Abstract
Impulse control disorders (ICDs) are potentially serious side effects of dopamine agonist therapy in Parkinson’s disease (PD), but prospective data are lacking about their incidence, time course, and risk factors. This work was a 4-year, prospective cohort study of outpatients with PD and no previous ICDs (N = 164). All subjects treated with a dopamine agonist during the study were followed longitudinally for new-onset ICDs. Baseline characteristics were compared in groups with (ICD+) and without (ICD−) subsequent ICDs. Forty-six subjects were treated with a dopamine agonist, including 25 who were newly treated and 21 who received ongoing dopamine agonist therapy. Of these 46 subjects, 18 (39.1%) developed new-onset ICDs. The timing of ICD onset varied from 3.0 to 114.0 months (median, 23.0) after initiation of dopamine agonist therapy. Baseline demographic characteristics were similar in ICD+ and ICD− groups. At baseline, ICD+ subjects had a greater prevalence of motor complications (61.1% versus 25.0%; P = 0.01) than ICD− subjects, despite comparable total dopaminergic medication usage in both groups (median, 150.0 versus 150.0 levodopa equivalents; P = 0.61). Compared with ICD− subjects, ICD+ subjects had a greater baseline prevalence of caffeine use (100% versus 66.7%; P = 0.007) and higher lifetime prevalence of cigarette smoking (44.4% versus 14.3%; P = 0.04). Peak dopamine agonist doses were higher in ICD+ than ICD− subjects (median 300.0 versus 165.0 L-dopa equivalents; P = 0.03), but cumulative dopamine agonist exposure was similar in both groups. In summary, the timing of new-onset ICDs in PD is highly variable. Risk factors include cigarette smoking, caffeine use, motor complications, and higher peak dopamine agonist dosage.
Keywords: dopamine agonist, dopamine agonist withdrawal syndrome, impulse control disorder, prospective, Parkinson’s disease
Dopamine agonists (DAAs) are a mainstay of treatment in Parkinson’s disease (PD) and are often favored as initial therapy because they are less likely to produce motor complications than levodopa.1–6 However, in recent years it has been increasingly recognized that DAAs can produce serious nonmotor complications, including impulse control disorders (ICDs) such as compulsive eating, pathological gambling, hypersexuality, and compulsive buying.7 Patients treated with dopaminergic medications may also develop repetitive, purposeless behaviors referred to as punding. In previous studies, punding behaviors have been more closely linked to use of L-dopa rather than DAAs,8–10 but have also been observed in combination with ICDs.11
ICDs have the potential to produce serious financial, medical, legal, or psychosocial consequences, but are not always recognized or acknowledged by affected patients. Moreover, although the primary treatment for ICDs is to taper or discontinue DAA therapy, not all patients are able to tolerate this because of motor worsening or because of dopamine agonist withdrawal syndrome (DAWS), a severe nonmotor drug withdrawal syndrome that mimics that of cocaine and other psychostimulants.12,13 Accordingly, there has been considerable interest in identifying risk factors for ICDs, to facilitate the prevention of ICDs and secondary consequences thereof.
Previous studies have shown that current DAA usage is the strongest, most consistent predictor of ICDs.12,14–16 In the largest cross-sectional study to date, ICDs were also independently associated with L-dopa use, younger age, family history of gambling disorders, concurrent cigarette smoking, unmarried status, combination DAA/L-dopa therapy, and higher L-dopa doses.14 Other studies have also shown an independent association between ICDs and factors such as higher DAA dosage,12,15 younger age of PD onset,17 previous ICD history,12 personal or family history of alcohol use disorders,17 and novelty-seeking behaviors.17 Psychiatric symptoms that have been associated with ICDs include depression, anxiety, obsessive-compulsive symptoms, disinhibition, irritability, and appetite disturbance.18,19 There are conflicting data about whether amantadine may be a treatment20 or a potential risk factor for ICDs.21,22
A major limitation of previous studies has been the use of a cross-sectional or retrospective study design. These methods are subject to treatment and recall bias, and can only identify the prevalence and clinical correlates of ICDs, but not their incidence or risk factors. The aim of the current study was to use a prospective cohort design to determine the frequency, time course, and risk factors for new-onset ICDs in PD.
Patients and Methods
Subjects
A cohort of 164 nondemented outpatients with PD was recruited from a tertiary movement disorders clinic between June 28, 2006 and November 1, 2010, as previously described.13,23,24 Inclusion criteria were idiopathic PD by United Kingdom Brain Bank criteria,25 capacity to provide written informed consent, and ability to complete a series of research questionnaires. Exclusion criteria included a previous history of ICDs, atypical clinical features, Mini–Mental State Examination (MMSE) score less than 25, clinical diagnosis of dementia, life expectancy <12 months, use of a dopamine receptor blocking agent, or previous PD neurosurgery.
Subjects were treated under routine clinical care and followed prospectively until they reached the first of the following predetermined endpoints: new-onset ICDs; discontinuation of DAA therapy; death or loss to follow-up; or June 30, 2011. Only those subjects who received a predefined minimum exposure to DAA after study enrollment (at least 50 L-dopa equivalent daily doses [LEDDs] of DAA for 3 or more consecutive months) were included in the analysis. The study was performed in accord with the Weill Cornell Institutional Review Board. Written informed consent was obtained from all subjects.
Assessments
At baseline, all subjects were evaluated by a movement disorders neurologist, who completed the Unified Parkinson’s Disease Rating Scale (UPDRS), Schwab and England Activities of Daily Living (ADL) Scale (S&E), and modified Hoehn and Yahr (H&Y) staging. Demographic and clinical features, medication usage, past medical history (PMH), and family history (FH) were recorded. A research assistant subsequently administered an MMSE, Beck Anxiety Inventory (BAI), and Beck Depression Inventory-II (BDI). Motor scores, clinical disease features, and medication usage were evaluated and prospectively recorded at all subsequent routine office visits.
Assessment for the presence of ICDs and punding behaviors occurred at the baseline visit and each subsequent office visit using a semistructured interview involving the subject and all available caregivers and outside observers. The interview included broad questions to identify symptoms suggestive of compulsive eating, pathological or problem gambling, compulsive shopping, hypersexuality, or other abnormal, repetitive behaviors. If a subject endorsed one or more repetitive behaviors, then follow-up questions were asked to determine the scope and consequences of these behaviors. Behaviors were classified as ICDs if they disrupted normal work, family, or social interactions or caused negative medical or psychological consequences. Subsyndromal ICDs were defined as repetitive behaviors that were similar to ICDs, but did not cause adverse consequences.
ICD status (presence or absence; ICD+ or ICD− groups, respectively) was determined at the time of each visit through clinical consensus between the subject, treating neurologist, and caregiver(s). Subjects were also queried about the presence of ICDs or punding at all telephone contacts with the treating physician. Data, including ICD and punding status, were entered prospectively into an electronic database (Microsoft Access 2010; Microsoft Corp., Redmond, Washington) as previously described.13,23,24 Database entries were verified by at least two research assistants.
L-dopa-LEDD and DAA-LEDD were calculated as previously described.13,23 Monoamine oxidase inhibitor LEDD (MAOI-LEDD) was calculated as follows: (100 times rasagiline dose) + (10 times oral selegiline dose) + (80 times sublingual selegiline dose).26 Total LEDD was calculated as the sum of L-dopa-LEDD + DAA-LEDD + MAOI-LEDD. Cumulative DAA-LEDD was estimated by multiplying the maintenance dosage of DAA by the duration of DAA exposure. Peak DAA dosage was defined as the highest dosage of DAA with which the subject was treated during the study period. Motor complications were defined as the presence of dyskinesias, end-of-dose wearing off, and/or “off” dystonia. A history of smoking was defined as lifetime usage of at least 5 pack-years of cigarettes.12 Caffeine usage was converted to coffee-cup equivalents using the following formula: (1 times the number of 8-ounce cups of caffeinated coffee) + (0.5 times the number of 8-ounce cups of caffeinated tea) + (0.25 times the number of 12-ounce cans of caffeinated soda).
Statistical Analysis
Cumulative incidence and incidence density of ICDs were calculated for the DAA-exposure cohort. The probability of ICD-free survival was estimated by Kaplan-Meier survival analysis, and median ICD-free survival time and the associated 95% confidence interval (95% CI) were estimated for the cohort. Descriptive statistics (including mean, standard deviation [SD], median, range, frequency, and percent), stratified by ICD development status (i.e., ICD+ versus ICD− group), were calculated for demographic, environmental, and clinical variables. Univariate relationships between these variables and ICD status were assessed as follows. For continuous variables, the two-sample t test (or nonparametric Wilcoxon rank-sum test) was used. For categorical variables, the chi-square test (or Fisher’s exact test) was used. Given the small sample size, no correction was made for multiplicity of risk factors. Multivariate regression analysis was not performed because of the small number of subjects with ICDs in the DAA-exposure cohort. All P values are two-sided, with statistical significance evaluated at the 0.05 alpha level. Analyses were performed in SPSS Version 19 (SPSS Inc., Chicago IL) and SAS Version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Frequency and Characteristics of New-Onset ICDs
One-hundred and sixty-four subjects enrolled in the study, of whom 46 were subsequently treated with the predetermined minimum dosage and duration of DAA therapy for inclusion in the analysis. This included 25 subjects who initiated DAA treatment after enrolling in the study and 21 subjects who continued DAA therapy (but had no previous ICDs). Of these 46 subjects, 18 (50% female) developed new-onset ICDs, establishing a cumulative frequency of 39.1% after a median DAA treatment duration of 21.0 months (range, 3.0–150.0 months). The incidence density of new-onset ICDs was 1 subject per 100 person-months of DAA exposure ([18 ICD cases per 1,654 total person-months exposure] × 100). Six subjects without ICDs were lost to follow-up before reaching one of the other study endpoints, including 1 who died. The mean interval between office visits (and thus formal evaluation of ICD status) was similar in ICD+ and ICD− subjects (mean, 142.1 ± 87.0 versus 124.1 ± 57.1 days; P = 0.44).
The most common ICD was compulsive eating, which affected 16 of 18 ICD subjects (7 female). Six subjects (1 female) experienced hypersexuality, 5 subjects (3 female) developed compulsive shopping or buying, and 1 female subject reported compulsive gambling. Eight of the sixteen ICD subjects (50%) reported the presence of two ICDs, and 1 subject had three ICDs. Concomitant punding was present in 12 subjects with ICDs (66.7%); these behaviors included excessive internet use (5 subjects), compulsive sorting or organizing (4 subjects), and 1 subject with each of the following: adding numbers or completing spreadsheets; cutting coupons; playing Sudoku; or crafting ceramics. None of the ICD− subjects reported punding behaviors.
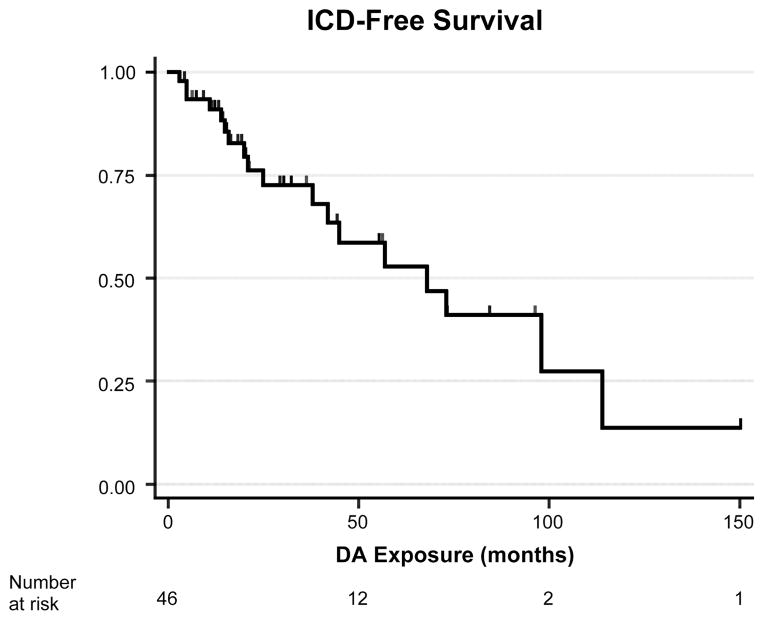
Time of onset of ICDs was highly variable, ranging from 3 months to almost 10 years (median, 23.0 months) after initiation of DAA therapy and 1 to 19 years (median, 5.5 years) after PD onset, with a median ICD-free survival time of 68 months after initiation of DAA treatment (95% CI = 34.8–101.2 months) (Fig. 1). Diagnosis of ICDs was often delayed, ranging from 0 to 15 months (median, 4 months) after ICD onset. In 4 subjects (22.2%), the presence of ICDs was elucidated only through interview of a caregiver or other outside observer.
FIG. 1.
ICD-free survival time relative to DAA treatment duration. Kaplan-Meier survival curve showing time to occurrence of ICDs in subjects treated with a DAA. ICD− subjects are included as censored observations based on time of last observation. Median ICD-free survival time was 68 months (95% CI = 34.8–101.2).
Baseline Characteristics of ICD+ Versus ICD− Groups
Baseline demographic characteristics, including sex, age, age of PD onset, disease duration, and marital status, were similar in ICD+ and ICD− groups (Table 1). However, the ICD+ group had a significantly higher lifetime prevalence of cigarette smoking, compared with the ICD− group (44.4% versus 14.3%; P = 0.04), and a trend toward greater median cumulative cigarette exposure (2.0 versus 0.0 pack-years; P = 0.07). Compared with the ICD− group, the ICD+ group also had a higher baseline prevalence of caffeine use (100% versus 66.7%; P = 0.07) and greater mean lifetime caffeine exposure (72.4 versus 37.5 cup-years; P = 0.04). Although overall alcohol usage in the cohort was low, there was a trend toward greater baseline daily use of alcohol in ICD+ subjects than ICD− subjects (median, 0.6 versus 0.3 drinks/day; P = 0.09). Previous alcoholism/problem drinking and illicit drug use were rare and of similar prevalence in both groups.
TABLE 1.
Baseline demographic and clinical characteristics of subjects by development of ICDs
| Subject Characteristics | ICD+ (N = 18) | ICD− (N = 28) | P Value |
|---|---|---|---|
| Demographics | |||
| Age (mean ± SD) | 62.4 (±10.2) | 61.9 (±10.5) | 0.86 |
| Age of PD onset (mean ± SD) | 56.7 (±10.4) | 57.0 (±9.0) | 0.92 |
| Disease duration, years (median, range) | 4.0 (1.0–19.0) | 4.5 (0.0–14.0) | 0.69 |
| Female sex, N (%) | 9 (50) | 9 (32.1) | 0.23 |
| Right-handedness, N (%) | 16 (88.9) | 26 (92.9) | 0.64 |
| Right-side onset, N (%) | 12 (66.7) | 17 (68.0) | 0.93 |
| Married, N (%) | 14 (77.8) | 22 (78.6) | 0.99 |
| Ethnicity (% white), N (%) | 16 (88.9) | 24 (85.7) | 0.99 |
| College (or higher) education, N (%) | 17 (94.4) | 25 (89.3) | 0.99 |
| Social history | |||
| Smoking historya, N (%) | 8 (44.4) | 4 (14.3) | 0.040 |
| Lifetime cigarette smoking, pack-years (median, range) | 2.0 (0.0–90.0) | 0.0 (0.0–32.4) | 0.070 |
| Current alcohol use, N (%) | 16 (88.9) | 18 (64.3) | 0.090 |
| Current alcohol use, drinks/day (median, range) | 0.6 (0.0–5.0) | 0.3 (0.0–3.5) | 0.090 |
| Current caffeine useb, N (%) | 18 (100) | 18 (66.7) | 0.007 |
| Current caffeine use, cups/day (median, range)b | 1.0 (0.3–4.0) | 0.8 (0.0–3.5) | 0.060 |
| Lifetime caffeine cup-years (median, range)b | 72.0 (19.8–124.3) | 37.5 (0.0–132.0) | 0.040 |
| Motor, disability, and ADL scores | |||
| UPDRS motor score (mean ± SD)c | 33.9 (±12.6) | 33.7 (±13.9) | 0.97 |
| Motor complications, N (%) | 11 (61.1) | 7 (25.0) | 0.01 |
| Dyskinesias, N (%) | 3 (16.7) | 3 (10.7) | 0.67 |
| Wearing off, N (%) | 4 (22.2) | 6 (21.4) | 0.95 |
| Off dystonia, N (%) | 8 (44.4) | 5 (17.9) | 0.05 |
| Modified H & Y stage (median, range) | 2.0 (1.0–2.0) | 2.0 (1.0–3.0) | 0.05 |
| UPDRS ADL score (median, range) | 9.0 (2.0–14.0) | 7.0 (1.0–24.0) | 0.56 |
| S&E ADL Scale (mean ± SD) | 88.6 (±14.3) | 91.3 (±14.2) | 0.54 |
| Nonmotor assessments | |||
| MMSE (mean ± SD) | 29.6 (±0.6) | 28.9 (±1.5) | 0.04 |
| BAI (mean ± SD) | 10.4 (±6.0) | 8.6 (±7.4) | 0.39 |
| BDI (mean ± SD) | 39.9 (±6.1) | 37.4 (±7.8) | 0.26 |
| Current sleep disorder, N (%) | 12 (66.7) | 13 (46.4) | 0.18 |
| Current visual hallucinations, N (%) | 3 (16.7) | 1 (3.6) | 0.28 |
| Medication usage | |||
| On any dopaminergic medication, N (%) | 12 (66.7) | 16 (57.1) | 0.52 |
| Total LEDD (median, range) | 150.0 (0.0–2,320.0) | 150.0 (0.0–1,510.0) | 0.61 |
| On DAA, N (%) | 10 (55.6) | 11 (39.3) | 0.28 |
| DAA-LEDD (median, range) | 106.3 (0.0–450.0) | 0.0 (0.0–450.0) | 0.15 |
| On L-dopa, N (%) | 7 (38.9) | 13 (46.4) | 0.62 |
| L-dopa-LEDD (median, range) | 0.0 (0.0–2,000.0) | 0.0 (0.0–1,450.0) | 0.64 |
| On amantadine, N (%) | 3 (16.7) | 0 (0.0) | 0.05 |
| On MAOI, N (%) | 5 (27.8) | 3 (10.7) | 0.23 |
| On antidepressants, N (%) | 5 (27.8) | 4 (14.3) | 0.28 |
| On benzodiazepines, N (%) | 5 (27.8) | 6 (21.4) | 0.73 |
| PMH | |||
| PMH anxiety, N (%) | 7 (38.9) | 13 (46.4) | 0.62 |
| PMH depression, N (%) | 7 (38.9) | 8 (28.6) | 0.47 |
| PMH alcoholism/problem drinking, N (%) | 3 (16.7) | 3 (10.7) | 0.67 |
| FH | |||
| PD, N (%) | 6 (33.3) | 8 (28.6) | 0.75 |
| Anxiety, N (%) | 6 (33.3) | 10 (35.7) | 0.99 |
| Depression, N (%) | 11 (61.1) | 9 (32.1) | 0.05 |
| Alcoholism/problem drinking, N (%) | 5 (27.8) | 8 (29.6) | 0.89 |
Excludes subjects with a trivial smoking history (<5 pack-years).
Data missing for one ICD− subject.
All subjects with motor fluctuations were evaluated in the “on” state.
At baseline, subjects in the ICD+ group had a greater prevalence of motor complications than the ICD− group (61.1% versus 25.0%; P = 0.01) and a trend toward lower H & Y scores (median, 2.0; range, 1.0–2.0 versus 2.0; range, 1.0–3.0; P = 0.05). In contrast, there were no significant differences in UPDRS motor scores in the two groups (P = 0.97) (Table 1). Quantitative and qualitative use of dopaminergic medications was similar in both groups, as was the prevalence of antidepressant and benzodiazepine usage.
MMSE scores were significantly higher in ICD+ than ICD− subjects (29.6 ± 0.6 versus 28.8 ± 1.5; P = 0.04), but the absolute difference was small. Other baseline nonmotor clinical features, as well as anxiety and depression scores, were similar in both groups (Table 1). FH of PD, anxiety, and alcoholism/problem drinking were also comparable in both groups, but there was a trend toward a greater prevalence of a FH of depression in the ICD+ versus the ICD− group (61.1% versus 32.1%; P = 0.05).
Endpoint Characteristics of ICD+ Versus ICD− Groups
At study endpoint, the major difference between the ICD+ and ICD− groups was a higher peak DAA dosage in the ICD+ versus ICD− group (median, 300.0 versus 165.0 LEDD; P = 0.03). In contrast, disease duration, DAA treatment duration, cumulative DAA exposure, the specific DAA that was used, concomitant L-dopa usage, L-dopa dosage, total LEDD, and duration of dopaminergic therapy did not significantly differ between the two groups (Table 2).
TABLE 2.
Endpoint demographic and clinical characteristics of PD subjects by ICD status
| Subject Characteristics | ICD+ (N = 18) | ICD− (N = 28) | P Value |
|---|---|---|---|
| Demographics | |||
| Disease duration at endpoint, years (median, range) | 5.5 (1.0–19.0) | 7.0 (2.0–18.0) | 0.84 |
| Motor assessments | |||
| Motor complications, N (%) | 11 (61.1) | 13 (46.4) | 0.33 |
| Dyskinesias, N (%) | 5 (27.8) | 4 (14.3) | 0.28 |
| Wearing off, N (%) | 5 (27.8) | 9 (32.1) | 0.75 |
| Off dystonia, N (%) | 9 (50.0) | 6 (21.4) | 0.04 |
| UPDRS ADL score (median, range) | 7.5 (2.0–25.5) | 9.5 (1.0–30.0) | 0.52 |
| Medications | |||
| Total dopaminergic medication usage | |||
| Total LEDD (median, range) | 422.5 (150.0–2,730.0) | 538.8 (40.0–1,380.0) | 0.91 |
| Months on dopaminergic therapy (median, range) | 28.5 (5.0–114.0) | 29.5 (4.0–150.0) | 0.95 |
| DAA usage | |||
| Peak DAA-LEDD (median, range) | 300.0 (75.0–450.0) | 165.0 (50.0–400.0) | 0.03 |
| Duration of DAA treatment, months (median, range) | 23.0 (3.0–114.0) | 20.5 (4.0–150.0) | 0.77 |
| Cumulative DAA-LEDD (median, range) | 4,706.3 (825.0–36,800.0) | 3,290.0 (337.5–24,300.0) | 0.39 |
| Predominant DAA used (pramipexole), N (%)a | 12 (66.7) | 19 (67.9) | 0.93 |
| On L-dopa, N (%) | 10 (55.6) | 17 (60.7) | 0.73 |
| L-dopa-LEDD (median, range) | 200.0 (0.0–2,520.0) | 300.0 (0.0–1,320.0) | 0.92 |
| Months on L-dopa (median, range) | 4.0 (0.0–82.0) | 9 (0.0–43.0) | 0.93 |
| On amantadine, N (%) | 1 (5.6) | 0 (0) | 0.39 |
| On MAOI, N (%) | 7 (38.9) | 11 (39.3) | 0.98 |
All other subjects were treated with ropinirole, except for one ICD− subject, who was treated with rotigotine.
Outcomes of ICD+ Subjects
Of the 18 subjects in the ICD+ group, outcomes were as follows: ICDs resolved in all 10 subjects who discontinued DAA therapy; in 3 of 5 subjects who reduced their DAA dose; and in 0 of 3 subjects who continued the same dosage of DAA. Concomitant punding occurred in 12 of the 18 subjects with ICDs and subsequently resolved in all 5 of those subjects who discontinued DAA therapy; 2 of 4 subjects who reduced the DAA dose; and 0 of 3 subjects who continued the same dosage of DAA. DAWS occurred in 6 (33.3%) of the ICD subjects: 4 who discontinued DAA usage; 1 who reduced the DAA dosage; and 1 who was unable to decrease the DAA dosage (because of severe DAWS symptoms). Four of the five subjects with DAWS developed secondary dopamine dysregulation syndrome (DDS) as they self-adjusted their L-dopa dosage in unsuccessful attempts to alleviate DAWS symptoms. None of the 6 ICD− subjects who tapered a DAA developed DAWS.
Discussion
ICDs have increasingly been recognized as a serious and relatively common side effect of DAAs, and one that may be irreversible because patients cannot always tolerate discontinuation of DAA therapy. For this reason, it is critical to identify risk factors for ICDs to facilitate the prevention of these behaviors in susceptible patients.
In this study, we used a prospective cohort study design to determine the frequency, time course, and risk factors for ICD development in DAA-treated subjects. We observed 1 new ICD case per 100 person-months of DAA exposure, with new-onset ICDs occurring in 39.1% of subjects after a median duration of 21 months of DAA therapy, and a 50% ICD-free survival time of 68 months. Time course of ICD onset was highly variable, ranging from just 3 months after initiation of DAA therapy to over 10 years, indicating that vigilant monitoring for ICDs is warranted throughout DAA therapy. As in the general population, patients were sometimes unaware of the pathological nature of their behaviors. This underscores the importance of including outside observers in assessment of ICD status.27
The major patient-specific risk factors for ICDs were caffeine and cigarette usage, with a similar trend for alcohol use. These findings suggest that (1) ICD patients have an underlying susceptibility to addiction disorders and/or (2) chronic exposure to addictive substances may sensitize subjects to subsequent development of ICDs. Based on the drug and behavioral addiction literature in PD and non-PD populations, a combination of both factors is likely.28,29 Accordingly, PD patients in this and previous studies frequently exhibit multiple concomitant ICDs.13,14,16,18,30
Interestingly, many of the factors that have been associated with the development of ICDs in this and previous studies are inverse risk factors for PD, including caffeine use, cigarette exposure,31 and novelty-seeking personality traits22,23 (in contrast with the harm-avoidance characteristics typically associated with PD).17,18,32 These findings suggest that PD patients susceptible to ICDs may have a distinct phenotype from other PD patients, perhaps the result of baseline abnormalities in mesocorticolimbic function. 13,33,34 Some subjects in the cohort did not develop ICDs despite long-term, high-dose DAA exposure, suggesting that only a subset of patients may be vulnerable to ICDs.
The most important modifiable risk factor for ICDs in the study was the peak dosage of DAA, which was twice as high in ICD+ than ICD− subjects. This is consistent with the dopamine overdose hypothesis of ICDs in PD, whereby adjustment of dopaminergic medications to optimize nigrostriatal dopaminergic function leads to an “overdose” of the relatively spared mesocortiocolimbic dopamine system.35 Given that the motor benefits of DAAs may saturate at lower than the maximum U.S. Food and Drug Administration–approved doses36 (e.g., ≤1.5 mg/day of pramipexole), and that lower DAA doses are efficacious in treating PD motor symptoms,36,37 the avoidance of high-dose DAAs might potentially reduce the risk of ICDs without compromising motor benefit. Although some previous studies have shown an association between ICDs and L-dopa dosage,14 we found no group differences in baseline or peak L-dopa dosage. However, we did find that DDS (and associated excess L-dopa usage) was a common consequence of attempted DAA taper. Thus, the previously reported correlation between L-dopa usage and ICDs may result, in part, from reverse causation, whereby attempted DAA taper leads to a secondary increase in L-dopa dosage.
Although previous literature has suggested that punding behaviors are more closely associated with L-dopa than with DAAs,8–10 we observed punding behaviors in the preponderance of subjects with ICDs versus none of the ICD− subjects. Moreover, punding behaviors resolved in all subjects who discontinued DAA therapy, despite concomitant increases in L-dopa dosage. These findings suggest that punding may be more closely related to DAA versus L-dopa therapy, and thus that ICDs and punding may share similar biological substrates.
Study strengths include the prospective cohort design, which enabled us to determine the frequency of new-onset ICDs and identify risk factors thereof with a reduced risk of recall bias. In addition, all subjects were treated with DAA therapy, eliminating concerns about treatment bias resulting from differential prescribing patterns for DAAs versus L-dopa. The frequency of new-onset ICDs—and, specifically, compulsive eating—was higher than might have been predicted from previous studies, presumably reflecting our inclusion of all compulsive eating behaviors16 and not just binge-eating disorder,14 as well as our repeated assessment of subjects and caregivers over time. Limitations include the observational nature of the study; relatively small sample size; use of clinical diagnosis rather than formal diagnostic criteria for the diagnosis of ICDs; and recruitment of subjects from a single tertiary academic medical center. Because we included subjects with current DAA use and no previous ICDs, but excluded those with past or current ICDs, we may also have overestimated the median latency between initiation of DAA therapy and ICD onset.
Conclusion
In summary, we have used a prospective cohort design to identify risk factors for ICDs in DAA-treated subjects with PD. Patient-specific risk factors for ICDs include greater usage of caffeine and cigarettes—substances with high potential for physiological and psychological dependence—consistent with a generalized susceptibility to addiction disorders in subjects with ICDs. Disease-related factors included a higher peak DAA dosage and the presence of baseline motor complications. DAWS was common in subjects with ICDs, often preventing discontinuation of DAA therapy and resulting in chronic ICDs. Together, these findings suggest that careful patient selection and avoidance of high peak DAA dosages may reduce the incidence and secondary consequences of ICDs in PD.
Acknowledgments
Funding agencies: This study was supported by a center grant from the Parkinson’s Disease Foundation. J.B. was supported by a 2011 American Academy of Neurology Medical Student Summer Research Scholarship. P.C. was partially supported by a grant from the Clinical Translational Science Center (2UL1 TR000457-06).
Footnotes
Relevant conflicts of interest/financial disclosures: Nothing to report. Full financial disclosures and author roles may be found in the online version of this article.
This article first published online ahead of print on January 2, 2013. It has since been updated. “Dopamine agonists (DA)” has been changed to “Dopamine agonists (DAAs)”. Also, the data in Figure 1 has been updated.
References
- 1.Lewitt PA. Levodopa for the treatment of Parkinson’s disease. N Engl J Med. 2008;359:2468–2476. doi: 10.1056/NEJMct0800326. [DOI] [PubMed] [Google Scholar]
- 2.Obeso JA, Grandas F, Vaamonde J, et al. Motor complications associated with chronic levodopa therapy in Parkinson’s disease. Neurology. 1989;39(11 Suppl 2):11–19. [PubMed] [Google Scholar]
- 3.Perez-Lloret S, Rey MV, Ratti L, Rascol O. Pramipexole for the treatment of early Parkinson’s disease. Expert Rev Neurother. 2011;11:925–935. doi: 10.1586/ern.11.75. [DOI] [PubMed] [Google Scholar]
- 4.Tsouli S, Konitsiotis S. How should we treat a patient with early Parkinson’s disease? Int J Clin Pract. 2010;64:1210–1219. doi: 10.1111/j.1742-1241.2010.02371.x. [DOI] [PubMed] [Google Scholar]
- 5.Miyasaki JM, Martin W, Suchowersky O, Weiner WJ, Lang AE. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11–17. doi: 10.1212/wnl.58.1.11. [DOI] [PubMed] [Google Scholar]
- 6.Nirenberg MJ, Fahn S. The role of levodopa and catechol-O-methyltransferase inhibitors. In: Schapira AHV, Olanow CW, editors. Principles of Treatment in Parkinson’s Disease. Philadelphia, PA: Butterworth Heinemann Elsevier; 2005. pp. 3–24. [Google Scholar]
- 7.Weintraub D, Nirenberg MJ. Impulse control and related disorders in Parkinson’s disease. Neurodegener Dis. 2013;11:63–71. doi: 10.1159/000341996. [DOI] [PubMed] [Google Scholar]
- 8.Evans AH, Katzenschlager R, Paviour D, et al. Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19:397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- 9.Friedman JH. Punding on levodopa. Biol Psychiatry. 1994;36:350–351. doi: 10.1016/0006-3223(94)90636-x. [DOI] [PubMed] [Google Scholar]
- 10.Fasano A, Ricciardi L, Pettorruso M, Bentivoglio AR. Management of punding in Parkinson’s disease: an open-label prospective study. J Neurol. 2011;258:656–660. doi: 10.1007/s00415-010-5817-8. [DOI] [PubMed] [Google Scholar]
- 11.Spencer AH, Rickards H, Fasano A, Cavanna AE. The prevalence and clinical characteristics of punding in Parkinson’s disease. Mov Disord. 2011;26:578–586. doi: 10.1002/mds.23508. [DOI] [PubMed] [Google Scholar]
- 12.Weintraub D, Siderowf AD, Potenza MN, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63:969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabinak CA, Nirenberg MJ. Dopamine agonist withdrawal syndrome in Parkinson disease. Arch Neurol. 2010;67:58–63. doi: 10.1001/archneurol.2009.294. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 15.Ondo WG, Lai D. Predictors of impulsivity and reward seeking behavior with dopamine agonists. Parkinsonism Relat Disord. 2008;14:28–32. doi: 10.1016/j.parkreldis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Nirenberg MJ, Waters C. Compulsive eating and weight gain related to dopamine agonist use. Mov Disord. 2006;21:524–529. doi: 10.1002/mds.20757. [DOI] [PubMed] [Google Scholar]
- 17.Voon V, Thomsen T, Miyasaki JM, et al. Factors associated with dopaminergic drug-related pathological gambling in Parkinson disease. Arch Neurol. 2007;64:212–216. doi: 10.1001/archneur.64.2.212. [DOI] [PubMed] [Google Scholar]
- 18.Voon V, Sohr M, Lang AE, et al. Impulse control disorders in Parkinson disease: a multicenter case—control study. Ann Neurol. 2011;69:986–996. doi: 10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- 19.Pontone G, Williams JR, Bassett SS, Marsh L. Clinical features associated with impulse control disorders in Parkinson disease. Neurology. 2006;67:1258–1261. doi: 10.1212/01.wnl.0000238401.76928.45. [DOI] [PubMed] [Google Scholar]
- 20.Thomas A, Bonanni L, Gambi F, Di Iorio A, Onofrj M. Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol. 2010;68:400–404. doi: 10.1002/ana.22029. [DOI] [PubMed] [Google Scholar]
- 21.Walsh RA, Lang AE. Multiple impulse control disorders developing in Parkinson’s disease after initiation of amantadine. Mov Disord. 2012;27:326–327. doi: 10.1002/mds.23964. [DOI] [PubMed] [Google Scholar]
- 22.Weintraub D, Sohr M, Potenza MN, et al. Amantadine use associated with impulse control disorders in Parkinson disease in cross-sectional study. Ann Neurol. 2010;68:963–968. doi: 10.1002/ana.22164. [DOI] [PubMed] [Google Scholar]
- 23.Khadem NR, Nirenberg MJ. Carbidopa/levodopa pharmacy errors in Parkinson’s disease. Mov Disord. 2010;25:2867–2871. doi: 10.1002/mds.23311. [DOI] [PubMed] [Google Scholar]
- 24.Liu AA, Boxhorn CE, Klufas MA, et al. Clinical predictors of frequent patient telephone calls in Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:95–99. doi: 10.1016/j.parkreldis.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein RZ, Craig AD, Bechara A, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence AD, Evans AH, Lees AJ. Compulsive use of dopamine replacement therapy in Parkinson’s disease: reward systems gone awry? Lancet Neurol. 2003;2:595–604. doi: 10.1016/s1474-4422(03)00529-5. [DOI] [PubMed] [Google Scholar]
- 29.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 30.Limotai N, Oyama G, Go C, et al. Addiction-like manifestations and Parkinson’s disease: a large single center 9-year experience. Int J Neurosci. 2012;122:145–153. doi: 10.3109/00207454.2011.633722. [DOI] [PubMed] [Google Scholar]
- 31.Tanner CM. Advances in environmental epidemiology. Mov Disord. 2010;25(Suppl 1):S58–S62. doi: 10.1002/mds.22721. [DOI] [PubMed] [Google Scholar]
- 32.Evans AH, Lawrence AD, Potts J, et al. Relationship between impulsive sensation seeking traits, smoking, alcohol and caffeine intake, and Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:317–321. doi: 10.1136/jnnp.2005.065417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- 34.Thobois S, Ardouin C, Lhommee E, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain. 2010;133:1111–1127. doi: 10.1093/brain/awq032. [DOI] [PubMed] [Google Scholar]
- 35.Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- 36.Safety and efficacy of pramipexole in early Parkinson disease. A randomized dose-ranging study. Parkinson Study Group. JAMA. 1997;278:125–130. doi: 10.1001/jama.1997.03550020057038. [DOI] [PubMed] [Google Scholar]
- 37.Kieburtz K. Twice-daily, low-dose pramipexole in early Parkinson’s disease: a randomized, placebo-controlled trial. Mov Disord. 2011;26:37–44. doi: 10.1002/mds.23396. [DOI] [PubMed] [Google Scholar]