Abstract
The optimal dose and schedule of thymoglobulin (ATG) for graft-versus-host disease prevention (GVHD) is unknown. We compared two doses of ATG (4.5 mg/kg and 7.5 mg/kg) in a Bayesian adaptively randomized fashion, and assessed whether ATG levels measured on days 0, 7, 14, and 28 were associated with clinical outcomes. Treatment success was defined as the patient being alive, engrafted, in remission, and without acute GVHD at day 100. Twenty patients received ATG 4.5 mg/kg (n=15) or 7.5 mg/kg (n=5) with reduced-intensity conditioning (RIC) followed by unrelated donor hematopoietic cell transplantation (HCT). The first 10 patients were randomized fairly. Then, based on data from the first 10 patients, the posterior probability that the 4.5 mg/kg dose is superior to the 7.5 mg/kg dose was found to be 0.94, and the next 10 patients were each assigned to the ATG 4.5 mg/kg arm. The posterior mean treatment success rates for the ATG 4.5 mg/kg and ATG 7.5 mg/kg arms were 0.73 and 0.45, respectively. The posterior probability that the success rate was greater in the 4.5 mg/kg arm than in the 7.5 mg/kg arm was 0.93. There was no difference in the overall survival (p=0.607), relapse-free survival (p=0.607), treatment-related mortality (p=0.131), or incidence of acute (p=0.303) or chronic GVHD (p=0.999) between the two doses. ATG levels were not associated with clinical outcomes. Thus, our results favor the use of ATG 4.5 mg/kg over ATG 7.5 mg/kg in patients undergoing unrelated donor HCT with RIC regimens.
Keywords: ATG, Graft-Versus-Host Disease, hematopoietic cell transplantation
Introduction
Rabbit antithymocyte globulin (ATG) (Genzyme Co., Cambridge, MA) has been increasingly used for the prevention of graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation (HCT).[1] The mechanism of action is multifaceted and involves T-cell depletion in the blood and lymphoid tissues;[2] apoptosis of naïve B cells, activated B cells, and plasma cells;[3,4] and enrichment of regulatory T cells and natural killer cells.[5] Randomized trials have shown that ATG use can decrease the incidence of acute (a) and chronic (c) GVHD after allogeneic HCT, however a survival benefit is generally not seen.[6,7] In the study by Finke et al, who compared rabbit anti-Jurkat ATG-Fresenius (ATG-F; Fresenius Biotech, Graefelfing, Germany) versus no ATG in patients undergoing unrelated donor allogeneic HCT, the cumulative incidence of grade II-IV aGVHD in the ATG-F group was 33% (95% CI 25.1–43.5) versus 51% (95% CI 42.0–61.9) in the control group (p=0.011). The 2-year cumulative incidence of extensive cGVHD in the ATG-F group was 12.2% (95% CI 7.0–21.3) versus 42.6% (95% CI 33.0–55.0) in the control group (p=<0.0001). Despite the decrease in the incidence of GVHD, the 2-year cumulative incidence of non-relapse mortality (NRM) was not statistically different between the two groups [(ATG-F group 19.6% (95% CI 13.2–29.0) versus 28.9% (95% CI 20.8–40.1) in the control group (p=0.20)]. The 2-year disease-free survival (DFS) rate [(ATG-F group 51.6% (95% CI 41.8–61.4) versus control group 47.5% (95% CI 37.1–57.9) p=0.65] and the overall survival (OS) rate [ATG-F group 59.2% (95% CI 49.5–68.9), versus control group 51.9% (41.3–62.5) p=0.47] were also not significantly different between the two arms.[6] In another study, Bacigalupo et al. reported the results of 2 consecutive randomized trials that compared rabbit ATG (Sangstat, Lyon, France) to no ATG in patients undergoing unrelated donor allogeneic HCT.[7] Grade II-IV aGVHD was diagnosed in 72% of patients in the ATG arm versus 69% (P=0.6) in the first and in 37% of patients in the ATG-arm versus 79% (P=0.001) in the second trial. Overall, extensive cGVHD developed more frequently in patients given no ATG (62% vs. 39%; p=.04). The actuarial event-free survival at 2-years in patients who did and did not receive ATG was 34% and 20%, respectively in the first trial (p=0.6) and 17% versus 15% (p=0.8), respectively in the second trial. The actuarial survival rates between the ATG and no ATG arms were 55% and 56% (p=0.8), respectively in the first trial and 43% in each arm in the second trial. On the other hand, in a prospective randomized study by Champlin et al., no difference in the probability of grade II-IV aGVHD at day 100 [18% (95% CI 10%–29%) versus 11% (95% CI 6%–22%)] and cGVHD at 5 years [21% (95% CI 12%–33%) versus 32% (95% CI 21%–44%)] were seen between the no ATG and the equine ATG arm (ATGAM; Upjohn, Kalamazoo, MI; or Lymphoglobulin Merieux, Laboratories Pasteur Merieux, Lyon, France), respectively.[8]
The optimal dose of ATG in the setting of reduced-intensity conditioning (RIC) allogeneic HCT is not clearly known. While effective in preventing GVHD, higher doses of ATG (>10 mg/kg) may be associated with higher rates of infection and in some cases higher treatment-related mortality (TRM) than lower doses of ATG or no ATG.[6,7,9,10] The risk of fatal infections was higher in the high-dose ATG arm (15 mg/kg) than in the no ATG arm in the study by Bacigalupo et al.[7] while the risks of infection with cytomegalovirus or herpes simplex virus and of Epstein-Barr virus–positive post-transplant lymphoproliferative disorder were higher in the ATG-F (20 mg/kg) arm than in the no ATG arm in the study by Finke et al.[6]
Here we sought to optimize the use of rabbit ATG in the setting of unrelated donor (UD) RIC allogeneic HCT. We proposed to compare the safety and efficacy of two low to intermediate dose levels of rabbit ATG (4.5 mg/kg and 7.5 mg/kg) in preventing aGVHD. We also proposed to assess whether ATG levels monitored at specific time points correlate with transplant outcome.
Materials and Methods
Patients
Patients with acute myelogenous leukemia or myelodysplastic syndrome were included if they met the following eligibility criteria: Zubrod performance status less than 3, adequate organ function (serum creatinine <2.0 mg/dL, left ventricular ejection fraction >40%, diffusing capacity of the lungs for carbon monoxide >40% of predicted, serum bilirubin <3 g/dL, and serum alanine amino transferase <3 times upper normal limit), and age less than 76 years. The protocol was approved by the institutional review board of The University of Texas MD Anderson Cancer Center, and all patients (or a legal guardian) provided written informed consent, in accordance with the Declaration of Helsinki.
Unrelated donor transplants and human leukocyte antigen typing
Peripheral blood or bone marrow cells were obtained through the National Marrow Donor Program. All donor-recipient pairs were matched using high-resolution allele level human leukocyte antigen (HLA) typing for HLA-A, HLA-B, HLA-DRB1, and HLA-DQB1 loci, as previously described.[11] High-resolution typing for HLA-C was available for 15 (75%) patients, while the other 5 (25%) patients had intermediate resolution HLA-C typing. As many as two mismatches were allowed.
Treatment plan
All patients received an RIC regimen of fludarabine 100–125 mg/m2 and melphalan 100–140 mg/m2 (n=20). Five patients also received bortezomib 0.7 mg/m2 on days 1, 4, 8, and 11 beginning on pre-transplant day -12 (n=2), post-transplant day 1 (n=2), or post-transplant day 30 (n=1), as part of a study protocol. Rabbit ATG was administered to all patients based on randomization to one of two arms: a lower dose (1.5 mg/kg/day on days -3, -2, and -1; total dose 4.5 mg/kg) or a higher dose (2.5 mg/kg/day on days -3, -2, and -1; total dose 7.5 mg/kg).
GVHD prophylaxis consisted of tacrolimus (Fujisawa Healthcare, Deerfield, IL) started on day -2 and administered for 6–9 months if no GVHD, and mini-methotrexate (5 mg/m2 on post-transplant days 1, 3, 6, and 11).[12] All supportive care measures were used according to extant institutional protocols. All patients received filgrastim (Amgen, Thousand Oaks, CA) 5 µg/kg subcutaneously daily from day 7 until absolute neutrophil count (ANC) exceeded 0.5 × 109/L for 3 consecutive days.
Engraftment
Neutrophil engraftment was defined as having occurred on the first of 3 consecutive days that the ANC exceeded 0.5 × 109/L. Platelet count recovery was defined as having occurred on the first of 7 consecutive days in which the platelet count exceeded 20 × 109/L without platelet transfusions.
Definition of infection
Infection was defined as an illness with signs or symptoms consistent with an infection along with microbiological detection of a pathogen. The pathogen could be isolated from a sterile site or a nonsterile site by culture or molecular/immunohistochemical testing. In case of isolation from a nonsterile site, the pathogen had to be considered clinically pathogenic. Culture-documented bacteremia, viremia, or fungemia in the absence of signs or symptoms was also counted.
Determination of ATG levels
Serum ATG levels were measured on day 0 (day of stem cell infusion) and on post-transplant days 7, 14, and 28. Total ATG was quantified by a sandwich ELISA method. Anti-rabbit IgG polyclonal antibodies were used to capture ATG in human serum (or plasma). Bound ATG was detected using HRP conjugated polyclonal anti-rabbit IgG. ATG levels (mg/mL) were interpolated from an ATG reference standard curve. This assay is specific for ATG and does not detect human IgG, IgM, or IgE when tested at levels expected to be present in serum. The limit of quantification (LOQ) was 3.9 mg/mL and accuracy (% recovery) was 80–120%. Overall precision was 5.7–17.4% throughout the range of the standard curve.
Active ATG (the component that binds to human peripheral blood mononuclear cells) was quantified using a flow cytometric method. Cell-bound ATG was detected using polyclonal anti-rabbit IgG FITC, and ATG levels in serum or plasma (mg/mL) were interpolated from an ATG reference standard curve. This assay is specific for active ATG and does not detect rabbit IgG against an irrelevant antigen in human serum. Throughout the quantification range, accuracy (% recovery) was 80–120% with LOQ at 0.20 mg/mL; overall assay precision was less than 20%.
Statistical methods
This study employed a Bayesian adaptive randomization design.[13] The first 10 patients were randomized so that 5 would be treated with 4.5 mg/kg ATG and 5 with 7.5 mg/kg ATG. Before enrolling the 11th and subsequent patients, the randomization probabilities were adapted to reflect the outcomes from the patients already on study and evaluated so that each patient would have a greater chance of being assigned to the treatment arm that appeared to be having better results. The randomization probabilities were equivalent to the probability that one treatment arm had a greater success rate than the other treatment arm. At no time, however, was the randomization probability to either treatment arm allowed to exceed 0.90 or be less than 0.10. Treatment success was defined as a patient being alive, engrafted, in remission, and without aGVHD at post-transplant day 100. We modeled the probability of treatment success, π, as logit(π) = β0Tk + β1(1-Tk) + β2Zk, where β0 is the treatment effect of the low dose, β1 is the treatment effect of the high dose, and β2 is the effect of the risk of failure (BMT/low risk, PBSC/high risk). We assumed that β0 and β1 each have a N(0, 1) prior distribution and that β2 has a N(0.40, 0.25) prior distribution, reflecting the expected 10% difference in success rates between the 2 risk groups. Prior distributions for the treatment success rates were updated with the outcomes from the patients enrolled on the trial to obtain posterior distributions of the treatment success rates. From these posterior distributions we calculated the mean posterior probability of treatment success for each treatment arm and its associated 90% credible interval. We also calculated the posterior probability that the ATG 4.5 mg/kg arm had a higher treatment success rate than the ATG 7.5 mg/kg arm.
The analysis of ATG serum levels was exploratory, with 24 tests for each assay, so we used a Bonferroni correction to the significance level and considered differences statistically significant if the p-value was less than 0.05/24 (0.002). The average ATG level for each patient was found as the area under the concentration time curve (AUC) using the trapezoidal rule and only those days on which an ATG level was measured. For example, if no ATG was measured at day 28, then the AUC was found through day 14. One patient treated with 4.5 mg/kg thymoglobulin had no ATG levels measured. Cox proportional hazards regression14 was then used to model the effect of the average ATG level on OS, relapse-free survival (RFS), time to neutrophil engraftment, and time to platelet engraftment.
We used descriptive statistics to summarize the demographic and clinical characteristics of the patients. We used the product limit estimator of Kaplan and Meier[14] to estimate median OS, RFS, and time to engraftment. All statistical analyses were performed using SAS 9.1 for Windows (Copyright © 2002–2003 by SAS Institute Inc., Cary, NC).
Results
A total of 20 patients were enrolled between January 2004 and January 2007. The study was stopped early by the institutional data safety monitoring board due to slow accrual. The median follow-up for 8 patients remaining alive at last contact was 49.5 months (range: 42.7–61.4). The demographic and clinical characteristics of patients are summarized in Table 1.
Table 1.
Patient demographic and clinical characteristics
| Dose of thymoglobulin | ||||
|---|---|---|---|---|
| All patients | 4.5 mg/kg | 7.5 mg/kg | p-value | |
| N (%) | N (%) | N (%) | ||
| Number of patients | 20 | 15 | 5 | |
| Sex | 0.6169 | |||
| Female | 9 (45.0) | 6 (40.0) | 3 (60.0) | |
| Male | 11 (55.0) | 9 (60.0) | 2 (40.0) | |
| Age at transplant (years) | 0.6674 | |||
| Median (range) | 60 (40–75) | 60 (40–75) | 62 (50–67) | |
| Diagnosis | 0.5395 | |||
| AML | 17 (85.0) | 12 (80.0) | 5 (100.0) | |
| MDS | 3 (15.0) | 3 (20.0) | 0 (0.0) | |
| CR at transplant | 0.3034 | |||
| No | 10 (50.0) | 9 (60.0) | 1 (20.0) | |
| Yes | 10 (50.0) | 6 (40.0) | 4 (80.0) | |
| Stem cell source | 0.1089 | |||
| Peripheral blood | 12 (60.0) | 11 (73.3) | 1 (20.0) | |
| Bone marrow | 8 (40.0) | 4 (26.7) | 4 (80.0) | |
| TNC (×108/kg) | 0.1227 | |||
| Median (range) | 9 (2.6–17.7) | 10.8 (3.1–17.7) | 4.4 (2.6–9.8) | |
| CD34+(×106/kg) | 0.467 | |||
| Median (range) | 5.6 (1.3–36.4) | 5.9 (1.3–36.4) | 5.3 (1.8–7.3) | |
| Acute GVHD | 0.3034 | |||
| No | 10 (50.0) | 6 (40.0) | 4 (80.0) | |
| Yes | 10 (50.0) | 9 (60.0) | 1 (20.0) | |
| Acute GVHD grade | 0.5240 | |||
| 0 | 10 (50.0) | 6 (40.0) | 4 (80.0) | |
| I | 6 (30.0) | 5 (33.3) | 1 (20.0) | |
| II | 2 (10.0) | 2 (13.3) | 0 (0.0) | |
| III | 2 (10.0) | 2 (13.3) | 0 (0.0) | |
| Neutrophilengraftment | 0.9999 | |||
| No | 1 (5.0) | 1 (6.7) | 0 (0.0) | |
| Yes | 19 (95.0) | 14 (93.3) | 5 (100.0) | |
| Platelet engraftment | 0.9999 | |||
| No | 4 (20.0) | 3 (20.0) | 1 (20.0) | |
| Yes | 16 (80.0) | 12 (80.0) | 4 (80.0) | |
| Limited chronic GVHD | 0.9999 | |||
| No | 8 (61.5) | 7 (63.6) | 1 (50.0) | |
| Yes | 5 (38.5) | 4 (36.4) | 1 (50.0) | |
| Extensive chronic GVHD | 0.9999 | |||
| No | 10 (76.9) | 8 (72.7) | 2 (100.0) | |
| Yes | 3 (23.1) | 3 (27.3) | 0 (0.0) | |
AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome; CR, complete remission; TNC, total nucleated cells; GVHD, graft-versus-host disease
Treatment success rate
The first 10 patients were randomized fairly between the two arms. Of the first 5 patients assigned to the lower dose, 100% (n=5) were successes. Of the 5 patients randomized to the higher dose, 40% (n=2) were successes and 60% (n=3) were failures. Based on data from the first 10 patients, the posterior probability that the 4.5 mg/kg dose is superior to the 7.5 mg/kg dose is 0.94. The next 10 patients were each assigned to the ATG 4.5 mg/kg arm. The probability of assignment to this arm for each of these 10 patients was 0.90, the maximum probability of assignment to either treatment arm according to the study design. Overall, of the 15 patients assigned to the ATG 4.5 mg/kg arm, 3 are considered treatment failures and 12 are considered treatment successes. Based on the data from all 20 patients, the posterior probability that the 4.5 mg/kg dose has a higher treatment success rate than the 7.5 mg/kg dose is 0.93, similar to the finding after the first 10 patients. The randomization sequence for the patients on the study is detailed in Table 2.
Table 2.
Randomization sequence
| Accrual | Treatment assignment |
Probability of randomization* |
Outcome at 100 days |
Probability of success† |
|---|---|---|---|---|
| 1 | 7.5 mg/kg | --- | Failure | -- |
| 2 | 4.5 mg/kg | --- | Success | -- |
| 3 | 4.5 mg/kg | --- | Success | -- |
| 4 | 4.5 mg/kg | --- | Success | -- |
| 5 | 7.5 mg/kg | --- | Success | -- |
| 6 | 7.5 mg/kg | --- | Failure | -- |
| 7 | 4.5 mg/kg | --- | Success | -- |
| 8 | 7.5 mg/kg | --- | Failure | -- |
| 9 | 7.5 mg/kg | --- | Success | -- |
| 10 | 4.5 mg/kg | --- | Success | 0.94 |
| 11 | 4.5 mg/kg | 0.9 | Success | 0.94 |
| 12 | 4.5 mg/kg | 0.9 | Success | 0.93 |
| 13 | 4.5 mg/kg | 0.9 | Failure | 0.93 |
| 14 | 4.5 mg/kg | 0.9 | Success | 0.93 |
| 15 | 4.5 mg/kg | 0.9 | Success | 0.93 |
| 16 | 4.5 mg/kg | 0.9 | Success | 0.93 |
| 17 | 4.5 mg/kg | 0.9 | Success | 0.95 |
| 18 | 4.5 mg/kg | 0.9 | Success | 0.95 |
| 19 | 4.5 mg/kg | 0.9 | Failure | 0.93 |
| 20 | 4.5 mg/kg | 0.9 | Failure | 0.93 |
The first 10 patients were assigned to treatment by sampling without replacement from a pool of 5 slots for 4.5 mg/kg and 5 slots for 7.5 mg/kg. The maximum randomization probability for either treatment arm is 0.9 per the study design.
Success defined as: 4.5 mg/kg is better than 7.5 mg/kg
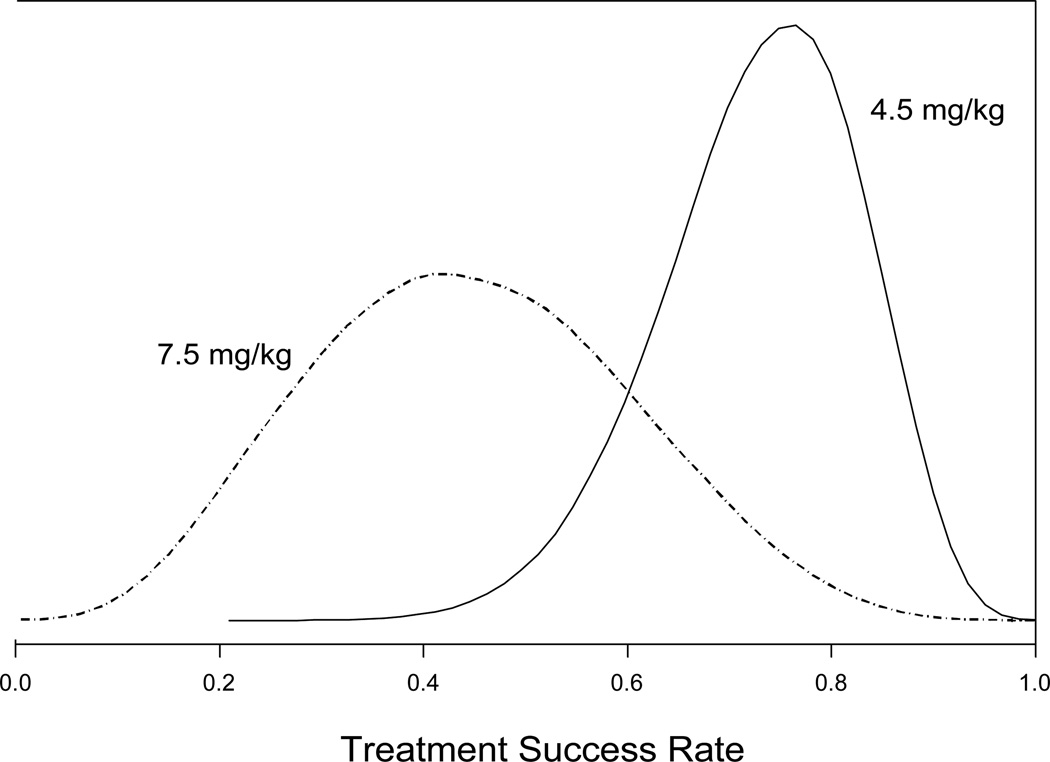
The posterior mean treatment success rate for the ATG 4.5 mg/kg treatment arm was 0.73 with a 90% credible interval of 0.56 to 0.87. The posterior mean treatment success rate for the ATG 7.5 mg/kg treatment arm was 0.45 with a 90% credible interval of 0.20 to 0.71. The posterior distributions of the success rates for the two treatment arms are shown in Figure 1.
Figure 1.
Posterior distributions of the success rates for the two treatment arms. The posterior mean treatment success for the lower dose arm (4.5 mg/kg) was 0.73 and the posterior mean treatment success rate for the higher dose arm (7.5 mg/kg) was 0.45.
Engraftment
Nineteen patients (95%) engrafted neutrophils. Median times to engraftment were 13 days and 13.5 days on the lower dose and higher dose arms, respectively (p=0.903). One patient on the 4.5 mg/kg dose did not engraft neutrophils before dying on post-transplant day 14. Twelve of 15 patients on the 4.5 mg/kg dose engrafted platelets at a median of 19 days, while 4 of 5 patients on the 7.5 mg/kg dose engrafted platelets, also at a median of 19 days (p=0.758).
Graft-vs-host disease
The cumulative incidence of grade II-IV aGVHD on the 4.5 mg/kg dose was 27% (n=4), while no patient on the 7.5 mg/kg dose developed grade II-IV aGVHD (p=0.530). Thirteen patients were evaluable for cGVHD. The incidence of limited and extensive cGVHD was 55% (6 of the 11 evaluable patients) on the 4.5 mg/kg dose and 50% (1 of the 2 evaluable patients) on the 7.5 mg/kg dose (p=0.999). One patient on the 4.5 mg/kg arm had both limited and extensive cGHVD.
Treatment-related mortality
Day 100 TRM was 20% (n=3) in the 4.5 mg/kg arm (due to infection and multi-organ failure, n=2; to disease relapse and GVHD, n=1), while it was 60% (n=3) in the 7.5 mg/kg arm (all due to severe infection and multi-organ failure). This difference was not statistically significant (p=0.131).
Infections
The incidence of infection was similar in both groups: 73% (n=11) in the 4.5 mg/kg arm and 80% (n=4) in the 7.5 mg/kg arm (p=0.999). There were no cases of Epstein-Barr virus–positive post-transplant lymphoproliferative disorder.
Relapse and survival
Of the 15 patients in the 4.5 mg/kg arm, 9 died, with a median OS of 20.1 months. Of the 5 patients in the 7.5 mg/kg arm, 3 died, with a median OS of 2.7 months. There was no difference in OS between the two arms (p=0.607; HR = 1.41; 95% CI 0.38 – 5.26). Survival probability at 1 year was 0.60 (95% CI 0.32 to 0.80) for the lower dose arm and 0.40 (95% CI 0.05 to 0.75) for the higher dose arm. Survival probability at 3 years was 0.40 (95% CI 0.16 to 0.63) for the lower dose arm and 0.40 (95% CI 0.05 to 0.75) for the higher dose arm. All 4 patients who had relapsed disease were in the 4.5 mg/kg arm, and all 4 subsequently died. The median RFS for the 4.5 mg/kg arm and 7.5 mg/kg arm were 15.5 months and 2.7 months, respectively (p=0.607; HR=1.41; 95% CI 0.38 – 5.26).
ATG level and outcomes
ATG levels were determined on transplant days 0, 7, 14, and 28. There were no statistically significant differences between blood levels of ATG and clinical outcomes (OS, RFS, TRM) or incidence of acute or chronic GVHD (data not shown). Moreover, there was no association between ATG levels and incidence of infections. However, these results should be interpreted with caution given the small sample size.
Discussion
We incorporated two intermediate doses of ATG in this first prospective adaptively randomized trial evaluating the role of ATG in aGVHD prophylaxis. The adaptive randomization method used here is advantageous since the participants were more likely to be exposed to the dose with the higher success rate. Moreover, success was defined as a composite of clinically relevant end points that included engraftment, survival, and remission status in addition to the absence of aGVHD. The success rates in the lower dose and the higher dose arms were 73% and 45%, respectively. Lowering the ATG dose to 4.5 mg/kg did not affect the time to engraftment, incidence of acute or chronic GVHD, RFS, or OS. Our study was terminated early because of the slow accrual and high TRM (60%) at 100 days in the ATG 7.5 mg/kg dose arm. These data highlight the safety and efficacy of the lower dose of ATG and also the early toxicity that can be associated with the higher dose of ATG.
Several randomized and nonrandomized trials have shown that addition of ATG to the conditioning regimen or its administration after transplant can decrease the incidence of acute and chronic GVHD.[6,7,9,15–19] Furthermore, some studies have shown that a higher blood ATG level after transplant is associated with lower incidence of grade II-IV acute and chronic GVHD.[20,21] In a study of 153 patients undergoing allogeneic HCT who received ATG (4.5 mg/kg) for GVHD prophylaxis, Podgorny et al. reported that the patients who developed grade II-IV aGVHD had significantly lower serum levels of ATG on day 7 and day 28 after transplant than the patients with grade 0-I aGVHD.[20] Similarly, in the study by Remberger et al., an ATG level less than 70 µg/mL at day 0 (prior to the transplant) was a predictor for significantly higher risk of grade II-IV aGVHD.[21] Moreover, a dose-dependent response was seen, with lower cumulative incidence of aGVHD in patients receiving a higher dose of ATG.
These same authors, in a previous retrospective study, had reported that ATG doses of 4 mg/kg and 10 mg/kg were associated with a higher TRM than ATG doses of 6 mg/kg or 8 mg/kg.[9] In that study, the incidence of grade II-IV aGVHD was highest in the ATG 4 mg/kg arm. We, on the other hand, did not see a higher TRM in the lower dose arm. Furthermore, we did not find the blood ATG levels obtained on different days to be predictive of development of acute or chronic GVHD or other clinical outcomes. As in the previous studies,[9,18,21] however, no patient in the higher dose arm developed grade II-IV aGVHD, which could be suggestive of a dose-response relationship.
High serum levels of ATG have been associated with development of post-transplant lymphoproliferative disorder.[20] We could not shed any light on this relationship since no patient in our study developed this complication. Moreover, it was not possible to draw concrete conclusions about the risk of infections for the two dose levels because of the small sample size.
We recognize the statistical limitations of our study; however some important lessons can be learned. For instance, one can infer from these results that it will be more beneficial to allow longer run-in periods to allow for more patients to be randomized fairly before adapting so that we have more reliable information on which to base the adapting. Also, performing the randomization in blocks would be a useful control against runs of patients assigned to one treatment arm or the other, as suggested by others[22,23]. Note that the chance of assigning 10 patients in a row to a treatment arm, if the probability of being assigned to that treatment arm is 0.90, is 0.9010 = 0.35.
In summary, our data suggest that the lower dose of ATG (4.5 mg/kg) is safe and yields clinical outcomes similar to those of the higher dose ATG (7.5 mg/kg). These results favor the use of lower dose ATG (4.5 mg/kg) for patients undergoing RIC allogeneic HCT. Whether the benefit of reducing the incidence of GVHD at the cost of higher incidence of serious infections and lack of a survival benefit justifies the use of ATG remains to be seen. Further randomized trials are needed to compare ATG-containing GVHD prophylaxis regimens with newer agents.
Acknowledgments
This work was supported in part by the NCI Cancer Center Support Grant to The University of Texas MD Anderson Cancer Center (P30 CA016672).
Footnotes
Conflict of interest
None.
References
- 1.Frassoni F. Anti-T-cell globulin: an essential ingredient for haematopoietic cell transplantation? Lancet Oncol. 2009;10:839. doi: 10.1016/S1470-2045(09)70233-5. [DOI] [PubMed] [Google Scholar]
- 2.Brayman K. New Insights Into the Mechanisms of Action of Thymoglobulin. Transplantation. 2007;84:S3–S4. [Google Scholar]
- 3.Zand MS, Vo T, Pellegrin T, Felgar R, Liesveld JL, Ifthikharuddin JJ, Abboud CN, Sanz I, Huggins J. Apoptosis and complement-mediated lysis of myeloma cells by polyclonal rabbit antithymocyte globulin. Blood. 2006;107:2895–2903. doi: 10.1182/blood-2005-06-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zand MS, Vo T, Huggins J, Felgar R, Liesveld J, Pellegrin T, Bozorgzadeh A, Sanz I, Briggs BJ. Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation. 2005;79:1507–1515. doi: 10.1097/01.tp.0000164159.20075.16. [DOI] [PubMed] [Google Scholar]
- 5.Lowsky R. Thymoglobulin and Regulatory T Cells in Organ and Hematopoietic Cell Transplantation. Transplantation. 2007;84:S20–S26. [Google Scholar]
- 6.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, Volin L, Ruutu T, Heim DA, Schwerdtfeger R, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 7.Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, Oneto R, Bruno B, Barbanti M, Sacchi N, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98:2942–2947. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- 8.Champlin RE, Perez WS, Passweg JR, Klein JP, Camitta BM, Gluckman E, Bredeson CN, Eapen M, Horowitz MM. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109:4582–4585. doi: 10.1182/blood-2006-10-052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remberger M, Svahn BM, Mattsson J, Ringden O. Dose study of thymoglobulin during conditioning for unrelated donor allogeneic stem-cell transplantation. Transplantation. 2004;78:122–127. [PubMed] [Google Scholar]
- 10.Seidel MG, Fritsch G, Matthes-Martin S, Lawitschka A, Lion T, Potschger U, Rosenmayr A, Fischer G, Gadner H, Peters C. Antithymocyte globulin pharmacokinetics in pediatric patients after hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2005;27:532–536. doi: 10.1097/01.mph.0000184575.00717.25. [DOI] [PubMed] [Google Scholar]
- 11.Parmar S, Del Lima M, Zou Y, Patah PA, Liu P, Cano P, Rondon G, Pesoa S, de Padua Silva L, Qazilbash MH, et al. Donor-recipient mismatches in MHC class I chain-related gene A in unrelated donor transplantation lead to increased incidence of acute graft-versus-host disease. Blood. 2009;114:2884–2887. doi: 10.1182/blood-2009-05-223172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Przepiorka D, Khouri I, Ippoliti C, Ueno NT, Mehra R, Korbling M, Giralt S, Gajewski J, Fischer H, Donato M, et al. Tacrolimus and minidose methotrexate for prevention of acute graft-versus-host disease after HLA-mismatched marrow or blood stem cell transplantation. Bone Marrow Transplant. 1999;24:763–768. doi: 10.1038/sj.bmt.1701983. [DOI] [PubMed] [Google Scholar]
- 13.Berry DA, Eick SG. Adaptive assignment versus balanced randomization in clinical trials: a decision analysis. Stat Med. 1995;14:231–246. doi: 10.1002/sim.4780140302. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL MP. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 15.Deeg HJ, Storer BE, Boeckh M, Martin PJ, McCune JS, Myerson D, Heimfeld S, Flowers ME, Anasetti C, Doney KC, et al. Reduced incidence of acute and chronic graft-versus-host disease with the addition of thymoglobulin to a targeted busulfan/cyclophosphamide regimen. Biol Blood Marrow Transplant. 2006;12:573–584. doi: 10.1016/j.bbmt.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 16.Schattenberg A, van der Meer A, Preijers F, Schaap N, Rinkes M, van der Maazen R, Allebes W, Joosten I, De Witte T. Addition of ATG to the conditioning regimen is a major determinant for outcome after transplantation with partially lymphocyte-depleted grafts from voluntary unrelated donors. Bone Marrow Transplant. 2004;33:1115–1121. doi: 10.1038/sj.bmt.1704490. [DOI] [PubMed] [Google Scholar]
- 17.Basara N, Baurmann H, Kolbe K, Yaman A, Labopin M, Burchardt A, Huber C, Fauser AA, Schwerdtfeger R. Antithymocyte globulin for the prevention of graft-versus-host disease after unrelated hematopoietic stem cell transplantation for acute myeloid leukemia: results from the multicenter German cooperative study group. Bone Marrow Transplant. 2005;35:1011–1018. doi: 10.1038/sj.bmt.1704957. [DOI] [PubMed] [Google Scholar]
- 18.Remberger M, Sundberg B. Rabbit-immunoglobulin G levels in patients receiving thymoglobulin as part of conditioning before unrelated donor stem cell transplantation. Haematologica. 2005;90:931–938. [PubMed] [Google Scholar]
- 19.Bacigalupo A, Lamparelli T, Milone G, Sormani MP, Ciceri F, Peccatori J, Locasciulli A, Majolino I, Di Bartolomeo P, Mazza F, et al. Pre-emptive treatment of acute GVHD: a randomized multicenter trial of rabbit anti-thymocyte globulin, given on day+7 after alternative donor transplants. Bone Marrow Transplant. 45:385–391. doi: 10.1038/bmt.2009.151. [DOI] [PubMed] [Google Scholar]
- 20.Podgorny PJ, Ugarte-Torres A, Liu Y, Williamson TS, Russell JA, Storek J. High rabbit-antihuman thymocyte globulin levels are associated with low likelihood of graft-vs-host disease and high likelihood of posttransplant lymphoproliferative disorder. Biol Blood Marrow Transplant. 16:915–926. doi: 10.1016/j.bbmt.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Remberger M, Sundberg B. Low serum levels of total rabbit-IgG is associated with acute graft-versus-host disease after unrelated donor hematopoietic stem cell transplantation: results from a prospective study. Biol Blood Marrow Transplant. 2009;15:996–999. doi: 10.1016/j.bbmt.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Karrison TG, Huo D, Chappell R. A group sequential, response-adaptive design for randomized clinical trials. Control Clin Trials. 2003;24:506–522. doi: 10.1016/s0197-2456(03)00092-8. [DOI] [PubMed] [Google Scholar]
- 23.Turnbull J. Group Sequential Methods wtih Applications to Clinical Trials. 2000:327–336. [Google Scholar]