine receptor antagonists attenuate conditioned place preference following sexual behavior in female Syrian hamsters. Eur J Pharm. 1996;309:21–24. doi: 10.1016/0014-2999(96)00389-5. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Mullins AJ. Sexual experience in female rodents: cellular mechanisms and functional consequences. Brain Res. 2006;1126:56–65. doi: 10.1016/j.brainres.2006.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A Stereotaxic Atlas of the Golden Hamster Brain. Academic Press; San Diego: 2001. [Google Scholar]
- Nestler EJ. Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. DeltaFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26:9196–9204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakman MC, Colby C, Perrotti LI, Tekumalla P, Carle T, Ulery P, Chao J, Duman C, Steffen C, Monteggia L, Allen MR, Stock JL, Duman RS, McNeish JD, Barrot M, Self DW, Nestler EJ, Schaeffer E. Inducible, brain region-specific expression of a dominant negative mutant of c-Jun in transgenic mice decreases sensitivity to cocaine. Brain Res. 2003;970:73–86. doi: 10.1016/s0006-8993(03)02230-3. [DOI] [PubMed] [Google Scholar]
- Pitchers KK, Balfour ME, Lehman MN, Richtand NM, Yu L, Coolen LM. Neuroplasticity in the mesolimbic system induced by natural reward and subsequent reward abstinence. Biol Psychiatry. 2010a;67:872–879. doi: 10.1016/j.biopsych.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Balfour ME, Lehman MN, Richtand NM, Yu L, Coolen LM. Neuroplasticity in the mesolimbic system induced by natural reward and subsequent reward abstinence. Biol Psychiatry. 2010b;67:872–879. doi: 10.1016/j.biopsych.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Frohmader KS, Vialou V, Mouzon E, Nestler EJ, Lehman MN, Coolen LM. DeltaFosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes Brain Behav. 2010c;9:831–840. doi: 10.1111/j.1601-183X.2010.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM. Natural and Drug Rewards Act on Common Neural Plasticity Mechanisms with DeltaFosB as a Key Mediator. J Neurosci. 2013;33:3434–3442. doi: 10.1523/JNEUROSCI.4881-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas FJ, Mir D. Accumbens lesion in female rats increases mount rejection without modifying lordosis. Rev Esp Fisiol. 1991;47:1–6. [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Meisel RL. Aggressive experience increases dendritic spine density within the nucleus accumbens core in female Syrian hamsters. Neuroscience. 2012;227:163–169. doi: 10.1016/j.neuroscience.2012.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61:1021–1029. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Vargas-Perez H, Mena-Segovia J, Giordano M, Diaz JL. Induction of c-fos in nucleus accumbens in naive male Balb/c mice after wheel running. Neurosci Lett. 2003;352:81–84. doi: 10.1016/j.neulet.2003.08.073. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolanos-Guzman CA. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, Brene S. Delta FosB regulates wheel running. J Neurosci. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DE, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]