Abstract
Objective measures of postural control that are sensitive to Parkinson's Disease (PD) progression would improve patient care and accelerate clinical trials. Although measures of postural sway during quiet stance in untreated PD have been shown to differ from age-matched control subjects, it is not known if sway measures change with disease progression in early PD. In this pilot study, we asked whether accelerometer-based metrics of sway could provide a practical tool for monitoring progression of postural dyscontrol in people with untreated or newly treated PD.
We examined 13 subjects with PD and 12 healthy, age-matched control subjects. The PD subjects had been recently diagnosed and had not started any antiparkinsonian medications at the baseline session. All subjects were tested 3-to-6 months and 12 months after the baseline session. Subjects were asked to stand quietly for two minutes while wearing an inertial sensor on their posterior trunk that measured trunk linear acceleration.
Our results suggested that objective sway measures deteriorated over one year despite minimal changes in UPDRS motor scores. Medio-lateral (ML) sway measures were more sensitive than antero-posterior sway measures in detecting progression. The ML JERK was larger in the PD group than the control group across all three testing sessions. The ML sway dispersion and ML sway velocity were also significantly higher in PD compared to control subjects by the 12-month evaluation. It is feasible to measure progression of PD prior to onset of treatment using accelerometer-based measures of quiet standing.
Keywords: Parkinson's disease progression, posture, accelerometry
Introduction
The defining motor features of Parkinson's disease (PD) are characterized by their insidious onset and inexorable, but variable progression. Reliable and validated, objective markers that can monitor PD progression throughout its course would dramatically improve patient care and accelerate clinical trials [1]. Clinical trials of neuroprotective interventions, in particular, require measures that are able to track PD progression, even in the early stage of the disease and before patients start taking antiparkinsonian medications.
Measures of postural control have not been considered viable candidates for measurement of progression in early-to-moderate, untreated PD because postural problems are not clinically apparent at this stage [2,3]. In fact, an abnormal response to the Pull test marks the progression from Stage II to Stage III of the Hoehn and Yahr scale and the PIGD (postural instability and gait disorder) subscore of the UPDRS is nearly normal in Stages I and II [3]. Postural problems in patients with PD in clinical trials is usually measured with the PIGD, that includes: sit-to-stand, posture, pull test, and gait evaluated on a 0 to 4 scale [4,5]. Although the PIGD is easy to use, does not require equipment, and is quick to administer, the results obtained are subjective and are not sensitive enough to detect early disease progression. Moreover, a recent study that evaluated the responsiveness of different PD scales to change over time showed only a small to moderate effect size of the UPDRS Motor part III [6].
Contrary to the general assumption that postural problems develop later in PD, several recent studies, utilizing force-plate measurements, have shown that postural sway is abnormal early in the disease, even in PD patients with mild symptoms (e.g., Motor UPDRS < 21) [7,8]. Quantitative measures of postural sway reflect how the nervous system controls the complex sensorimotor task of maintaining bipedal equilibrium [9]. The basal ganglia play an important role in control of axial tone, postural response amplitude, and interpretation of somatosensory information [10-12]. Thus, postural abnormalities in early PD are not surprising. Although abnormalities in postural sway have been shown to predict past and future falls [13,14], it is not possible for clinicians to rate measures of postural sway (e.g. sway amplitude, velocity, frequency, or jerkiness), because these are subtle abnormalities that are not always evident or quantifiable by clinical observation.
Our recent studies have shown that neural control of postural sway is compromised in subjects with untreated PD [15] and levodopa administration increases several postural sway parameters [16]. We recently demonstrated that accelerometer-based measures of postural sway can distinguish subjects with untreated PD from age-matched control subjects. Sway jerkiness was shown to be one of the most discriminative measures in differentiating untreated PD from age-matched control subjects. JERK is an indicator of sway smoothness; a higher value indicates more jerky or less smooth sway (i.e. more postural sway corrections). It reflects corrections made by the nervous system to control sway while maintaining a quiet, upright posture.
In the current pilot study, we investigated whether this type of accelerometer-based analysis of spontaneous sway could provide a feasible tool for monitoring progression of postural dyscontrol in people with untreated or newly treated PD. Monitoring progression of postural control with accelerometer-based measures may represent a practical, inexpensive alternative to force plate measures of postural sway because of its unobtrusiveness and portability, key factors for use in clinical or community setting.
Methods
Subjects
We followed 13 subjects with idiopathic PD (7 male and 6 female, 60.4±8.5 years) and 12 healthy, age-matched control subjects (5 male and 7 female, 60.2±8.2 years) for 12 months. Control subjects were free of any neurological or musculoskeletal impairment that could affect postural control and were either spouses or friends of subjects with PD. A movement disorders neurologist diagnosed our PD subjects. The PD subjects were free of musculoskeletal disorders and, as inclusion criteria, were not taking any antiparkinsonian medications at the beginning of the study [15]. All subjects were tested at baseline, a second time 3 to 6 months after the baseline session (3-6m), and a third time 12 months after the baseline session (12m). Eight out of thirteen PD subjects started on low dose antiparkinsonian medication before the second test session, and were subsequently tested in the practical ‘off’ state, after withholding their medication for approximately 12 hours overnight. Some of the subjects were untreated because their disease was mild, while others were untreated due to personal choice, despite clinicians' concerns. A summary of the subject characteristics and their antiparkinsonian medication, as well as start of medication are summarized in Table 1.
Table 1.
Subject characteristics (individual and group means ± SEM).
Abbreviations: H&Y=Hoehn and Yahr scale, PIGD=Postural Instability and Gait Disability subscore.
Dopamine agonist: ROP=Ropinirole, PRM=Pramipexole, ROT=Rotigine DOPA=Carbidopa-Levodopa
| Subj | UPDRS III | H&Y | Bradykinesia | Rigidity | PIGD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | 3-6m | 12m | Base | 3-6m | 12m | Base | 3-6m | 12m | Base | 3-6m | 12m | Base | 3-6m | 12m | 3-6m | |
| P1 | 32 | 35 | 46 | 2 | 2 | 2 | 13 | 14 | 18 | 9 | 8 | 12 | 1 | 2 | 2 | Donepezil 5mg/day |
| P2 | 33 | 33 | 39 | 2 | 2 | 2 | 17 | 17 | 20 | 9 | 9 | 12 | 0 | 0 | 0 | |
| P3 | 45 | 46 | 46 | 2.5 | 2.5 | 2 | 19 | 18 | 18 | 10 | 12 | 13 | 2 | 2 | 2 | ROP .25mg/day |
| P4 | 21 | 32 | 30 | 2 | 2 | 2 | 9 | 12 | 8 | 7 | 10 | 9 | 1 | 2 | 2 | PRM 3mg/day |
| P5 | 33 | 32 | 39 | 2 | 2 | 2 | 16 | 12 | 14 | 8 | 8 | 10 | 2 | 2 | 3 | PRM .125mg/day |
| P6 | 12 | 13 | 13 | 1 | 1 | 1 | 4 | 5 | 5 | 3 | 4 | 3 | 0 | 0 | 0 | |
| P7 | 29 | 25 | 2 | 2 | 22 | 18 | 2 | 2 | 0 | 0 | PRM .75mg/day | |||||
| P8 | 17 | 15 | 19 | 1 | 1 | 1 | 10 | 8 | 12 | 1 | 1 | 1 | 0 | 0 | 0 | |
| P9 | 46 | 52 | 49 | 3 | 3 | 2.5 | 21 | 24 | 22 | 12 | 16 | 15 | 5 | 7 | 5 | DOPA 75mg/day |
| P10 | 18 | 22 | 23 | 1.5 | 1.5 | 1.5 | 8 | 10 | 10 | 4 | 4 | 4 | 0 | 1 | 1 | PRM .75mg/day |
| P11 | 35 | 34 | 2 | 2 | 19 | 18 | 5 | 4 | 1 | 1 | ||||||
| P12 | 18 | 9 | 14 | 1 | 1 | 1 | 10 | 5 | 9 | 5 | 2 | 2 | 0 | 0 | 0 | ROT 5mg/day |
| P13 | 7 | 15 | 9.5 | 1 | 2 | 1 | 1 | 3 | 2 | 1 | 5 | 1 | 0 | 1 | 0 | |
|
|
||||||||||||||||
| Mean | 26.6 | 28.2 | 29.4 | 1.8 | 1.8 | 1.7 | 13.0 | 12.2 | 13.0 | 5.8 | 6.9 | 7.0 | 0.9 | 1.5 | 1.3 | |
| SEM | 3.5 | 3.9 | 4.1 | 0.2 | 0.2 | 0.2 | 1.9 | 1.8 | 1.9 | 1.0 | 1.3 | 1.5 | 0.4 | 0.6 | 0.5 | |
Patients were clinically rated on the Motor Section (III) of the Unified Parkinson's Disease Rating Scale and the Hoehn and Yahr Scale by a trained examiner immediately before each experimental session as summarized in Table 1. All participants provided informed consent for a protocol approved by the Oregon Health & Sciences University Institutional Review Board.
Protocol and Postural Measures
Subjects were asked to stand quietly for 2 minutes while looking at an art poster 6 meters ahead. Their arms were crossed and heel-to-heel distance was standardized at 10 cm [17]. The feet were externally rotated to a comfortable position for each subject. Each trial was repeated 3 times and mean values for these 3 trials were reported for all subjects.
Subjects wore one MTX Xsens sensor (49A33G15, XSens, Enschede, NL) with 3-D accelerometers (±1.7g range) and 3-D gyroscopes, (±300°/s range) mounted on the posterior trunk at the level of L5, near the body center of mass. The sensing axes were oriented along the anatomical antero-posterior (AP), medio-lateral (ML), and vertical directions. The sensor was connected via a cable to a data transmitter located on a belt around the waist.
Pre-processing of acceleration signals has been described previously in Mancini et al. [15]. We computed 4 measures of postural sway from the acceleration signals, including: 1) Sway dispersion, as the root mean square relative to the mean (RMS), 2) Sway velocity, from the mean velocity (MV), 3) Frequency of Sway, as the highest frequency of sway comprising the 95% of the power (F95%), and 4) Jerkiness of sway (JERK), from the first derivative of the acceleration signal [15]. This set of parameters was computed independently for the antero-posterior (AP) and medio-lateral (ML) directions of sway.
Statistical analyses
To assess the longitudinal changes in postural sway measures (baseline, 3-6m, and 12m) and the differences between the two groups (PD and control), we performed a linear mixed model analysis followed by a Bonferroni pair-wise correction for multiple comparisons.
Since 8 patients started medication between baseline and 3-6m and 5 did not, PD subjects were then clustered into two subgroups for further analysis (PD Med and PD noMed). As a secondary analysis sensitivity to change between baseline and 12m (both for sway measures and clinical scores) was investigated by the Standardized Response Mean (SRM) which is the mean change (d) reported in units of standard deviation of change (SDdiff), SRM=d/SDdiff[18]. For SRM, a value of 0.20 represents a small change, 0.50 a moderate change, and 0.80 a large change [18]. The linear mixed model analysis and Pearson's correlation were performed with NCSS Software, Kaysville, Utah.
Results
Changes in sway measures over one year
Trunk accelerations from a representative control subject and an untreated PD subject are shown at baseline, 3-6m and 12m in Figure 1. While traces of the control subject did not change across time, there was a progressive increase in the AP and ML acceleration excursions for the PD subject (P13).
ML sway measures detected more differences between control and PD subjects than the AP sway measures (see Table 2). The ML JERK was significantly larger in the PD group compared to the control group at all three time points (p=0.04, p=0.002, p=0.01). The ML sway dispersion (p=0.04) and sway velocity (p=0.03) were significantly larger in the PD group compared to the control group at 12m. In addition, the AP JERK was larger in the PD group compared to the control group at baseline (p=0.03). Longitudinally, the ML JERK (p=0.04) and AP sway frequency (p=0.01) were different from baseline to 3-6m testing in the PD group.
Sway measures did not change in the healthy control subjects across time and showed low variability between subjects and across the three evaluations during the 12-month period (Figure 2).
Untreated PD subjects showed an increase in ML JERK, sway dispersion, velocity, and frequency between the baseline and 12m session (SRM=.56, .90, .53, .59, respectively), suggesting a progressive deterioration of their postural control. In contrast, the treated PD subjects had a slight decrease in ML sway dispersion and ML sway velocity from baseline to 12m (SRM=-.38, -.39 respectively).
Figure 1.
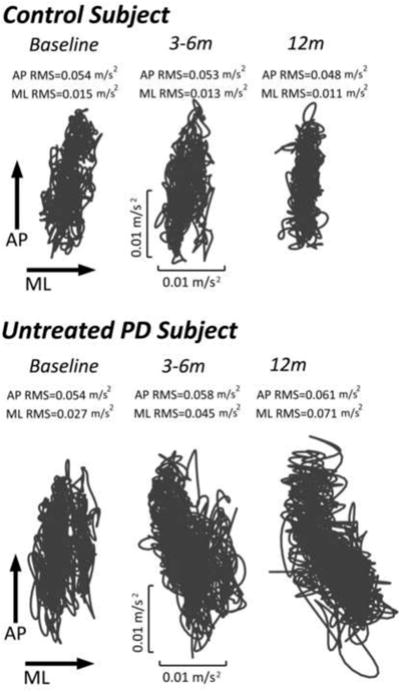
Lower trunk acceleration trajectories in the horizontal plane for representative control and PD (P13) subjects across time.
Table 2.
Mean (S.E.M) of trunk acceleration measures in control and PD subjects across time. The F-values and P-values for each sway measure and statistical differences, between groups (*) and differences between time-points (#) are shown.
| Repetitions:Baseline | 3-6m | 12m | Repetitions | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | |||||
| AP JERK(m2/s5) | CTR | 0.0104 | 0.0013 | 0.0093 | 0.0012 | 0.0084 | 0.0007 | F-value | 0.67 | |
| PD | 0.0181 | 0.0031 | 0.0205 | 0.0051 | 0.0147 | 0.0025 | P-value | 0.5 | ||
|
| ||||||||||
| ML JERK(m2/s5) | CTR | 0.0067 | 0.0021 | 0.0038 | 0.0005 | 0.0032 | 0.0002 | F-value | 3.5 | |
| PD | 0.0169 | 0.0038 | # | 0.0348 | 0.0092 | 0.0281 | 0.0113 | P-value | 0.04 | |
|
| ||||||||||
| AP RMS(m/s2) | CTR | 0.075 | 0.007 | 0.076 | 0.012 | 0.072 | 0.008 | F-value | 0.18 | |
| PD | 0.096 | 0.011 | 0.083 | 0.009 | 0.094 | 0.010 | P-value | 0.82 | ||
|
| ||||||||||
| ML RMS(m/s2) | CTR | 0.028 | 0.003 | 0.019 | 0.002 | 0.019 | 0.002 | F-value | 2.23 | |
| PD | 0.040 | 0.008 | 0.033 | 0.004 | 0.034 | 0.003 | P-value | 0.11 | ||
|
| ||||||||||
| AP MV(m/s) | CTR | 1.19 | 0.23 | 1.46 | 0.44 | 1.14 | 0.18 | F-value | 0.09 | |
| PD | 1.60 | 0.30 | 1.59 | 0.31 | 1.88 | 0.30 | P-value | 0.9 | ||
|
| ||||||||||
| ML MV(m/s) | CTR | 0.47 | 0.08 | 0.31 | 0.06 | 0.31 | 0.05 | F-value | 0.64 | |
| PD | 0.1.5275 | 0.14 | 0.69 | 0.13 | 0.71 | 0.11 | P-value | 0.53 | ||
|
| ||||||||||
| AP F95%(Hz) | CTR | 1.52 | 0.11 | 1.57 | 0.11 | 1.52 | 0.09 | F-value | 3.25 | |
| PD | 1.28 | 0.15 | # | 1.94 | 0.18 | 1.42 | 0.16 | P-value | 0.04 | |
|
| ||||||||||
| ML F95%(Hz) | CTR | 2.25 | 0.16 | 2.40 | 0.12 | 2.52 | 0.09 | F-value | 1.03 | |
| PD | 2.59 | 0.09 | 2.76 | 0.11 | 2.63 | 0.13 | P-value | 0.36 | ||
Figure 2.
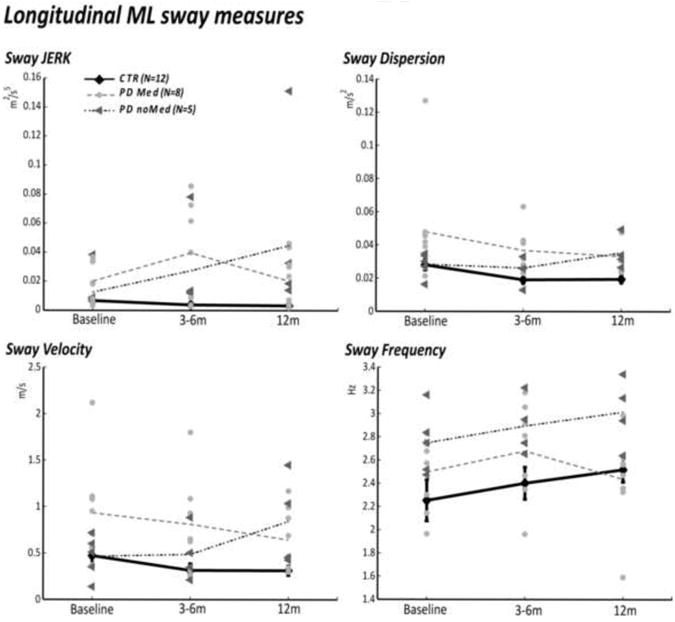
Comparison of group mean sway measures in PD treated (PD Med), non-treated (PD noMed), and control subjects across time. Note that the PD Med subjects were tested OFF medication.
Changes in Clinical Measures over one year
In contrast to the inertial sensor sway measurements, the Motor UPDRS, the PIGD sub-scores, the Bradykinesia and Rigidity sub-scores did not show change over time (p>0.05). When the PD groups were divided into the treated and untreated subgroups (Figure 3), the treated subgroup showed a tendency to have more severe UPDRS scores than the untreated group at all 3 measurements times. No worsening was observed in UPDRS sub-scores between the baseline and 12m sessions.
Figure 3.

Left panel: Mean clinical measures in PD subjects across time.
Right panel: Comparison of group mean clinical measures in PD treated (PD Med) and non-treated (PD noMed) across time.
Discussion
Results of this pilot longitudinal study suggest that quantitative postural sway measures are able to identify postural dyscontrol early in PD progression and thus may be useful to study progression of Parkinson's disease in early stages of the disease. However, trends toward a slowing in progression of postural sway dyscontrol with initiation of levodopa suggests that more studies are needed to determine if initiation of medication in early PD masks progression of disease.
In this pilot study, we did not find a significant interaction between longitudinal repetition and groups, which would have supported a significant decline in control of postural sway in the PD, but not the control group. This may be due differences in the longitudinal changes in postural sway between the patients who started antiparkinsonian medication during the 12 months and those who remained drug free. The qualitative analysis carried out with the Standardize Response Mean suggests a progressive worsening in sway measures in untreated PD subgroup only. However, more studies are needed to determine whether progression of postural sway in PD is affected by postural control at baseline or by initiating levodopa treatment. Interestingly, the UPDRS Motor Score didn't show any change across time.
These results suggest accelerometer-based technology might be a useful means of monitoring disease progression in untreated subjects during quiet standing. However, a larger sample is needed with untreated PD subjects to confirm these results, as well as a longer observation period.
In this pilot study, we were not able to find a sample of untreated PD subjects who were willing to withhold starting antiparkinsonian medication therapy for one year. In fact, only 5 of the 13 subjects did not start medication over the 12 months after their baseline measure, and the UDPRS scores at baseline of these untreated subjects were lower than those who did start medication. Unlike the group that were untreated, the PD subgroup that started medication at 3-6 months, showed trends toward improvements in postural sway jerk, velocity and RMS. This might suggest that disease progression will not be tracked by these measurements once the subjects start antiparkinsonian medications.
The PD subjects who started medication after baseline were clinically more affected than those who did not. Subjects who started medication were taking very small doses of levodopa/carbidopa or dopamine agonists. Although this group of 8 subjects started with significantly worse postural sway and clinical signs, they did not show the same negative progression of postural control as the untreated subjects, although they were tested after withholding their medication for at least 12 hours. This lack of deterioration of postural sway might be related to improved central control of posture from increasing daily activity and exercise due to the medication, masking decline in postural control associated with PD progression.
The fact that lateral sway measures identify more differences than the antero-posterior measures between PD and control groups is consistent with previous studies showing that ML sway is more predictive of future falls [19-21] and sensitive to moderate-severe PD [7,22]. Differences in the results of the two directions of sway to PD may be related to the fact that lateral sway involves control of hip and trunk muscles whereas antero-posterior sway involves control of ankle muscles, that may be less affected by PD [23,24]. A decrease in sway frequency, as seen in the more affected PD subjects in this study, has been associated with an inverted pendulum, ankle sway strategy rather than a more normal, multi-segmental, hip strategy [25]. These changes in postural sway across 12 months appear to be more sensitive to disease duration than the Motor UPDRS, PIGD, bradykinesia or rigidity sub-scores.
To determine whether postural sway or other objective measures of posture control could be used as a biomarker for neuroprotective interventions, additional longitudinal studies are needed to characterize postural instability progression in a larger sample of subjects with early PD who have never taken antiparkinsonian medication. Also, other studies are needed to characterize the effect of antiparkinsonian medication on progression of postural control in PD.
Accelerometer-based analysis of postural sway could provide a simple, but sensitive, tool to monitor progression of early PD. Also, the standing test with an accelerometer on the belt is so simple that it is feasible to ask patients to measure their own postural sway at home. If our results are confirmed in larger studies, this new technology has the potential to ultimately aid in the clinical care of patients and serve as a key instrument to assess postural abnormalities in clinical trials.
Highlights.
Objective sway measures deteriorated over one year
UPDRS motor scores didn't show significant changes over one year
Medio-lateral (ML) sway measures were more sensitive than antero-posterior sway measures in detecting progression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marek K, Jennings D, Tamagnan G, Seibyl J. Biomarkers for Parkinson's [corrected] disease: tools to assess Parkinson's disease onset and progression. Ann Neurol. 2008 Dec;64(2):S111–21. doi: 10.1002/ana.21602. [DOI] [PubMed] [Google Scholar]
- 2.Rossi M, Soto A, Santos S, Sesar A, Labella T. A prospective study of alterations in balance among patients with Parkinson's Disease. Protocol of the postural evaluation. Eur Neurol. 2009;61(3):171–6. doi: 10.1159/000189270. [DOI] [PubMed] [Google Scholar]
- 3.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967 May;17(5):427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 4.Fahn S, Elton R. The UPDRS Development Committee Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent Developments in Parkinson's Disease. Florham Park, New Jersey: Macmillan Healthcare Information; 1987. pp. 153–63. [Google Scholar]
- 5.Bloem BR, Beckley DJ, van Hilten BJ, Roos RA. Clinimetrics of postural instability in Parkinson's disease. J Neurol. 1998 Oct;245(10):669–73. doi: 10.1007/s004150050265. [DOI] [PubMed] [Google Scholar]
- 6.Schrag A, Spottke A, Quinn NP, Dodel R. Comparative responsiveness of Parkinson's disease scales to change over time. Mov Disord. 2009 Apr 30;24(6):813–8. doi: 10.1002/mds.22438. [DOI] [PubMed] [Google Scholar]
- 7.Chastan N, Debono B, Maltete D, Weber J. Discordance between measured postural instability and absence of clinical symptoms in Parkinson's disease patients in the early stages of the disease. Mov Disord. 2008 Feb 15;23(3):366–72. doi: 10.1002/mds.21840. [DOI] [PubMed] [Google Scholar]
- 8.Beuter A, Hernandez R, Rigal R, Modolo J, Blanchet PJ. Postural sway and effect of levodopa in early Parkinson's disease. Can J Neurol Sci. 2008 Mar;35(1):65–8. doi: 10.1017/s0317167100007575. [DOI] [PubMed] [Google Scholar]
- 9.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002 Sep;88(3):1097–118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 10.Maurer C, Mergner T, Peterka RJ. Abnormal resonance behavior of the postural control loop in Parkinson's disease. Exp Brain Res. 2004 Aug;157(3):369–76. doi: 10.1007/s00221-004-1852-y. [DOI] [PubMed] [Google Scholar]
- 11.Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38(1):35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- 12.Wright WG, Gurfinkel VS, Nutt J, Horak FB, Cordo PJ. Axial hypertonicity in Parkinson's disease: direct measurements of trunk and hip torque. Exp Neurol. 2007 Nov;208(1):38–46. doi: 10.1016/j.expneurol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visser JE, Carpenter MG, van der Kooij H, Bloem BR. The clinical utility of posturography. Clin Neurophysiol. 2008 Nov;119(11):2424–36. doi: 10.1016/j.clinph.2008.07.220. [DOI] [PubMed] [Google Scholar]
- 14.Piirtola M, Era P. Force platform measurements as predictors of falls among older people - a review. Gerontology. 2006;52(1):1–16. doi: 10.1159/000089820. [DOI] [PubMed] [Google Scholar]
- 15.Mancini M, Horak FB, Zampieri C, Carlson-Kuhta P, Nutt JG, Chiari L. Trunk accelerometry reveals postural instability in untreated Parkinson's disease. Parkinsonism Relat Disord. 2011 Aug;17(7):557–62. doi: 10.1016/j.parkreldis.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002 Sep;73(3):267–74. doi: 10.1136/jnnp.73.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIlroy WE, Maki BE. Preferred placement of the feet during quiet stance: development of a standardized foot placement for balance testing. Clin Biomech (Bristol, Avon) 1997 Jan;12(1):66–70. doi: 10.1016/s0268-0033(96)00040-x. [DOI] [PubMed] [Google Scholar]
- 18.Moe-Nilssen R, Nordin E, Lundin-Olsson L. Criteria for evaluation of measurement properties of clinical balance measures for use in fall prevention studies. J Eval Clin Pract. 2008 Apr;14(2):236–40. doi: 10.1111/j.1365-2753.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- 19.Melzer I, Benjuya N, Kaplanski J. Postural stability in the elderly: a comparison between fallers and non-fallers. Age Ageing. 2004 Nov;33(6):602–7. doi: 10.1093/ageing/afh218. [DOI] [PubMed] [Google Scholar]
- 20.Melzer I, Kurz I, Oddsson LI. A retrospective analysis of balance control parameters in elderly fallers and non-fallers. Clin Biomech (Bristol, Avon) 2010 Dec;25(10):984–8. doi: 10.1016/j.clinbiomech.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994 Mar;49(2):M72–84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell SL, Collins JJ, De Luca CJ, Burrows A, Lipsitz LA. Open-loop and closed-loop postural control mechanisms in Parkinson's disease: increased mediolateral activity during quiet standing. Neurosci Lett. 1995 Sep 8;197(2):133–6. doi: 10.1016/0304-3940(95)11924-l. [DOI] [PubMed] [Google Scholar]
- 23.Kuo AD, Speers RA, Peterka RJ, Horak FB. Effect of altered sensory conditions on multivariate descriptors of human postural sway. Exp Brain Res. 1998 Sep;122(2):185–95. doi: 10.1007/s002210050506. [DOI] [PubMed] [Google Scholar]
- 24.Henry SM, Fung J, Horak FB. Effect of stance width on multidirectional postural responses. J Neurophysiol. 2001 Feb;85(2):559–70. doi: 10.1152/jn.2001.85.2.559. [DOI] [PubMed] [Google Scholar]
- 25.Creath R, Kiemel T, Horak F, Peterka R, Jeka J. A unified view of quiet and perturbed stance: simultaneous co-existing excitable modes. Neurosci Lett. 2005 Mar 29;377(2):75–80. doi: 10.1016/j.neulet.2004.11.071. [DOI] [PubMed] [Google Scholar]


