Abstract
A total of 149 patients with multiple myeloma (MM) who received allogeneic hematopoietic stem cell transplantation (allo-HCT) with myeloablative (MAC; n=38) or reduced-intensity conditioning (RIC; n=110) regimens at MD Anderson Cancer Center were evaluated. Of the total, 120 (81%) patients had relapsed or had refractory disease. Median age of MM patients was 50 (28-70) years with a follow-up time of 28.5(3-164) months. The 100-day and 5-year treatment related mortality (TRM) rates were 17% and 47%, respectively. TRM was significantly lower with RIC regimens (13%) vs. 29% for MAC at 100 days (p=0.012). The cumulative incidence of grade II-IV acute graft-versus-host disease (GVHD) was 35% and chronic GVHD was 46%. PFS and OS at 5 years were 15% and 21%, respectively. In multivariate analysis, allo-HCT for primary remission consolidation was associated with longer PFS (HR 0.35; 95% CI, 0.18–0.67) and OS (HR 0.29; 95% CI 0.15–0.55), while absence of high-risk cytogenetics was associated with longer PFS only (HR 0.59; 95% CI 0.37–0.95). We observe that TRM has decreased with the use of RIC regimens and long-term disease control can be expected in a subset of MM patients undergoing allo-HCT. Further studies should be conducted in carefully designed clinical trials in this patient population.
Keywords: Multiple Myeloma, Allogenic transplant, Survival
INTRODUCTION
Absence of contaminating tumor cells in the graft and the potential for a graft- versus-myeloma (GVM) effect make allogeneic hematopoietic stem cell transplantation (allo-HCT) a potentially valuable treatment option for patients with multiple myeloma (MM).1-5 Studies with myeloablative conditioning (MAC) have shown that patients undergoing allo-HCT have lower relapse rates than patients receiving autologous (auto)-HCT,6 and achievement of a durable molecular remission can virtually eliminate the risk of relapse.7 Nevertheless, the relapse rate remains high, and the results are further marred by a high treatment-related mortality (TRM).8,9
Introduction of reduced-intensity conditioning (RIC) allo-HCT has led to more acceptable TRM rates of approximately 15-20%, while preserving the GVM effect.10-16 However, large prospective trials have shown conflicting results with improved survival seen after allo-HCT in some studies, but not in others.17-21 Therefore, the appropriate use of this treatment modality remains unclear. We previously reported that RIC allo-HCT offers long-term survival in a subset of patients with relapsed or refractory MM.22 Here we evaluate the outcome of all patients with MM who received allo-HCT at our institution in the last 25 years. In addition, we also investigate factors that may help predict survival outcomes in multiple myeloma, allo-HCT recipients.
METHODS
Patients
All patients older than 18 years with MM who underwent allo-HCT at MD Anderson Cancer Center between November 1985 and June 2010, with the exception of the patients treated on a tandem auto-auto versus auto-allo-HCT trial [Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0102 Trial,20 were included in this analysis. These patients met the general eligibility criteria to undergo allo-HCT, including adequate cardiac, pulmonary, renal, and hepatic function, and absence of uncontrolled infection.22 Fifty-one patients have previously been reported.22 All patients gave written informed consent according to the institutional guidelines. This retrospective analysis was approved by the Institutional Review Board at MD Anderson Cancer Center.
Hematopoietic progenitor cell collection
Hematopoietic progenitor cells (HPCs) were collected from the bone marrow or peripheral blood using standard approaches.23 Granulocyte-colony stimulating factor (G-CSF) was used for peripheral blood stem cell mobilization. HPCs from unrelated donors were obtained through the National Marrow Donor Program according to the standard guidelines.
Conditioning regimens and supportive care
A number of different conditioning regimens were used (Table I). Thirty-eight patients (26%) received MAC regimens, and 110 patients (74%) received RIC regimens. Preparative regimen information was not available for one patient. Thirty-four patients (23%) received anti-thymocyte globulin (ATG) for in vivo T-cell depletion, while alemtuzumab was used in five patients (3%). In addition, rituximab was used in 18 patients (12%). Graft-versus-host disease (GVHD) prophylaxis consisted of a variety of combinations (Table I). Most patients received a combination of tacrolimus and methotrexate (n=104; 70%). All supportive care was provided according to extant institutional guidelines.
Table I.
Conditioning regimens and graft-versus-host disease prophylaxis.
| Regimen* | N (%) |
|---|---|
| Myeloablative conditioning regimens | 38 (26) |
| Fludarabine-Melphalan | 7 (5) |
| Melphalan | 6 (4) |
| Melphalan-TBI | 10 (7) |
| Busufan-Cyclosporin-Thiotepa | 6 (4) |
| Others | 9 (6) |
| Reduced-intensity conditioning regimens | 110 (74) |
| Fludarabine-Melphalan | 99 (67) |
| TBI | 6 (4) |
| Others | 5 (3) |
|
| |
| Graft-versus-host disease prophylaxis | 147(100) |
| Cyclophosphamide | 4 (3) |
| Cyclophosphamide-MTX | 12 (8) |
| Cyclophosphamide-MMF | 6 (4) |
| None** | 6 (4) |
| Tacrolimus | 6 (4) |
| Tacrolimus-MTX | 104 (70) |
| Others | 11 (7) |
| Missing | 2 (1) |
TBI, total-body irradiation; MTX, methotrexate; MMF, mycophenolate mofetil;
One patient could not be assigned to myeloablative or reduced intensity conditioning due to missing data.
These patients received transplantation from human leukocyte antigen–identical twin siblings.
Engraftment and Disease Response
The day of neutrophil engraftment was defined as the first of 3 consecutive days on which the absolute neutrophil count was >0.5×109/L. Primary graft failure was defined as the failure to achieve neutrophil engraftment by day 30. The day of platelet engraftment was defined as the first of 7 consecutive days on which the platelet count was ≥20×109/L, independent of platelet transfusion.
Response, relapse, and disease progression were defined according to the International Myeloma Working Group (IMWG) uniform response criteria.24
Statistical analysis
Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan-Meier method.25 OS was measured from the day of stem cell infusion (day 0) to death from any cause, with censoring performed at the date of last contact. PFS was determined from the day of stem cell infusion to the day of documented relapse, progression or death. Death from any cause other than relapse was classified as TRM. GVHD was diagnosed and graded according to the standard criteria.26 Comparisons of OS, PFS, and TRM between groups of patients were carried out using the log-rank test. A Cox proportional hazard model was used for each of the survival endpoints to identify significant factors in a multivariate model. Correlation of clinical variables and outcome was tested using the Fisher’s exact test. All tests were two-sided, and p-values of 0.05 or less were considered to be statistically significant. Statistical analysis was carried out using SAS version 9 (SAS Institute, Cary, NC) and S-Plus 7 (Insightful Inc., Seattle, WA).
RESULTS
Patient and disease characteristics
A total of 149 patients with MM underwent allo-HCT between November 1985 and June 2010 at MD Anderson Cancer Center. Patient characteristics are summarized in Table II. Median age was 50 years (range, 28-70). The patients who received allo-HCT during or after the year 2000 were significantly older than the patients transplanted before the year 2000 (52 years vs. 48 years; p=<0.0001). In addition, significantly higher number of patients received MAC regimens before the year 2000 compared to the time period after that (66% before the year 2000 vs. 7% in 2000 and later; p=<0.0001). At the time of transplantation, the disease status was partial response (PR) or better in 51% (n=76) of the patients [complete response (CR) 2%; very good partial response (VGPR) 11%; PR 38%]. Ninety-one patients had received one or more prior auto-HCT. Of these, 10 patients received allo-HCT for first remission consolidation, while 80 patients received allo-HCT for relapsed or refractory disease. Results of conventional chromosomal analysis performed at some point between diagnosis and allo-HCT were available for 112 patients (75%). Deletion of chromosome 13q was detected in 26 patients (17%), and deletion of chromosome 17p was detected in 16 patients (11%). Overall, one or more high-risk cytogenetic abnormalities [t(4;14), t(14;16), del 17p, del 13q, 1q amplification, or hypodiploidy]27-30 were detected in 36 patients (24%).
Table II.
Patient and disease characteristics.
| Characteristic | Median (range) or n (%) |
|---|---|
| Age, years | 50 (28-70) |
| Age > 50 years | 84 (56) |
| Age ≤ 50 years | 65 (44) |
| Gender | |
| Female | 62 (42) |
| Male | 87 (58) |
| Myeloma subtype | |
| IgG | 78 (52) |
| IgA | 32 (21) |
| IgM | 3 (2) |
| IgD | 3 (2) |
| Light chain only | 25 (17) |
| Nonsecretory | 6 (4) |
| Plasma cell leukemia | 2 (1) |
| Durie-Salmon stage at initial diagnosis | |
| I | 16 (11) |
| II | 60 (40) |
| III | 71 (48) |
| Unknown | 2 |
| Prior response to therapy at allogenic-HCT | |
| Complete response | 3 (2) |
| Very good partial response | 17 (11) |
| Partial response | 56 (38) |
| Stable disease | 29 (20) |
| Progressive disease | 43 (29) |
| Disease status at allogenic-HCT | |
| Primary sensitive | 26 (17) |
| Relapsed/refractory | 121 (81) |
| Poor-risk cytogenetic features | |
| Yes | 36 (24) |
| No | 76 (51) |
| Unknown | 37 (25) |
| Prior autologous-HCT | |
| None | 57 (38) |
| 1 | 70 (47) |
| 2 | 20 (13) |
| 3 | 1 |
| Prior allogenic-HCT | |
| None | 142 (95) |
| 1 | 6 (4) |
| Time from diagnosis to allogenic-HCT, months | 30 (3-229) |
| HCT source | |
| Peripheral blood | 100 (67) |
| Bone marrow | 46 (31) |
| Cord blood | 3 |
| CD34+ cell count (×106/kg) | 4.4 (0.3-54) |
| TNC (×108/kg) | 5.4 (0.13-415) |
| Donor type | |
| Related donor | 114 (77) |
| Unrelated donor | 35 (23) |
| Donor lymphocyte infusion | |
| Yes | 6 (4) |
| No | 143 (96) |
HCT, hematopoietic stem cell transplant; TNC, total nucleated cells;
Engraftment
One patient failed to engraft and received infusion of autologous stem cells, resulting in autologous reconstitution. Six patients experienced early death prior to engraftment. Overall, 142 patients (95%) achieved neutrophil engraftment. The median time to neutrophil engraftment was 12 days (range: 8-24). Platelet engraftment was seen in 133 patients (89%), with a median time to engraftment of 13 days (range: 0-70).
Response
The overall response rate, assessed at approximately 3-months after allo-HCT, was 78% (CR 12%; VGPR 26%; PR 40%). Nine patients (6%) had stable disease (SD). The response rate in patients with progressive disease (PD) at allo-HCT (n=43) was 61% (CR 12%; VGPR 21%; PR 28%). In contrast, the response rate in patients in ≥PR at allo-HCT (n=76) was 91% (CR 15%; VGPR 29%; PR 47%). The response rate in patients treated with MAC regimens (n=38) was 68% (CR 8%; VGPR 26%; PR 34%) and the response rate in patients treated with RIC regimens (n=110) was 82% (CR 14%; VGPR 25%; PR 43%). This difference was not statistically significant (p=0.25).
TRM
Overall, the 100-day and 5-year TRM rates were 17% and 47%, respectively. The most common causes of death were recurrent disease 52 (35%), acute GVHD 15 (10%), chronic GVHD 15 (10%), infection 10 (7%), and multi-organ failure 9(6%). The following variables emerged as predictors of higher TRM in a univariate analysis: allo-HCT performed before 2000 (26% vs. 13% for allo-HCT during or after 2000; p=0.0013), no prior auto-HCT (23% vs. 13% for ≥ 1 prior auto-HCT; p=0.0062), bone marrow as the stem cell source (29% vs. 10% for peripheral blood stem cells; p=0.002), use of MAC regimens (29% vs. 13% for RIC regimens; p=0.012), disease status less than PR at allo-HCT (25% vs. 9% for patients with disease status ≥ PR; p=0.0164), and the presence of high-risk cytogenetic features (27% vs. 5% for patients without high-risk cytogenetics; p=0.013).
GVHD
The cumulative incidence of grade II-IV acute GVHD at 5 years was 35%. Grade III-IV acute GVHD was seen in 17% of patients. The cumulative incidence of limited or extensive chronic GVHD at 5 years was 46%. The use of peripheral blood stem cells or unrelated donors did not increase the risk of acute or chronic GVHD. There was no correlation between development of GVHD and response to allo-HCT.
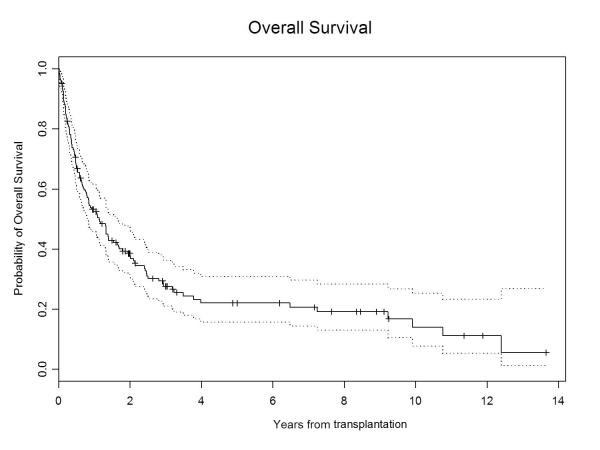
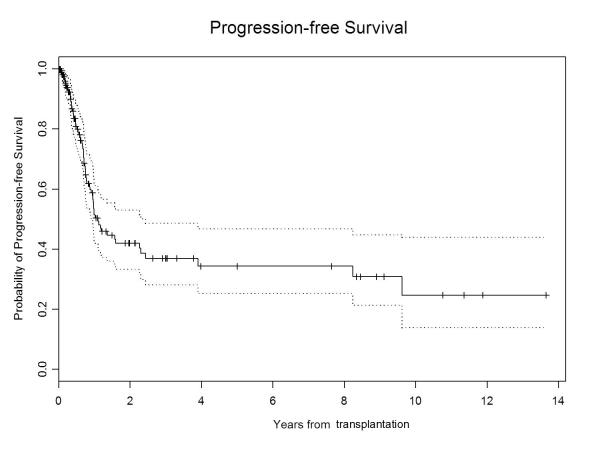
Survival
The median follow-up for all surviving patients was 28.5 months (range, 3-164). The 5-year PFS and OS were 15% (95% CI, 0.09–0.23) and 21% (95% CI, 0.15–0.31), respectively (Figure 1A & 1B). At the time of last follow-up, 40 patients were still alive, with 28 in remission, the longest remission being 164 months. On univariate analysis, ≥PR at allo-HCT, the use of peripheral blood as stem cell source, allo-HCT for first remission consolidation, and the absence of high-risk cytogenetic features were associated with longer PFS and OS (Table III). In addition, allo-HCT in or after the year 2000 emerged as a predictor of longer OS. On multivariate analysis, allo-HCT for first remission consolidation was associated with longer PFS (HR 0.35; 95% CI 0.18–0.67; p=0.0016) and OS (HR 0.29; 95% CI 0.15–0.55; p=0.0002), while absence of high-risk cytogenetic features was associated with longer PFS only (HR 0.59; 95% CI 0.37–0.95; p=0.03). Age, immunoglobulin subtype, β2 microglobulin level, serum albumin level, serum lactate dehydrogenase level, hemoglobin level, serum calcium level, interval between diagnosis and allo-HCT, interval between auto-HCT and allo-HCT, number of prior auto-HCTs, development of chronic GVHD, and administration of donor lymphocyte infusion (DLI) did not emerge as significant predictors of PFS or OS.
Figure1A.
Overall survival in multiple myeloma patients
Figure 1B.
Progression free survival in multiple myeloma patients
Table III.
Univariate analysis of factors affecting PFS and OS.
| Variable | N | PFS* % (95% CI) |
p-value | OS* % (95% CI) |
p-value |
|---|---|---|---|---|---|
| All patients | 149 | 15 (9-23) | 21 (15-31) | ||
| Age, years | |||||
| ≤ 50 | 84 | 14 (8-25) | 0.197 | 18 (11-30) | 0.138 |
| > 50 | 65 | 11 (4-34) | 26 (15-45) | ||
| Stage | |||||
| I | 16 | 10 (2-57) | 0.523 | 28 (10-79) | 0.258 |
| II | 60 | 21 (12-35) | 21 (12-39) | ||
| III | 71 | 11 (6-23) | 18 (11-31) | ||
| Poor-risk cytogenetics | |||||
| No | 76 | 18 (9-33) | 0.0005 | 29 (19-45) | 0.004 |
| Yes | 36 | NA | 13 (4-39) | ||
| Prior autologous-HCT† | |||||
| No | 57 | 17 (9-31) | 0.71 | 23 (14-38) | 0.68 |
| Yes | 91 | 11 (5-24) | 18 (10-31) | ||
| Prior allogenic-HCT | |||||
| None | 142 | 15 (9-23) | 0.881 | 22 (15-31) | 0.58 |
| 1 | 6 | ||||
| Conditioning | |||||
| MAC | 38 | 17 (8-36) | 0.20 | 16 (7-35) | 0.075 |
| RIC | 110 | 14 (8-24) | 23 (15-35) | ||
| Stem cell source | |||||
| PB | 100 | 18 (10-30) | 0.0002 | 26 (17-39) | < 0.001 |
| CB | 3 | NA | NA | ||
| BM | 46 | 9 (3-22) | 13 (6-28) | ||
| Donor type** | |||||
| MRD | 105 | 16 (10-27) | 0.34 | 23 (15-35) | 0.27 |
| MUD | 30 | 10 (3-32) | 19 (8-46) | ||
| Transplantation year | |||||
| Before 2000 | 47 | 15 (8-29) | 0.133 | 17 (9-32) | 0.041 |
| Since 2000 | 102 | 13 (6-26) | 22 (14-36) | ||
| Disease status at transplant | |||||
| Less than PR | 72 | 8 (3-19) | 0.0007 | 13 (7-26) | 0.001 |
| PR or better | 76 | 20 (11-35) | 28 (18-43) | ||
| Primary sensitive disease*** | |||||
| No | 121 | 8 (4-16) | < 0.0001 | 13 (7-22) | < 0.0001 |
| Yes | 26 | 38 (21-70) | 51 (31-82) | ||
| Deletion 13q | |||||
| No | 86 | 16 (8-30) | 0.016 | 26 (17-41) | 0.008 |
| Yes | 26 | NA | 15 (5-46) | ||
| Deletion 17p | |||||
| No | 96 | 15 (8-28) | 0.04 | 25 (16-38) | 0.023 |
| Yes | 16 | NA | 17 (5-56) |
PFS, progression free survival; OS, overall survival; HCT: hematopoietic stem cell transplantation; MAC, myeloablative conditioning; RIC, reduced-intensity conditioning; PB, peripheral blood; CB, cord blood; BM, bone marrow; MRD, matched related donor; MUD, matched unrelated donor; DLI, donor lymphocyte infusion. All p-values are based on the log-rank test.
Only three patients underwent planned autologous-allogeneic HCT. The median time between previous autologous HCT and allogeneic HCT was 18 (1.5 – 92) months.
At 5 years.
Includes only completely matched donors.
Includes newly diagnosed patients who had chemosensitive disease at the time of allogeneic HCT.
NA: Number of patients not sufficient to measure survival
DISCUSSION
We present here our experience with a large cohort of MM patients who underwent allo-HCT at MD Anderson Cancer Center in the last 25 years. In this heavily pretreated patient population (81% with relapsed or refractory disease), we have identified several factors that affect allo-HCT outcome and may help in identifying patients who may benefit the most from this treatment modality. Overall, we noticed a gradual decline in the use of MAC regimens beyond the year 1999. This trend is in concordance with the reported literature.31 For instance, in a report from the European Group for Blood and Marrow Transplantation (EBMT) that compared 320 RIC with 196 MAC allo-HCTs, performed between 1998 and 2002, a significant shift toward RIC allo-HCT was seen after the year 1999.8 This gradual shift away from MAC allo-HCT stems partly from a high TRM ranging from 30-50% in prospective studies.32,33 RIC regimens, on the other hand, were mainly developed to reduce the TRM.34 Consistent with this approach, we noticed a trend towards lower early TRM with RIC regimens (13% vs. 29% with MAC, p=0.001). Furthermore, RIC allo-HCT can be safely performed in older patients with co-morbid conditions, which is an important consideration in myeloma, a disease with median age at diagnosis of 65 years. Indeed, we noticed that the patients transplanted after the year 1999 were significantly older than the patients treated before that time period, suggesting the applicability of this treatment modality to a broader age range of patients.
Since the emergence of RIC regimens, several groups have sought the best way to utilize RIC allo-HCT, with some success. A CR rate ranging from 53% to 73% and a TRM of approximately 10% are generally reported when allo-HCT is used in tandem with an auto-HCT.35-38 The results of randomized trials comparing tandem auto-auto with auto-allo approaches for newly diagnosed MM, however, have failed to show a consistent survival benefit in favor of allo-HCT.17-21 Two studies that showed superior OS and PFS with allo-HCT were the Italian study and the study by Björkstrand et al, which included all patients irrespective of prognostic factors.18,21 Overall, while these studies highlight the low TRM associated with RIC regimens, conclusive evidence that RIC allo-HCT leads to superior outcome compared to auto-HCT in the up-front setting is lacking.
In addition to its use for the treatment of newly diagnosed MM, several studies have also highlighted the feasibility of RIC allo-HCT for relapsed/refractory disease.11,15,39-41 Our report includes a relatively heavily pretreated patient group, as the majority of patients received allo-HCT in the salvage setting, and the median time from diagnosis to allo-HCT was 30 months. Furthermore, 49% of patients had SD or PD at the time of allo-HCT. The TRM is generally higher in this patient population compared to the upfront approach.15,16,39,40,42 Consistent with this trend the TRM at 2-years in our study was 40% in patients with relapsed/refractory disease, compared to 24% in patients who received allo-HCT for first remission consolidation (p=0.036). Moreover, the prognosis of patients who undergo allo-HCT for relapsed disease is less favorable than other patients.11,39,41 Kröger et al. reported that, in patients undergoing RIC allo-HCT, relapse after prior high-dose chemotherapy was the most significant factor for event-free survival (EFS) and OS.16 However, prolonged survival and a clear plateau in PFS can be seen in a subset of such patients.39 Indeed, in our study, 27% of patients were alive at last follow-up with 19% in remission, with the longest remission being 14 years. These data suggest that RIC allo-HCT can salvage a subset of myeloma patients with relapsed/refractory disease for whom the conventional therapeutic options are otherwise limited.43-45
In recent years, cytogenetic abnormalities have emerged as important predictors of relapse and survival in myeloma. Whether allografting can overcome the poor prognosis associated with high-risk cytogenetics remains undetermined. In the study by Bruno et al.,37 del 13q was detected in 13 of 39 patients studied. There was no difference in OS; however, the EFS was better in patients without del 13q. In another study, Kröger et.al.46 retrospectively compared the outcomes of 31 patients with del 13q, and 37 patients without del 13q. At 2 years, patients with del 13q had significantly lower EFS (18% vs. 42%) and OS (18% vs. 67%). In addition, the relapse rate was higher in patients with del 13q (77% vs. 44%), but the TRM at 1 year was similar (24% vs. 18%). In our study, pre-transplantation cytogenetic information was available in 75% of patients. Patients with one or more of the known high-risk cytogenetic features [t(4;14), t(14;16), del 17p del 13q, 1q amplification, or hypodiploidy],27-30 had significantly lower 5-year PFS and OS compared to the patients without these features. This was particularly true for the patients who had either del 13q or del 17p abnormalities. Taken together, these data suggest that cytogenetics continue to play an important role in determining the outcome, and it remains uncertain whether RIC allo-HCT can overcome the risk posed by these high-risk cytogenetic abnormalities.
In summary, in this large, single-center study we observe a reduced TRM in patients undergoing RIC allo- HCT and conclude that MM patients with chemo-sensitive disease may achieve long PFS and OS with this treatment modality. However, allo-HCT with RIC should be further studied in carefully designed clinical trials to identify the subset of MM patients who may benefit the most from this approach.
ACKNOWLEDGEMENTS
This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (CA016672).
Footnotes
Disclosure Statement: Conflict of Interest? No If yes, please state:
REFERENCES
- 1.Tricot G, Vesole DH, Jagannath S, et al. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87:1196–8. [PubMed] [Google Scholar]
- 2.Alyea E, Weller E, Schlossman R, et al. Outcome after autologous and allogeneic stem cell transplantation for patients with multiple myeloma: impact of graft-versus-myeloma effect. Bone Marrow Transplant. 2003;32:1145–51. doi: 10.1038/sj.bmt.1704289. [DOI] [PubMed] [Google Scholar]
- 3.Lokhorst HM, Wu K, Verdonck LF, et al. The occurrence of graft-versus-host disease is the major predictive factor for response to donor lymphocyte infusions in multiple myeloma. Blood. 2004;103:4362–4. doi: 10.1182/blood-2003-11-3862. [DOI] [PubMed] [Google Scholar]
- 4.Bellucci R, Wu CJ, Chiaretti S, et al. Complete response to donor lymphocyte infusion in multiple myeloma is associated with antibody responses to highly expressed antigens. Blood. 2004;103:656–63. doi: 10.1182/blood-2003-07-2559. [DOI] [PubMed] [Google Scholar]
- 5.Salama M, Nevill T, Marcellus D, et al. Donor leukocyte infusions for multiple myeloma. Bone Marrow Transplant. 2000;26:1179–84. doi: 10.1038/sj.bmt.1702685. [DOI] [PubMed] [Google Scholar]
- 6.Bjorkstrand BB, Ljungman P, Svensson H, et al. Allogeneic bone marrow transplantation versus autologous stem cell transplantation in multiple myeloma: a retrospective case-matched study from the European Group for Blood and Marrow Transplantation. Blood. 1996;88:4711–8. [PubMed] [Google Scholar]
- 7.Corradini P, Cavo M, Lokhorst H, et al. Molecular remission after myeloablative allogeneic stem cell transplantation predicts a better relapse-free survival in patients with multiple myeloma. Blood. 2003;102:1927–9. doi: 10.1182/blood-2003-01-0189. [DOI] [PubMed] [Google Scholar]
- 8.Crawley C, Iacobelli S, Bjorkstrand B, et al. Reduced-intensity conditioning for myeloma: lower nonrelapse mortality but higher relapse rates compared with myeloablative conditioning. Blood. 2007;109:3588–94. doi: 10.1182/blood-2006-07-036848. [DOI] [PubMed] [Google Scholar]
- 9.Bensinger WI, Buckner CD, Anasetti C, et al. Allogeneic marrow transplantation for multiple myeloma: an analysis of risk factors on outcome. Blood. 1996;88:2787–93. [PubMed] [Google Scholar]
- 10.Giralt S, Aleman A, Anagnostopoulos A, et al. Fludarabine/melphalan conditioning for allogeneic transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2002;30:367–73. doi: 10.1038/sj.bmt.1703652. [DOI] [PubMed] [Google Scholar]
- 11.Einsele H, Schafer HJ, Hebart H, et al. Follow-up of patients with progressive multiple myeloma undergoing allografts after reduced-intensity conditioning. Br J Haematol. 2003;121:411–8. doi: 10.1046/j.1365-2141.2003.04299.x. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Simon JA, Martino R, Alegre A, et al. Chronic but not acute graft-versus-host disease improves outcome in multiple myeloma patients after non-myeloablative allogeneic transplantation. Br J Haematol. 2003;121:104–8. doi: 10.1046/j.1365-2141.2003.04237.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee CK, Badros A, Barlogie B, et al. Prognostic factors in allogeneic transplantation for patients with high-risk multiple myeloma after reduced intensity conditioning. Exp Hematol. 2003;31:73–80. doi: 10.1016/s0301-472x(02)01010-x. [DOI] [PubMed] [Google Scholar]
- 14.Peggs KS, Mackinnon S, Williams CD, et al. Reduced-intensity transplantation with in vivo T-cell depletion and adjuvant dose-escalating donor lymphocyte infusions for chemotherapy-sensitive myeloma: limited efficacy of graft-versus-tumor activity. Biol Blood Marrow Transplant. 2003;9:257–65. doi: 10.1053/bbmt.2003.50009. [DOI] [PubMed] [Google Scholar]
- 15.Crawley C, Lalancette M, Szydlo R, et al. Outcomes for reduced-intensity allogeneic transplantation for multiple myeloma: an analysis of prognostic factors from the Chronic Leukaemia Working Party of the EBMT. Blood. 2005;105:4532–9. doi: 10.1182/blood-2004-06-2387. [DOI] [PubMed] [Google Scholar]
- 16.Kroger N, Perez-Simon JA, Myint H, et al. Relapse to prior autograft and chronic graft-versus-host disease are the strongest prognostic factors for outcome of melphalan/fludarabine-based dose-reduced allogeneic stem cell transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. 2004;10:698–708. doi: 10.1016/j.bbmt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Garban F, Attal M, Michallet M, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107:3474–80. doi: 10.1182/blood-2005-09-3869. [DOI] [PubMed] [Google Scholar]
- 18.Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–20. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 19.Rosinol L, Perez-Simon JA, Sureda A, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008;112:3591–3. doi: 10.1182/blood-2008-02-141598. [DOI] [PubMed] [Google Scholar]
- 20.Amrita Krishnan MD, Pasquini Marcelo C, Ewell Marian, et al. Tandem Autologous Hematopoietic Stem Cell Transplants (AuHCT) with or without Maintenance Therapy (auto-auto) Versus Single AuHCT Followed by HLA Matched Sibling Non-Myeloablative Allogeneic HCT (auto-allo) for Patients with Standard Risk (SR) Multiple Myeloma (MM): Results From the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0102 Trial. Blood (ASH Annual Meeting Abstracts) 2010;116 Abstract 41. [Google Scholar]
- 21.Bjorkstrand B, Iacobelli S, Hegenbart U, et al. Tandem autologous/reduced-intensity conditioning allogeneic stem-cell transplantation versus autologous transplantation in myeloma: long-term follow-up. J Clin Oncol. 2011;29:3016–22. doi: 10.1200/JCO.2010.32.7312. [DOI] [PubMed] [Google Scholar]
- 22.Efebera YA, Qureshi SR, Cole SM, et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed multiple myeloma. Biol Blood Marrow Transplant. 2010;16:1122–9. doi: 10.1016/j.bbmt.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bashir Q, De Lima MJ, McMannis JD, et al. Hematopoietic progenitor cell collection in patients with chronic myelogenous leukemia in complete cytogenetic remission after imatinib mesylate therapy. Leuk Lymphoma. 51:1478–84. doi: 10.3109/10428194.2010.501534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, M P. Nonparametric estimation from incomplete observations. J. Amer. Statist. Assn. 1958;53 [Google Scholar]
- 26.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 27.Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109:3489–95. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 28.Fonseca R, Blood E, Rue M, et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood. 2003;101:4569–75. doi: 10.1182/blood-2002-10-3017. [DOI] [PubMed] [Google Scholar]
- 29.Hanamura I, Stewart JP, Huang Y, et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: incidence increases from MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantation. Blood. 2006;108:1724–32. doi: 10.1182/blood-2006-03-009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonseca R, Barlogie B, Bataille R, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64:1546–58. doi: 10.1158/0008-5472.can-03-2876. [DOI] [PubMed] [Google Scholar]
- 31.Blade J, Rosinol L, Cibeira MT, et al. Hematopoietic stem cell transplantation for multiple myeloma beyond 2010. Blood. 115:3655–63. doi: 10.1182/blood-2009-08-238196. [DOI] [PubMed] [Google Scholar]
- 32.Barlogie B, Kyle RA, Anderson KC, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24:929–36. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 33.Lokhorst HM, Segeren CM, Verdonck LF, et al. Partially T-cell-depleted allogeneic stem-cell transplantation for first-line treatment of multiple myeloma: a prospective evaluation of patients treated in the phase III study HOVON 24 MM. J Clin Oncol. 2003;21:1728–33. doi: 10.1200/JCO.2003.04.033. [DOI] [PubMed] [Google Scholar]
- 34.Champlin R, Khouri I, Shimoni A, et al. Harnessing graft-versus-malignancy: non myeloablative preparative regimens for allogeneic haematopoietic transplantation, an evolving strategy for adoptive immunotherapy. Br J Haematol. 2000;111:18–29. doi: 10.1046/j.1365-2141.2000.02196.x. [DOI] [PubMed] [Google Scholar]
- 35.Kroger N, Schwerdtfeger R, Kiehl M, et al. Autologous stem cell transplantation followed by a dose-reduced allograft induces high complete remission rate in multiple myeloma. Blood. 2002;100:755–60. doi: 10.1182/blood-2002-01-0131. [DOI] [PubMed] [Google Scholar]
- 36.Kroger N, Sayer HG, Schwerdtfeger R, et al. Unrelated stem cell transplantation in multiple myeloma after a reduced-intensity conditioning with pretransplantation antithymocyte globulin is highly effective with low transplantation-related mortality. Blood. 2002;100:3919–24. doi: 10.1182/blood-2002-04-1150. [DOI] [PubMed] [Google Scholar]
- 37.Bruno B, Rotta M, Patriarca F, et al. Nonmyeloablative allografting for newly diagnosed multiple myeloma: the experience of the Gruppo Italiano Trapianti di Midollo. Blood. 2009;113:3375–82. doi: 10.1182/blood-2008-07-167379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rotta M, Storer BE, Sahebi F, et al. Long-term outcome of patients with multiple myeloma after autologous hematopoietic cell transplantation and nonmyeloablative allografting. Blood. 2009;113:3383–91. doi: 10.1182/blood-2008-07-170746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimoni A, Hardan I, Ayuk F, et al. Allogenic hematopoietic stem-cell transplantation with reduced-intensity conditioning in patients with refractory and recurrent multiple myeloma: long-term follow-up. Cancer. 2010;116:3621–30. doi: 10.1002/cncr.25228. [DOI] [PubMed] [Google Scholar]
- 40.de Lavallade H, El-Cheikh J, Faucher C, et al. Reduced-intensity conditioning allogeneic SCT as salvage treatment for relapsed multiple myeloma. Bone Marrow Transplant. 2008;41:953–60. doi: 10.1038/bmt.2008.22. [DOI] [PubMed] [Google Scholar]
- 41.Kroger N, Shimoni A, Schilling G, et al. Unrelated stem cell transplantation after reduced intensity conditioning for patients with multiple myeloma relapsing after autologous transplantation. Br J Haematol. 2010;148:323–31. doi: 10.1111/j.1365-2141.2009.07984.x. [DOI] [PubMed] [Google Scholar]
- 42.Kroger N, Shimoni A, Schilling G, et al. Unrelated stem cell transplantation after reduced intensity conditioning for patients with multiple myeloma relapsing after autologous transplantation. Br J Haematol. 148:323–31. doi: 10.1111/j.1365-2141.2009.07984.x. [DOI] [PubMed] [Google Scholar]
- 43.Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 44.Barlogie B, Tricot G, Anaissie E, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354:1021–30. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 45.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79:867–74. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 46.Kroger N, Schilling G, Einsele H, et al. Deletion of chromosome band 13q14 as detected by fluorescence in situ hybridization is a prognostic factor in patients with multiple myeloma who are receiving allogeneic dose-reduced stem cell transplantation. Blood. 2004;103:4056–61. doi: 10.1182/blood-2003-12-4435. [DOI] [PubMed] [Google Scholar]